Abstract
Verthimia iphionoides extract from Palestine was tested in vitro for its antioxidant, antibacterial, and anticancer activities. Total phenolic content (TPC) and total flavonoid content (TFC) measurements were made concurrently. By using FRAP and DPPH methods, the antioxidant activity were measured spectrophotometrically. By using HPLC-PDA, phenolic and flavonoid compounds of the extract were determined. Results showed strong antioxidant activity of the plant extract revealed by inhibition of stable free radicals (DPPH test) and strong reducing ability (FRAP test). According to spectrophotometric methods for total phenolic compounds and total flavonoids content, the extracts were also found to be rich in polyphenolic compounds and flavonoids. Verthimia iphionoides extract had high antibacterial activity against three bacterial strains (Escherichia coli, Staphylococcus aureus, and Streptococcus aureus), with inhibition zone values of 14 mm, 25 mm, and 27 mm, respectively. Bioactivities were primarily attributed to plants’ abundant phenol-based chemical composition. Additionally, the extract was found to be abundant in phenolic and flavonoids, which improved its reducing activity and capacity to scavenge free radicals. Plant extracts were subjected to HPLC analysis, which identified different flavonoids and phenolic compounds in the extracts.
1. Introduction
In the Middle East, particularly in Palestine, herbs are utilized medicinally in addition to being a source of food [1]. Palestine has a diversified ecosystem due to its geographic location. Palestine possesses a wide range of folklore herbal remedies [2,3,4]. Varthemia iphionoides is a plant that grows in Palestine and has recently attracted attention. Like many other plants, V. iphionoides has a wide range of therapeutic activities, including the treatment of diabetes, stomach aches, issues with male and female fertility, and many more. However, more studies must be done on this plant in order to identify additional treatment applications [2].
Many studies have been carried out on plants, during which the active compounds and the role of these compounds in anticancer, antioxidant, and antibacterial effects are clarified [5,6]. Anticancer compounds have the power to both trigger apoptosis and stop the growth of cancer cells [7]. Numerous infections are fought off by antimicrobial action, especially now that many microorganisms have developed a resistance to synthetic antibiotics. According to the US Center for Disease Control and Prevention (CDC), more than 2 million Americans contract antibiotic-resistant illnesses each year [8]. In addition, it was estimated that 40,000 illness and fatalities in Europe were brought on by bacteria that were multidrug resistant each year [7,8]. Due to their antibacterial properties, medicinal plants have recently attracted the attention of many scientists and researchers [9,10,11].
Plants are investigated for their antioxidant activity, which counteracts oxidation, in addition to their antibacterial activity. Oxidative alterations are brought on by the formation of free radicals and reactive oxygen species (ROS) [12]. A buildup of free radicals damages DNA, enzymes, cellular proteins, and membrane lipids, which can lead to cellular damage. The negative side effects of excessive oxidation are combated by antioxidants [13,14].
Cancer treatment options include surgery, radiotherapy, and chemotherapy. However, there has been an emphasis on the use of plants in anti-cancer treatment due to the different negative effects linked to these alternatives [15]. The focus has shifted back to plant extracts and their phytochemicals because the chemically synthesized medications are typically dangerous [16]. Numerous studies have provided sufficient proof that plant extracts and phytochemicals are effective treatments with few side effects [17,18].
The active substances found in plants, known as secondary metabolites, include phenolics and flavonoids, alkaloids, glycosides, and terpenoids, which are crucial for a variety of vital biological processes [19]. More than 50,000 secondary metabolites from the plant kingdom have been identified. Depending on the requirements of the plants, they might be viewed as either attractants or repellents [19,20]. Both the plant and other living organisms benefit from the presence of these secondary metabolites. These secondary metabolites, which can function as antimicrobials, antioxidants, anticancer compounds, and anti-inflammatories, are what medicinal herbs rely on. Antioxidants and other significant bioactive substances, such as phenolic compounds, are well-known for their positive effects on human health [20,21]. They are employed in both the treatment and prevention of many disorders. They also have antibacterial, anticancer, cardioprotective, and anti-inflammatory actions in addition to their antioxidant activities. Additionally, they support the immunological system [20]. Flavonoids make up the biggest class of phenolic compounds that are found in nature. Fruits, vegetables, cereals, steams, flowers, and roots all contain flavonoids [22,23,24]. The antioxidative, anti-inflammatory, anticarcinogenic, and antimutagenic effects of flavonoids are well established [25]. Additionally, they have a variety of biochemical and antioxidant properties linked to illnesses such as cancer, Alzheimer’s disease, atherosclerosis, and many others [26,27,28].
V. iphionoides belong to the Asteraceae family, the genus Chiliadenus, and is growing all throughout the Eastern Mediterranean. The 20–50-cm-long V. iphionoides has small leaves and a woody base with several branches. It features tubular yellow blooms and sticky stems. The period of blooms for this plant is in September and December [29]. Wild populations of V. iphionoides can be found in rocky terrain, deserts, and harsh deserts in the Mediterranean, Sahara, and Iran. It is found in Palestine, Jordan, Syria, and Lebanon, among others. Traditional medicine has frequently employed V. iphionoides for a variety of medical conditions, including kidney stones, stomach disorders, diabetes, eye infections, and problems with male and female fertility. Additionally, V. iphionoides has demonstrated a variety of pharmacological therapeutic activities, including anticancer, antibacterial, antioxidant, and antiplatelet activity and in the treatment of inflammation that leads to cancer, especially prostate cancer [30,31,32].
Studying the antioxidant, antibacterial, and anticancer activities of the extract of V. iphionoides is consequently one of the study’s goals.
2. Materials and Methods
2.1. Plant Material
The plant was collected by herbalists from Palestine’s southern West Bank in September 2021 (Figure 1). The specimens were deposited in the herbarium of the biodiversity and environmental research center, BERC, Til Village, Nablus, under the reference numbers « BERC-BX-C-0135 ». Then, plant aerial parts were ground after being dried in the shade to a uniform weight.
Figure 1.
(a) Verthimia iphionoides plant and (b) flower of the Verthimia iphionoides plant.
2.2. Chemical and Instrument
Ethanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), catechin, gallic acid, acetic acid, sodium nitrite, aluminum chloride, copper chloride, ammonium acetate, neocuproine, sodium bicarbonate, and Mueller Hinton were purchased from Sigma-Aldrich. HPLC grade acetonitrile, methanol (MeOH) and water (H2O) were purchased from Sigma-Aldrich. Membrane filters (0.45 μm pore size) were purchased from Sigma-Aldrich. The acetonitrile and water were of an HPLC grade from Sigma. Phenolic and flavonoids standards vanillic acid, ferulic acid, syringic acid, trans-cinnamic acid, catechin, p-coumaric acid, sinapic acid, 4-hydroxyphenylacetic acid, rutin hydrate, caffeic acid, quercetin, gallic acid, 3,4-dihydroxyphenylacetic acid, chlorogenic acid, taxifolin, luteolin 7-glucoside, apigenin 7-glucoside, luteolin, and quercetin 3-D-galactose were from Sigma.
The instruments used in this study were specord40 UV VIS spectrum, versatile single-beam spectrophotometer for the measurement of 190–1100 nm (Analytikjena Company), rotary evaporator, water bath, ultrasonic homogenizer, and autoclave. The analytical HPLC was Waters Alliance (e2695 separations module), equipped with 2998 Photodiode Array (PDA) detector. Data acquisition and control were carried out using Empower 3 chromatography data software (Waters, Germany).
2.3. Plant Extraction
First, 100 g of powdered plant material was soaked for two hours in 1 L of 100% and 50% ethanol using an ultrasonic bath. The mixture was then filtered, and the solvent was evaporated under reduced pressure at 45 °C using rotary evaporator, and then the concentrated paste extract was dissolved in methanol or dimethyl sulfoxide (DMSO; 2%) to a final concentration of 0.1 g/mL. The extracts were dissolved in dimethyl sulfoxide to study the anticancer and antibacterial activities, and in methanol to study the antioxidant activity.
2.4. Anticancer Testing
For the anticancer testing, the whole experiment was conducted in a tissue culture hood. HT29 cells and MCF7 were cultured in RPMI media and incubated for 24 h before treatment with the extracts for the proliferation and growth of the cells. The extracts were prepared in DMSO (2%) 0.1 g/mL. Then, 20,000 cell/100µL media was seeded in 96-microtiter plates. The cells were incubated with two volumes of each extract (50 µL and 100 µL) for 72 h at 37 °C [18]. Finally, 100 µL of DMSO (2%) was used as control.
2.5. Antimicrobial Testing
For the antimicrobial testing, Gram-positive bacteria Streptococcus faecalis and Staphylococcus aureus and the Gram-negative Escherichia coli were used to test the activity of V. iphionoides plant extract. The Mueller Hinton agar media was prepared by weighing 19 g of the agar media and 500 mL of distilled water in an Elynmeyer flask. The mixture was boiled and autoclaved for 15 min at 121 °C. After the autoclave, the Mueller Hinton agar media was left to cool down and was poured into six the culture Petri dishes to solidify. E.coli, S. faecalis, and S. aureus were cultured onto petri dishes by obtaining pure colonies and inoculating them into small vinyl tubes with distilled water. The inoculum was prepared according to the 0.5 McFarland turbidity standard (1.5 × 108 CFU/mL). As the inoculum was prepared, the turbidity was regularly assessed using the McFarland card or a white card that has horizontal black lines. The standard can be compared to the inoculum until it matches the 0.5 McFarland turbidity standard. Using an inoculating loop sample of the inoculum were swabbed and streaked onto the surface of the agar media in the six petri dishes [21].
The testing for the assessment of the antimicrobial activity was conducted using the well diffusion method; the wells were formed on the media using the back of the 100 µL pipette tip, in which it was pushed into the agar media, then removed to pulls the contents out leaving behind a circular whole for the extract to be pipetted in. The extract of the V. iphionoides was filled in the wells. After that, the Petri dishes were incubated in the incubator at 37 °C for 24 h. In this experiment, the DMSO (2%) was used as control.
2.6. Total Phenolic Content (TPC) Determination
Folin–Ciocâlteu reagents were used to calculate the total phenolics [22]. A volume of 1.8 mL of Folin–Ciocâlteu reagent was combined with 50 µL of plant extracts, and the mixture was let to stand at room temperature for 5 min. After that, 1.2 mL of 7.5% sodium bicarbonate was added. At 765 nm, absorbance was determined after standing at room temperature for 60 min. For calibration curve, aqueous solutions with known concentrations of gallic acid between 20 and 500 mg/L were utilized. Gallic acid equivalents (GAE) were used to express the results of total phenolic content.
2.7. Total Flavonoid Content (TFC) Determination
The spectrophotometric method was used to determine TFC using aluminum chloride [22]. In a test tube, 1 mL of the extract was combined with 5 mL of water. Then, 0.3 mL of a 10% aluminum chloride solution and 0.3 mL of a 5% sodium nitrite solution were added. After 5 min of ambient temperature incubation, 2 mL of 1 M sodium hydroxide was added to the test tubes. With distilled water, the reaction mixture’s volume was immediately made to 10 mL. Using a test tube shaker, the mixture was well stirred, and the absorbance of the pink color that resulted was measured at 510 nm. For calibration curve, aqueous solutions with different concentrations of catechin between 20 and 100 mg/L were used. The results are reported as mg catechin per g of extract.
2.8. Free Radical Scavenging Activity Using DPPH
The basis of the DPPH assay is the evaluation of antioxidants’ capacity to scavenge the stable DPPH free radical [24]. To 0.1 mL of the extract, 3.9 mL of a 0.062 mM DPPH solution in 95% methanol was added. For 10 s, the mixture was vortexed. Until the sample extract’s absorbance reached a steady state after 30 min, the change in absorbance was monitored at 515 nm. As a blank, we used 95% methanol. The standard curve was created utilizing various Trolox concentrations (20 to 200 ppm). The results of DPPH assay were given as µmol Trolox/g.
2.9. Ferric Reducing/Antioxidant Power (FRAP Assay)
The assay of FRAP was used to measure the extracts’ antioxidant activity. First, 3.0 mL of freshly made FRAP reagent was warmed to 37 °C for 4 min and combined with 40 µL of the extract before being incubated at this temperature. The absorbance at 593 nm was read using water as blank. For calibration curve, aqueous solutions with known concentrations of Fe+2 in the range of 2–8 mM were used, and results were represented as mmoL Fe+2/g [23].
2.10. Analysis of Phytochemicals by HPLC
2.10.1. HPLC Conditions
Polyphenolic substances were analyzed using HPLC Waters Alliance with a PDA detector. Software called Empower 3 for chromatographic data was used for data collecting. A reversed phase HPLC method with a C18 column (25 cm, 3.6 m inner diameter), a blend of 0.5% acetic acid (solution A) and acetonitrile (solution B), and a linear gradient mode in accordance with the table below was used to analyze different polyphenolic chemicals and flavonoids (Table 1). The column temperature was 25 °C, the flow rate was set at 0.5 mL/min, and the injection volume was 20 µL. A disposable 0.45 µm filter was used to filter all samples. A photodiode array detector with a 210–400 nm wavelength range was used.

Table 1.
The gradient mobile phase conditions that were utilized for the RP-HPLC analysis of the polyphenolic chemicals and flavonoids in the plant extracts.
2.10.2. Standard Solutions Preparation
The standards that were used in HPLC analysis: gallic acid, caffeic acid, syringic acid, trans cinnamic acid, 3,4-dihydroxybenzoic acid, 3,4-dihydroxyphenylacetic acid, 4-hydroxyphenylacetic acid, rutin, ferrulic acid, quercetin, vanillic acid, isovanallic acid, kampferol, chlorogenic acid, verbacoside, sinapic acid, and p-coumaric acid. A measure of 20 µL of each standard (1 mg/mL) was injected into the HPLC chromatography.
2.10.3. Sample Preparation of the Extracts
First, 100 mg of dry extract was dissolved in 100 mL of ethanol (60%). Then, 20 µL of solution was injected in HPLC chromatography.
3. Results and Discussion
3.1. Analysis of Polyphenolic Standards
The HPLC chromatograph was used to analyze 20 µL of the combination of 17 standards using the RP-phase analysis technique that was previously discussed. Due to the fact that each compound has a different wavelength of peak absorption, the photodiode array detector was used at several wavelengths (Table 2). Figure 2 displays the chromatograms of the standards mixture at different wavelengths, including 300 nm (a), 323 nm (b), 270 nm (c), and 290 nm (d). Figure 2a–d makes clear that the 17 compounds were separated when various wavelengths were applied. In Table 2, the standards’ retention times are shown together with the standards’ maximum wavelengths of absorption.

Table 2.
HPLC retention times for selected standard compounds.
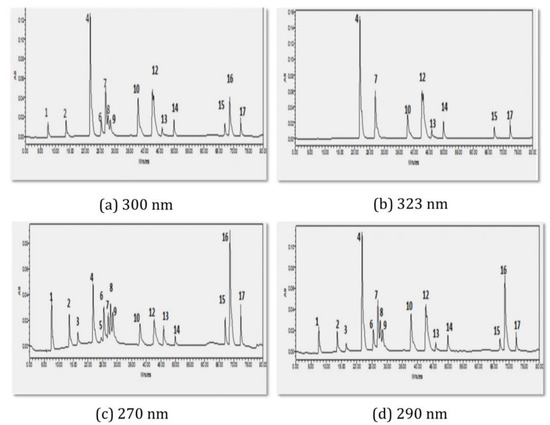
Figure 2.
HPLC chromatogram of polyphenolic and flavonoid standards analysed using RP-HPLC method at (a) 300 nm; (b) 323 nm; (c) 270 nm; and (d) 290 nm.
Figure 3 shows the chromatogram for V. iphionoides extract (50% ethanolic extract) at 2 wavelengths (300 and 250 nm). At 300 nm, 3,4-dihydroxybenzoic acid, kapmpferol, chlorogenic acid, quercetin, and syringic acid were detected in the plant extract, while at 250 nm, rutin, verbascoside, kampferol, quercetin, and chlorognic acid were detected in the chromatogram of the ethanolic extract. According to a study conducted by Abdelhalim and Al-Munawarah, the pharmacological properties of V. iphionoides (water and ethanolic extracts) are due to the existence of flavonoids and phenolic compounds [2].
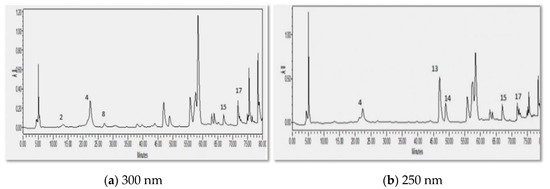
Figure 3.
HPLC chromatogram of V. iphionoides extract analyzed using RP-HPLC method at two different wavelengths, (a) 300 nm and (b) 250 nm.
3.2. Total Phenolic Content
Using a calibration curve of the absorbance at various contents of gallic acid, the total phenolic content of the extracts was determined using the Folin–Ciocâlteu method. Results were represented in mg of gallic acid/g extract. Table 3 displays the total phenolic content of the ethanolic extracts of V. iphionoides.

Table 3.
Bioactive compounds determination and antioxidant activity of V. iphionoides extract.
Total phenolic content for the extract with 50% ethanol concentration was found to be 88.2 ± 1.5 mg gallic acid/g. However, the extract with 100% ethanol concentration had a total phenolic content of 68.2 ± 1.9 (mg gallic acid/g). Results indicated that 50% has greater extractive potential than 100% ethanol extract for total phenolic compounds. This behavior may be attributed to the mixed mode of 50% ethanol containing water and ethanol which gives higher amounts of polyphenolic compounds. This has to do with polarity and the solubility properties of the active compounds in plant with the solvent.
A study by Abdelhalim and Al-Munawarah showed that different extracts and active ingredients of Chiliadenus iphinoides had anticancer, antimicrobial, antioxidant, and antiplatelet activities which might be due to the presence of flavonoids and phenolic compounds [2]. These results are in agreement with the study conducted by Al-Dabbas et al., whose polyphenol content was 72.37 ± 1.63 mg/g extract. Also during his study, he showed that the ethanol extract is better than other extracts, such as water, ethyl acetate, chloroform and hexane, to extract the phenolic compounds [21]. Moreover, the polyphenol content of 50% ethanol extract was higher with those reported by Fernandes et al. who showed in his study the polyphenol content ranged between 5.98 and 74.01 mg GAE/g when 13 plants were extracted using acetone, ultrapure water, and glacial acetic acid [33].
3.3. Total Flavonoid Content (TFC)
Total flavonoids content of the extracts was carried out using aluminum chloride method and results were expressed in mg catechin/g using calibration curve of the absorbance of different concentrations of catechin. Results showed that TFC was found to be 26.3 ± 1.2 and 15.8 ± 1.3 mg catechin/g for 50% and 100% ethanolic extract, respectively, Table 3. As for TPC, total flavonoids content was found to be higher for 50% ethanolic solvent compared to 100% ethanol solvent which can be explained by the polarity of the solvent and solubility of flavonoids in the solvent. These results show that the plant ethanolic extract is rich with phenolics and flavonoids. Al-Dabbas et al. showed that ethyl acetate extract has the highest content of flavonoids, followed by ethanol. The flavonoids content in his study was 24.26 ± 2.15 mg/g extract and this is consistent with the results in our study [21].
Several flavonoids have been isolated from C. iphionoides. Afifi et al. reported the isolation of xanthomicrol, kumatakenin, jaceidine, and 3,3′-di-O-methylquercetin [30].
3.4. Antioxidant Activity
DPPH radical scanning test is widely used to determine the antioxidant capacity of natural extracts [34]. The results of this test activity was found to be higher for 50% ethanolic extract compared to 100% ethanolic extract where the results obtained using the DPPH assay are 220.6 ± 3.29 and 188.6 ± 2.5 µmol Trolox/g, respectively, Table 3. This high antioxidant activity reflects that this ethanolic plant extract is rich with antioxidants which inhibit the free radical DPPH. These antioxidants can be polyphenolic compounds and flavonoids which are well known as efficient free radical scavengers. Polyphenolic and flavonoids antioxidants are primarily due to their redox properties which make them serve as reducing agents, donors of hydrogen, and singlet oxygen quenchers. Al-Dabbas et al. has studied DPPH activity of different extracts of V. iphionoides, and results showed high DPPH radical-scavenging activity in the water and ethanol extracts and were correlated to the contents of phenolic compounds [21]. Isolated flavonoids from C. iphionoides were found to exhibit a potent free radical scavenging activity by DPPH assay with inhibition of more than 60% at 200 μg/mL [31,35]. In addition, the results of DPPH assay in the present work were better than those conducted by Wojdyło et al. on 32 plants, in which the values ranged from 7.34 to 2021 µM trolox/100 g [36]. Another study carried out by Hamada on 14 plants, the results of scavenging free radicals ranged from 0.50 to 9.06 g trolox/100 g.
The results obtained using the FRAP assay are 9.56 ± 1.1 and 7.74 ± 1.3 mmol Fe + 2/g, respectively. From the results, it was found that the extracts have higher capacity in reducing the ferric ion (Fe3+) to ferrous ion (Fe2+) compared to scavenging free radicals. This is consistent with what Fernandes et al. explained in his study, as well the results of FRAP assay in his study ranged between 43.61 and 472.32 μmol trolox/g [33], and these values are lower than those in our study.
3.5. Anticancer Activity
The anticancer activity of the V. iphionoides plant extracts was tested for MCF7 breast cancer cell line as well as HT29 colon cancer cell line. DMSO was used as negative control for both cancer cell lines (Figure 4a) which has no activity against these cancer cell lines. Increased performance against cancer cell lines was assessed using cell count and calculating % of cell inhibition of cell line upon use of plant extract compared to the negative control (DMSO). Results showed that there is significant decrease in the cancer cell lines counts using both 50% and 100% extracts compared to negative control. This performance is higher at higher concentration (100 µL) compared to lower ones (50 µL) as shown in Table 4. The performance of the extract against MCF7 breast cancer cell line was found to be higher for 100% ethanolic extract compared to 50% ethanolic extract where the number of dead cancer cell lines increases (Figure 4 b–e). Table 4 summarizes the % of dead cancer cell lines for both MCF7 and HT29 cell lines. For MCF7 cancer cell lines, the highest % of dead cells (46%) was found to be for 100% ethanolic extract of V. iphionoides when 100 µL of extract was used.
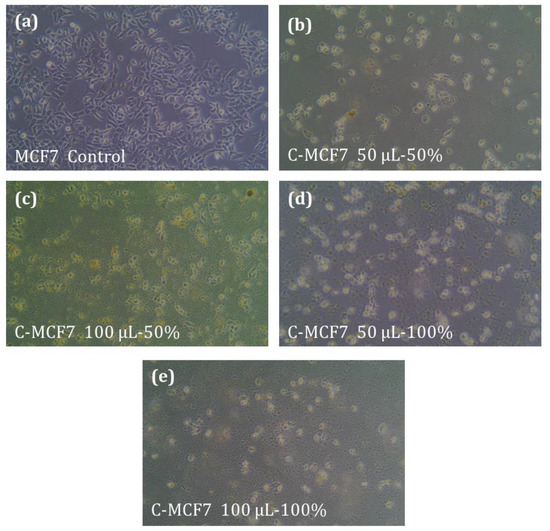
Figure 4.
Effect of plant extract on MCF7 cancer cell lines. (a) The control samples (DMSO); (b) 50 μL of 50% extract; (c) 100 μL of 50% extract; (d) 50 μL of 100% extract; (e) 100 μL of 100% extract. The figures are at magnification 400×.

Table 4.
Percentage of dead cells results by extracts.
In addition to MCF7 breast cancer cell lines, V. iphionoides extracts showed increased performance against HT29 colon cancer cell lines. As for MCF7 colon cancer cell lines, the performance of the extract against HT29 cancer cell line was found to be higher for 100% ethanolic extract compared to 50% ethanolic extract (Figure 5 b–e) where the highest % of dead cells was found to be 50% when 100 µL of 100% ethanolic extract was used, Table 4.
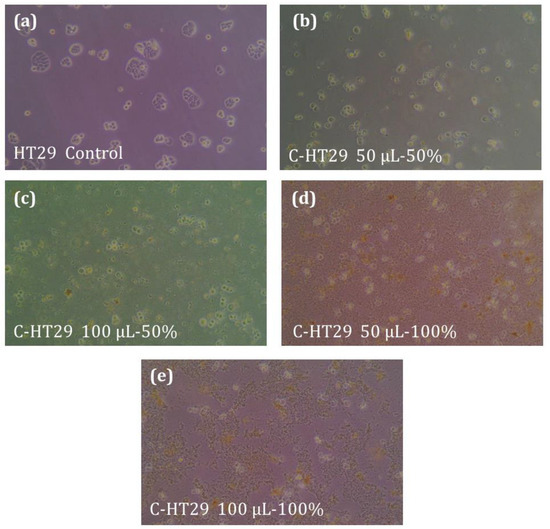
Figure 5.
Effect of plant extract on HT29 cancer cell lines. (a) The control samples (DMSO); (b) 50 μL of 50% extract; (c) 100 μL of 50% extract; (d) 50 μL of 100% extract; (e) 100 μL of 100% extract. The figures are at magnification 400×.
Comparing the performance of plant extract against the two cancer cell lines investigated in this study, results showed that the performance of V. iphionoides plant extract against HT29 is higher than that for MCF7 colon cancer cell lines using the same amount of extract.
It is interesting to compare the anticancer activity of the extract in this study with the extracts of this plant in other studies. Al-Dabbas et al. have studied different extracts against human myelocytic leukemia (HL-60) cells; and results showed a pronounced cytotoxic effect on HL-60 cells in the hexane, chloroform, and ethanol extracts, with inhibition of 89.0, 68.4, and 62.3%, respectively, at a concentration of 200 μg extract/mL [21]. Other studies also proved the anticancer activity of V. iphionoides extracts [37,38]. The V. iphionoides extract was showed to exhibit growth inhibition against human cancer cell lines related to the prostate (PC3) and breast (MCF7) in a study conducted by Abbas et al. [39].
3.6. Antimicrobial Activity
The disk diffusion method was used to test the plant extracts’ antibacterial activity against two Gram-positive bacteria (S. aureus and S. faecalis) and one Gram-negative bacteria (E. coli). With a zone of inhibition of 10, 15, and 16 mm, respectively, the results demonstrated high antibacterial activity of 100% ethanol extract against E. coli, S. aureus, and S. faecalis. In comparison to 100% ethanolic extract, 50% ethanol extract produced similar results against the tested bacteria but with a somewhat smaller zone of inhibition. The plant extract works better against Gram-positive bacteria than Gram-negative bacteria, as expected. This is explained by the fact that Gram-negative bacteria have a cell wall that is different from Gram-positive bacteria and more resistant to the plant extract. This is consistent with many studies that showed that Gram-positive bacteria are more sensitive than Gram-negative [40,41].
V. iphionoides had genuinely encouraging antibacterial activity against both Gram-positive and Gram-negative bacteria. V. iphionoides plant extracts have a highly promising effect on many microorganisms, especially in light of the advent of S. faecalis resistance to penicillin and other antibiotics as well as S. aureus resistance to various medicines [42], including methicillin-resistant S. aureus (MRSA). The presence of secondary metabolites in the plant, such as polyphenolic compounds and flavonoids, may be responsible for this antibacterial effect. The observed antimicrobial potential of V. iphionoides indicated that this plant possesses bioactive compounds that are able to combat pathogenic microorganisms and support its traditional use in the treatment of pathogen infection. Aabed et al. showed in his study that some phenolic compounds such as benzoic acids and fulvic acids increase the permeability of the plasma membrane, which leads to disturbances in cellular osmolarity and thus cell lysis [43]. In addition, many studies have shown the mechanism by which plant extracts work against bacteria such as degradation of cell wall and plasma membrane, Influence on genetic material and translation of proteins and also impact in amino acids and phospholipid chains in the membrane [44].
Al-Dabbas et al. studied the antibacterial activity of V. iphionoides plant extracts against different bacterial strains, and results showed activity against S. aureus, Bacillus subtilis, Micrococcus luteus, E. coli, Bacillus cereus, and Salmonella enteritides in the ethyl acetate and chloroform extracts [21]. Another study by Haddad et al. described the antibacterial activity of the methanolic extract of V. iphionoides against different standard bacterial species and was found to exhibit high antibacterial activity against Klebsiella pneumoniae ATCC 13883, Proteus vulgaris ATCC 13315, methicillin-resistant S. aureus ATCC 95047, and E. coli O157:H7 ATCC43895 [45]. Low sensitivity against E. coli of C. iphionoides was reported by Masadeh et al. [46], while the ethanolic extract did not exert an antibacterial effect against the Gram-negative Pseudomonas aeruginosa [47].
4. Conclusions
Overall, studies on V. iphionoides plant extract have revealed that it contains a variety of polyphenolic compounds and flavonoids, which are vital plant secondary metabolites with a variety of biological activities. Due to their presence, the plant has an antioxidant activity. Additionally, the plant’s antibacterial activities have shown promise, as it has a strong inhibitory effect on bacteria (Gram-positive and Gram-negative). Additionally, the effect on breast cancer cell lines (MCF7) and colon cancer cell lines (HT 29) have demonstrated that it has a good anticancer activity.
Author Contributions
Conceptualization, F.A.-R. and H.I.; methodology, F.A.-R. and H.I.; formal analysis, F.A.-R. and M.K.; resources, M.K.P. and O.A.k.; data curation, Z.S. and H.I.; writing—original draft preparation, F.A.-R.; writing—review and editing, M.K.P., A.S., C.S.D. and O.A.k. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R141), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors also extend their appreciation to the Researchers Supporting Project number (RSP-2021/379) of King Saud University, Riyadh.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R141), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors also extend their appreciation to the Researchers Supporting Project number (RSP-2021/379) of King Saud University, Riyadh.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abu-Rabia, A. Herbs as a Food and Medicine Source in Palestine. Asian Pac. J. Cancer Prev. 2005, 6, 404–407. [Google Scholar] [PubMed]
- Abdelhalim, A.; Al-Munawarah, A.-M. Pharmacological Properties and Chemical Constituents of Chiliadenus Iphionoides (Syn. Varthemia Iphionoides): A Review. Eur. J. Med. Plants 2020, 31, 84–97. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Zaid, A.N.; Al-Ramahi, R.; Alqub, M.A.; Hussein, F.; Hamdan, Z.; Mustafa, M.; Qneibi, M.; Ali, I. Ethnopharmacological Survey of Medicinal Plants Practiced by Traditional Healers and Herbalists for Treatment of Some Urological Diseases in the West Bank/Palestine. BMC Complement. Altern. Med. 2017, 17, 255. [Google Scholar] [CrossRef]
- State of Palestine Fifth National Report Preparation Team Members. Available online: https://www.cbd.int/doc/world/ps/ps-nr-05-en.pdf (accessed on 30 October 2022).
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Altun, İ.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran J Public Health 2018, 47, 1218–1219. [Google Scholar]
- CDC What Exactly Is Antibiotic Resistance? Available online: https://www.cdc.gov/drugresistance/about.html (accessed on 30 October 2022).
- Teoh, E.S. Medicinal Orchids of Asia; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Tarfaoui, K.; Brhadda, N.; Ziri, R.; Oubihi, A.; Imtara, H.; Haida, S.; Al kamaly, O.M.; Saleh, A.; Parvez, M.K.; Fettach, S.; et al. Chemical Profile, Antibacterial and Antioxidant Potential of Zingiber Officinale Roscoe and Elettaria Cardamomum (L.) Maton Essential Oils and Extracts. Plants 2022, 11, 1487. [Google Scholar] [CrossRef]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants vs. Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer. Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for Testing Antioxidant Activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant Derived Substances with Anti-Cancer Activity: From Folklore to Practice. Front. Plant. Sci. 2015, 6, 799. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty. Biomed. Res. Int. 2020, 2020, 8602879. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L. Herbal Extracts and Phytochemicals: Plant Secondary Metabolites and the Enhancement of Human Brain Function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef]
- Dekebo, A. Introductory Chapter; IntechOpen: London, UK, 2019; ISBN 978-1-83881-889-0. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Suganuma, T.; Kitahara, K.; Hou, D.-X.; Fujii, M. Cytotoxic, Antioxidant and Antibacterial Activities of Varthemia Iphionoides Boiss. Extracts. J. Ethnopharmacol. 2006, 108, 287–293. [Google Scholar] [CrossRef]
- Imtara, H.; Kmail, A.; Touzani, S.; Khader, M.; Hamarshi, H.; Saad, B.; Lyoussi, B. Chemical Analysis and Cytotoxic and Cytostatic Effects of Twelve Honey Samples Collected from Different Regions in Morocco and Palestine. Evid. -Based Complement. Altern. Med. 2019, 2019, e8768210. [Google Scholar] [CrossRef]
- Bakour, M.; da Campos, M.G.; Imtara, H.; Lyoussi, B. Antioxidant Content and Identification of Phenolic/Flavonoid Compounds in the Pollen of Fourteen Plants Using HPLC-DAD. J. Apic. Res. 2020, 59, 35–41. [Google Scholar] [CrossRef]
- Sadeq, O.; Mechchate, H.; Es-Safi, I.; Bouhrim, M.; Jawhari, F.Z.; Ouassou, H.; Kharchoufa, L.; AlZain, M.; Alzamel, N.; Mohamed Al Kamaly, O.; et al. Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria Fruticosa, Achillea Fragrantissima, and Phoenix Dactylifera. Plants 2021, 10, 676. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Jawhari, F.Z.; El Moussaoui, A.; Bourhia, M.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A. Anacyclus Pyrethrum (L): Chemical Composition, Analgesic, Anti-Inflammatory, and Wound Healing Properties. Molecules 2020, 25, 5469. [Google Scholar] [CrossRef] [PubMed]
- Bakour, M.; Al-Waili, N.S.; El Menyiy, N.; Imtara, H.; Figuira, A.C.; Al-Waili, T.; Lyoussi, B. Antioxidant Activity and Protective Effect of Bee Bread (Honey and Pollen) in Aluminum-Induced Anemia, Elevation of Inflammatory Makers and Hepato-Renal Toxicity. J. Food Sci. Technol. 2017, 54, 4205–4212. [Google Scholar] [CrossRef] [PubMed]
- Touzani, S.; Embaslat, W.; Imtara, H.; Kmail, A.; Kadan, S.; Zaid, H.; ElArabi, I.; Badiaa, L.; Saad, B. In Vitro Evaluation of the Potential Use of Propolis as a Multitarget Therapeutic Product: Physicochemical Properties, Chemical Composition, and Immunomodulatory, Antibacterial, and Anticancer Properties. BioMed Res. Int. 2019, 2019, 4836378. [Google Scholar] [CrossRef]
- Al-Bakheit, A.; Abu-Romman, S.; Sharab, A.; Alshhab, M. Anti-Inflammatory Effect of Varthemia Iphionoides Extracts against Prostate Cancer in Vitro. Eur. J. Inflamm. 2017, 15, 8–14. [Google Scholar] [CrossRef]
- Afifi, F.Ü.; Al-Khalil, S.; Abdul-Haq, B.K.; Mahasneh, A.; Al-Eisawi, D.M.; Sharaf, M.; Wong, L.K.; Schiff, P.L. Antifungal Flavonoids FromVarthemia Iphionoides. Phytother. Res. 1991, 5, 173–175. [Google Scholar] [CrossRef]
- Al-DABBAS, M.M.; Kitahara, K.; Suganuma, T.; Hashimoto, F.; Tadera, K. Antioxidant and α-Amylase Inhibitory Compounds from Aerial Parts of Varthemia Iphionoides Boiss. Biosci. Biotechnol. Biochem. 2006, 70, 2178–2184. [Google Scholar] [CrossRef]
- Abu-Romman, S.; Haddad, M.; Al-Hadid, K. The Potential Allelopathic Effects of Varthemia Iphionoides and the Identification of Phenolic Allelochemicals. Jordan J. Biol. Sci. 2015, 8, 301–306. [Google Scholar] [CrossRef][Green Version]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of Antioxidant Capacity of 13 Plant Extracts by Three Different Methods: Cluster Analyses Applied for Selection of the Natural Extracts with Higher Antioxidant Capacity to Replace Synthetic Antioxidant in Lamb Burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Khan, A.N.; Ali Aldowairy, N.N.; Saad Alorfi, H.S.; Aslam, M.; Bawazir, W.A.; Hameed, A.; Soomro, M.T. Excellent Antimicrobial, Antioxidant, and Catalytic Activities of Medicinal Plant Aqueous Leaf Extract Derived Silver Nanoparticles. Processes 2022, 10, 1949. [Google Scholar] [CrossRef]
- Al-Dabbas, M.; Al-Ismail, K.; Al-Qudah, Y.H. Antioxidant Activity of Different Extracts from Varthemia Iphionoides. Riv. Ital. Delle Sostanze Grasse 2010, 87, 243–249. [Google Scholar]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Thoppil, R.J.; Harlev, E.; Mandal, A.; Nevo, E.; Bishayee, A. Antitumor Activities of Extracts from Selected Desert Plants against HepG2 Human Hepatocellular Carcinoma Cells. Pharm. Biol. 2013, 51, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, L.; Bari, G.; Hamias, R.; Maor, H.; Budovsky, A.; Wolfson, M.; Fraifeld, V.; Danilenko, M.; Ben-Shabat, S. Preferential Anti-Proliferative Activity of Varthemia Iphionoides (Chiliadenus Iphinoides). Isr. J. Plant Sci. 2015, 62, 229–233. [Google Scholar] [CrossRef]
- Abbas, M.; Abbas, M.; Kandil, Y. Cytotoxic activity of varthemia iphionoides essential oil against various human cancer cell lines. Acta Pol. Pharm. Drug Res. 2019, 76, 701–706. [Google Scholar] [CrossRef]
- Ousaaid, D.; Imtara, H.; Laaroussi, H.; Lyoussi, B.; Elarabi, I. An Investigation of Moroccan Vinegars: Their Physicochemical Properties and Antioxidant and Antibacterial Activities. J. Food Qual. 2021, 2021, e6618444. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey Antibacterial Effect Boosting Using Origanum Vulgare L. Essential Oil. Evid. -Based Complement. Altern. Med. 2018, 2018, 7842583. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Aabed, K.; Mohammed, A.E.; Benabdelkamel, H.; Masood, A.; Alfadda, A.A.; Alanazi, I.O.; Alnehmi, E.A. Antimicrobial Mechanism and Identification of the Proteins Mediated by Extracts from Asphaltum Punjabianum and Myrtus Communis. ACS Omega 2020, 5, 31019–31035. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef]
- Haddad, M.; Abu-Romman, S.; Sharab, A. In Vitro Antimicrobial Activity of Methanolic Extract from Varthemia Iphionoides Leaves. J. Agric. Sci. 2016, 8, 178. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alkofahi, A.S.; Tumah, H.N.; Mhaidat, N.M.; Alzoubi, K.H. Antibacterial Activity of Some Medicinal Plants Grown in Jordan. Pak. J. Pharm. Sci. 2013, 26, 267–270. [Google Scholar] [PubMed]
- Khalil, A.; Dababneh, B.; Al-Gabbiesh, A. Antimicrobial Activity against Pathogenic Microorganisms by Extracts from Herbal Jordanian Plants. J. Food Agric. Environ. 2009, 7, 103–106. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).