Abstract
Frozen food is exposed to inevitable temperature changes during its storage, transport, and at the point of sale, which implies a significant impact on its properties and quality. Thus, the study of the effect of the formation of crystals on both the surface of the meat and the container when it is kept frozen, involving the thermodynamic analysis and changes that occurred at the structural level, is necessary. In this research, pork meat from Longissimus thoracis muscle was used, which was cut into plates and packed with two types of food-grade films: (1) Polyvinyl Chloride (PVC) and (2) low-density polyethylene (LDPE). Samples were frozen by indirect contact with nitrogen up to −40 ± 0.5 °C and subsequently stored at −20 ± 1 °C in a chamber from 0 to 15 days. The frost thickness was evaluated by the image superposition method. FTIR spectra were obtained by means of an Attenuated Total Reflectance (ATR) accessory, and thermal changes were determined by Modulated Differential Scanning Calorimetry (MDSC). It was found that the thickness of the frost on the surface of the meat is less when it is packaged with PVC due to the characteristics of the polymer matrix of the package. Furthermore, there were important changes at the molecular level identified by FTIR and MDSC, indicating significant differences (p < 0.05) between the samples. In general, PVC films were more stable at lower temperatures, allowing a small number of changes in the meat surface due to temperature fluctuations.
1. Introduction
Frozen meat is packaged for display, preserving the sanitary quality and allowing the product to be observed for purchase. However, during storage, transport, retail sale, or storage at home, repeated cycles of defrosting–freezing occur, breaking the cold chain, which leads to temperature fluctuations and changes in the relative humidity of the environment, causing the gradual development of ice crystals and the eventual formation of frost on the surface [1]. Thermoformed, heat-sealed, and extensible film containers with spaces having frost are generally used, making these products not well accepted by the consumer. Frost is a porous medium consisting of air and ice [2,3,4], and, in the case of packaged food, its formation is related to the concentration of vapor inside it. If this concentration is lower than that of saturated vapor on the surface of the product, the ice may sublimate [5,6], and the water vapor is transferred to the air until it reaches a cold region which causes frost formation, both in the food and inside the packaging.
The damage by freezing, due to storage time and frost formation in food, is centered on the surface, either with dehydration [7,8], color degradation [9], texture changes [10,11], or flavor [12,13]; however, in some cases, the effect of frost has not been considered. When Campañone et al. [14] studied the effects of frost formation on unpackaged meat products, they cited that there was also a loss of weight.
A completely frozen product consists of ice and non-frozen water with a high concentration of dissolved solutes, so that air and food temperatures may change when subjected to external air cycles; if the vapor concentration in the surrounding air is lower than the saturated vapor concentration on the surface of the product, the ice may sublimate, and water vapor is transferred to the air inside the container containing the food. In this case, the latent heat needed to sublimate the water is provided by the product, thus, the water vapor is transported through the air by diffusion and free convection until it reaches a cold region (with a product temperature lower than the dew point of the air), causing frost formation on the food surface [15].
The choice of packaging intended to protect food is based primarily on offering the consumer a product with similar characteristics to a fresh product. To achieve this, it is necessary to consider the specific properties of the product to be packaged, e.g., raw meat must have a bright red surface that is attractive to the naked eye and less frost presence. Packaging is the container or wrapper that is in direct contact with the product for the purpose of protecting it. However, the selection of the proper packaging must also consider the climatic and mechanical conditions to which the finished product will be subjected along the distribution chain in addition to the product–packaging compatibility to guarantee the conservation and safety of the food. Studies by Navid et al. [16], Rafati et al. [17], Nompumelelo et al. [18], and Medic et al. [19] have shown that the transfer of moisture through a membrane or plastic coating can prevent or delay the formation of frost by changing the parameters of temperature, relative humidity, the structure of materials, and the thickness of the coating.
For raw, processed, and frozen meat, a wide variety of flexible materials are applied, focused on preserving the hygienic quality of this type of product, and this has advantages over the use of glass, metal, and paper due to their structural, mechanical, and thermal properties. Among the containers applied for meat are the: (1) Polyolefins such as high and low-density polyethylene (PE) (HDPE/LDPE) and nylon polypropylene (PP); (2) Styrene such as polystyrene (PS) and expanded polystyrene (EPS); (3) Polyesters such as polyethylene terephthalate (PET), polycarbonates (PC), and vinyl [20], materials that allow the conservation of myoglobin on the surface of it.
However, no research has been conducted related to the exchange of substances between the product and the packaging, in conjunction with the ability of the plastic material to migrate substances between the environment and the product in which the barrier properties of the packaging have the key role.
Consequently, this study’s aims were to evaluate the effect of two plastic films on the formation of surface frost in frozen meat to select the packaging that provides greater stability and protection to the product according to the water vapor permeance and barrier property, involving thermodynamic and molecular analysis. The identification of compounds, transitions, and energy changes in polymers is based on infrared spectroscopy FTIR-ATR and modulated differential scanning calorimetry (MDSC).
2. Materials and Methods
2.1. Raw Material
Longissimus thoracis muscle of castrated males (Pietrain breed) with 6 months of age weighing approximately 110 ± 2 kg and 48 h rigor mortis, from a Federal Inspection Type (FIT) facility located in Cuautitlan Izcalli, State of Mexico, was used. Five pieces of muscle were obtained from the ninth to thirteenth rib section from which cuttings into experimental units were made with plate geometry in dimensions of 0.05 × 0.03 × 0.03 m (length × width × thickness), with an approximate weight of 7 ± 3 g for each unit. As a control, pH was measured as a parameter. It was verified that raw samples were within the normal range (5.4–5.7) for this type of meat [21]. The HI99163 potentiometer (Hanna Instruments, Woonsocket, RI, USA) was used, calibrated at three points of 4.01, 7.00, and 10.01 with buffer solutions, and the values obtained were automatically adjusted at 25 °C. The blade was used to pierce the sample and insert the electrode perpendicular to the direction of the meat fibers. The meat samples were frozen with liquid nitrogen by indirect contact with a stainless-steel base to a temperature of −40 ± 0.5 °C, packed and stored in a freezing chamber (Torrey, Mexico City, Mexico) at −20 ± 1 °C for 2, 7, and 15 days.
After the end of the storage period, samples were removed from the freezing chamber and kept exposed to environmental conditions for frost formation (relative humidity of 55 ± 3% and room temperature of 21 ± 1.5 °C). The surface temperature of the meat was recorded with an Infrared Thermometer model 39650-3 (Cole Palmer, Vernon Hills, IL, USA) and the %RH inside the container with an Extech HD500 psychrometer (Flir, Nashua, NH, USA).
Packages commonly used in the storage of food were selected: (a) Low-Density Polyethylene (LDPE) bags that have excellent chemical resistance [22] and (b) Polyvinyl chloride (PVC), a self-adhesive film, resistant to high temperatures [23].
The thickness of each container was determined with a Mitutoyo H-2780 digital micrometer (Uline, Kenosha, WI, USA) in 10 different cuts.
2.2. Experimental Determinations
2.2.1. Water Vapor Transmission and Permeance
The water vapor transmission rate (WVT) and permeance (P) of the used films were evaluated by the adapted ASTM E-95 gravimetric technique method [24]. Both the capsules (with a circular exposure area of 1.256 × 10−3 m2, leaving a gap of 0.026 m between the film and the desiccant) and the desiccant were kept at constant weight until treatment was initiated. To maintain homogeneous control under experimental conditions, the capsules were inserted into an acrylic box with adjustment ventilation and placed inside on a climate chamber CH-6090 (Figursa Industries, Mexico City, Mexico) at 20 ± 1 °C. An Extech anemometer (Flir, Nashua, NH, USA) was used to determine the velocity of air circulation.
For 6 weeks, the weight (g) of the LDPE and PVC capsules, the relative humidity inside the capsules (%RH), and the temperature of the acrylic chamber were recorded with an HD500 Extech psychrometer.
After the measurement time, a graph of weight gain versus time was made, and the necessary change ratio for the determination of the WVT was obtained with the following equations:
where WVT is the water vapor transmission rate (g/s·m2), J is the weight change g/s (dependent on the weight gain curve), t is the elapsed time (s), and A is the area for vapor transmission (m2).
The permeance was determined considering the partial water pressures on both sides of the film and their thickness:
where L is the film thickness (m), and Pw represents %RH over the desiccant and the acrylic chamber [24].
2.2.2. Frost Evaluation
Optical Analysis
An optical microscopy analysis was performed on the frost formed on the surface and inside of the container where the frozen meat was found. Images were taken with an EZ4D stereomicroscope (Leica, Germany) at 2, 7, and 15 days of storage with the two films (PVC and LDPE); likewise, it was also performed with a stainless-steel plate of 0.10 × 0.10 × 0.01 m. The samples were cooled with liquid nitrogen for 10 min near to −60 °C and after melting the frost. The samples were placed on a blue plate to cause a contrast effect with the Leica Application Suite V3, and a 12.5× approach was applied for a greater focus. Following the methodology of Meléndez et al. [25], images were superimposed to determine the maximum thickness. The frost formed on the stainless-steel plate against a sample of ultra-pure water (UW, Ultrapure Water for HPLC Analysis—METRIX Laboratory, Mexico City, Mexico) was examined to determine if there were differences in specific properties.
The water obtained from the fusion of the meat frost and the stainless-steel plate was separated for further analysis by MDSC and FTIR.
Frosting on Packages
Although it was not possible to quantify the thickness of the frost formed on the inner surface of the containers, it was collected for further analysis by MDSC and FTIR-ATR.
2.2.3. Effects on Frost Formation by Freezing Process and Storage Days on Meat and Films
Structural Components
Comparison of the structural components recorded in the infrared spectra of the pack samples (LDPE and PVC) after the freezing treatment of the meat samples, the frost water formed on the surface of the meat, and the inner surface of the pack was carried out against the HPLC (UW) grade spectrum as a reference for checking component migration using the Frontier SP8000, Perkin Elmer (Waltham, MA, USA) coupled to an attenuated total reflectance (ATR) device in a wavenumber range of 4000 to 400 cm−1 [26].
Structural Behavior by MDSC
The MDSC 2920, TA Instruments series (New Castle, DE, USA) with a cooling system (RSC) was used to analyze the structural behavior of the films used and the frost water. Thermograms were obtained and analyzed in the TA Universal Analysis software for the flow signals, where the total heat flow, reversible heat flow, non-reversible heat flow, and specific heat (Cp) were examined as a function of the temperature. To identify the number of transitions, both in the cooling and heating zones of the samples, the experimental conditions of the tests were: modulation: ±1.592 °C every/60 s, heating rate: 10 °C/min, nitrogen flow: 60 mL/min.
For analysis of the packages, the weight of the samples was between 4.8 ± 2 mg and, for frost water on the meat surface and inside the packages, the weight was between 5.6 ± 2 mg; the cooling and heating conditions for packages were between −50 and 300 °C and, for frost water, they were between −50 and 50 °C.
It was considered that since the films were used for the preservation of frozen meat, the results of the MDSC technique were analyzed in a temperature range of −45 to 20 °C, simulating the behavior of the meat during freezing with nitrogen, storage in a chamber (−20 °C), and thawing. The derivative of the heat flow was determined to improve the interpretation of the observed changes and to relate them to the vibration effects that were observed in the FTIR technique [25].
2.3. Statistical Analysis
The evaluation of tests for the periods of 2, 7, and 15 days in storage was conducted four times, where mean, standard deviation, one-way, and two-way ANOVA were obtained for each sample. Minitab 16.0.1 software (Penn State University, University Park, PA, USA) was used to identify significant differences (p < 0.05) between means applying Tukey’s test.
3. Results and Discussion
3.1. Determination of WVT Rate and Permeance of Containers
The diffusion of water vapor through a container gives an estimate of the strength of the polymer, as a difference in partial pressures is generated in the structure of the container. Figure 1 shows that in PVC film, because a slower increase was observed for PVC film from three to four weeks, the polar character of the Cl- increases the weight of the capsules containing the desiccant, thus proving to have a lower surface resistance. It can be established that there is a counter diffusion in the LDPE film when the vapor begins to permeate [27,28], causing the relative humidity to be higher now inside the device, which can be observed from the second to the fourth weeks and in the fifth week of the permeance study in Figure 1.
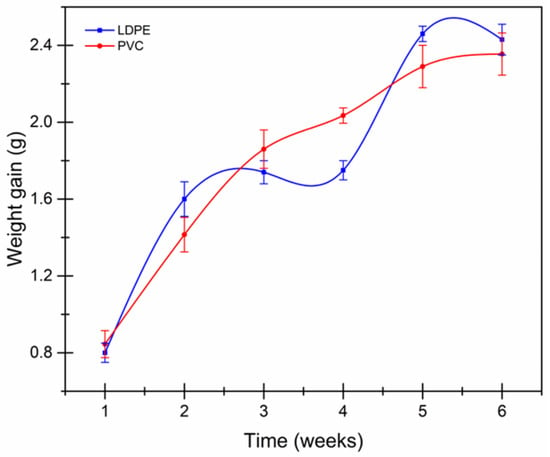
Figure 1.
Weight gain behavior of LDPE and PVC films.
Table 1 shows data from WVT and P of LDPE and PVC films. These results are consistent with those reported by Bertuzzi et al. [27] and Thomas et al. [28]. Both polymers seem to suggest a similar WVT. The P in the PVC film is an order of magnitude smaller; since it contains a greater quantity of phobic additives in its structure [29], it encourages an increase in the density of the molecules by promoting a lower free volume and, in turn, reducing the ability of the permeating molecule to move. Quintana [30] states that the LDPE film has regions with a lower proportion of crystalline fraction and which are mostly amorphous, with energy sufficiently able to change from one conformation to another by forming and destroying free volume or empty space that the water can occupy, thus facilitating the permeation of more water molecules.

Table 1.
Water vapor transmission rate (WVT) and permeance (P) of containers.
The permeance implies the relationship of the thickness of the films because the incorporation of plasticizer modifies the molecular organization. The thickness of the films was from 2 × 10−5 m of PVC to 6 × 10−4 m in the LDPE, where, in the latter, despite having a more significant thickness, the relationship of some hydrophilic part of the additive with the molecules of water vapor increases the possibility of a bond formation that improves its permeability. The results show that the composition of a film, the orientation of the molecules, and the structural arrangement are decisive for its interaction with water vapor.
3.2. Frost Formed on the Surface of the Meat
As shown in Table 2, the greatest thickness of the frost occurred on the stainless-steel plate, followed by unpackaged meat. With the passage of days of storage in the freezing chamber at a temperature of −20 °C, fluctuations due to the opening and closing of the door and the operation of the compressor increased the exchange of temperature and relative humidity, causing the reduction in the thickness on the surface frost in the meat where thickness is strongly related to the type of packaging [31,32].

Table 2.
Thickness of frost on the surface of the metal plate, unpacked meat, and packaged meat with respect to storage time.
Figure 2 shows the behavior of the frost formed where PVC is less thick, with solid and amorphous crystals.
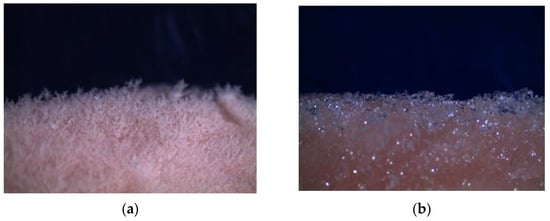
Figure 2.
Frost thickness on the surface of the meat at 15 days: (a) LDPE film; (b) PVC film.
3.3. Frost Formed Inside the Containers
It was not possible to quantify due to the melting of the ice, however, it was observed that LDPE films deposited a higher amount of frost on the outer surface and even inside the container compared to PVC, where frost was not detected on the surface but inside the container.
3.4. Identification of Functional and Compound Groups by Fourier Transform Infrared Spectrophotometry (FTIR-ATR)
3.4.1. Identification of Functional Groups and Compounds of Films before Freezing–Thawing and after Thawing
Some precedents confirm structural changes in polymers tested at high temperatures [33,34,35]. The spectra of the plastic films before freezing and after thawing are presented (Figure 3) where no differences were found in the bands; however, there were small variations in the relative transmittance of some bands, indicating that the freezing process in the chamber did not significantly affect the molecular structure of the containers used for this research.
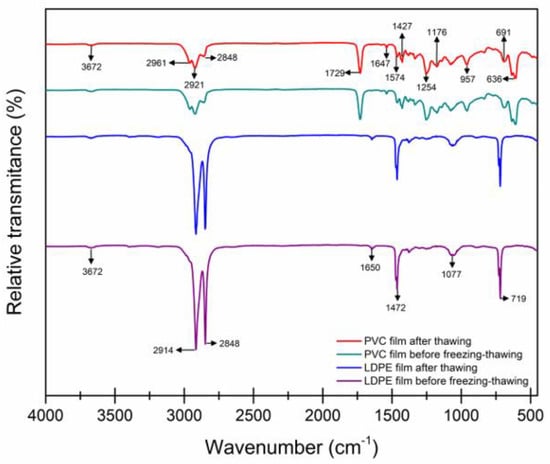
Figure 3.
Infrared spectrum of a commercial low-density polyethylene (LDPE) and polyvinyl chloride (PVC) films.
Figure 3 shows that, for LDPE, characteristic bands are presented at 2914 cm−1, 2848 cm−1, 1650 cm−1, 1463 cm−1, and 1379 cm−1 that are attributed to asymmetric methylene (-CH2), symmetric -CH2, stretching frequency of the ethylene monomer (C=C), and vibrations due to the symmetric deformation of methyl radical (-CH3), respectively, according to previous studies by Abdel et al. [26]. Meanwhile, at 729 and 719 cm−1, they are related to deformation vibrations and even bands of possible hydroxyl radical (-OH) groups [26]. At 3672, 3396, and 3360 cm−1, due to vibrations ≈1060 being associated with additive groups such as graphene oxide, phthalates, and even combination with ethylene–vinyl alcohol copolymer (EVOH), a semi-crystalline random copolymer of ethylene and vinyl alcohol is widely used and is more commonly in packaging structures for perishable foods due to their superior oxygen barrier [35].
It should be noted that the vibration band of the symmetric deformation of -CH3 at 1376 cm−1 was also one of the most important indicators used to distinguish polyolefin, which is a group of plastics of great commercial and economic importance in which LDPE is found, corroborating what was reported by Dogana et al. [35], where they verify, through similar bands, the band of polyolefin corresponding to LDPE. In this way, the other vibrations with lower intensity are interpreted as structural modifications in the crystalline parts of the polymer.
Regarding the spectrum of the PVC film presented in Figure 3, bands of different intensities were found among those that stand out as asymmetric -CH2 bonds at 2961 and 2921 cm−1, -CH2 symmetric 2871 and 2858 cm−1, the double bond C=C is confirmed at 1647 cm−1 as well as the terminal vinyl C=C-Cl at 1574 cm−1, which are the characteristics of the main PVC molecule.
The other records in the spectrum are not exclusive to PVC but include some additives, such as lubricants. Intense bands were present at 1729 and 1254 cm−1 as well as weak bands around 810–950 cm−1, which are characteristic of ester groups such as calcium stearate, used to provide greater conformity in the polymer matrix at the time of processing as well as give high clarity to PVC [34].
Plasticizers such as the mixture of several phthalates, such as Di-octyl (DOP) at 2859, 1722, 1462, 1381, 1123 cm−1, and Diethylhexyl Phthalate (DEHP), were also detected at 1029–1157 cm−1, which gives PVC greater thermal stability and flexibility at high and low temperatures. According to the report by Inca et al. [34], this may be the epoxidized soybean oil, widely used in PVC to provide stability to heat and light as well as having zero volatility.
3.4.2. Identification of Functional Groups and Compounds of Ice Water
In the case of frost water, as shown in Figure 4, it was compared with water commonly used for HPLC studies to have a standard behavior without impurities. Characteristic traces of water were found, the first one with greater energy due to the O-H bond that generates a very wide and intense band between 3500 and 3200 cm−1 for all samples, and the second one at 1630–1637 cm−1, assigned to the H-O-H bond [36].
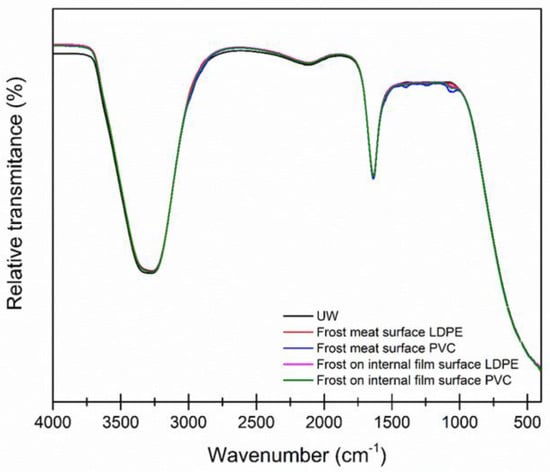
Figure 4.
Infrared spectrum of frost water in the containers.
When analyzing Figure 5 profile a, the frost water on the surface of the containers in the fingerprint area was found to be bands approximating those of the UW, where traces of SO2, CO2, and O3 were detected from about 1460 to 1024 cm−1 in both the PVC and LDPE containers, suggesting the use of additives that have functional groups in their structure, ether and epoxy for both polymers, and even the probability of a common additive.
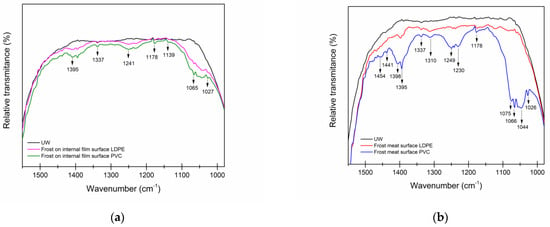
Figure 5.
Infrared spectrum of frost water on the (a) surface of LDPE and PVC containers and (b) surface of meat packaged in LDPE and PVC, in contrast to ultra-pure water.
The most used phthalate in food packaging materials or containers is Di (2-ethylhexyl) phthalate (DEHP) (Di-(2-ethylhexyl)-phthalate), where its main compounds are observed between 1029 and 1157 cm−1, as observed in the intensity of the bands presented in Figure 5b. Results reported by Dogana et al. [35] indicate that this compound migrated to meat, butter, margarine, and ready-to-eat foods, and in cheese, in addition, DEHP levels increased when food was heated, increasing the rate of migration.
Other phthalates detected in both packaging and food were Di-n-butyl, butylbenzyl, and diethyl-phthalate. Therefore, the water on the surface of the containers has a greater record of molecules in the study, as it is subjected to storage and prone to the permeation of molecules where the frost settled on the surface of the containers, resulting in film components migrating at the time of recrystallization.
3.4.3. Identification of Functional Groups and Compounds of Meat Surfaces at Frost
Figure 5b shows the effect that the packaging had on the frost formed on the meat, where some additive molecules were also recorded, such as C-Cl interactions, -CH3 groups, and C-H groups associated with alkene and alkenyl side chains. This part is essential because it corroborates the migration not only of vapor-permeating molecules but also of environmental pollution and release of component compounds from the packaging. However, a limiting factor of commonly used additives for commercial polymers is their unavoidable physical loss by polymer matrix migration that may occur during compositional processes, as well as during consumer storage and end-use.
3.5. Kinetic and Structural Behavior, Analysis with Modulated Differential Calorimeter (MDSC)
3.5.1. Kinetic and Structural Behavior of PVC and LDPE Films
The heat flow signal of the containers (Figure 6a) shows that the most conspicuous and energy-intensive transitions occur when the state change happens in the polymers, i.e., when the melting temperature (Tf) is reached, characteristic of the crystalline part of the polymers where their structural order is lost, collapses, and even transforms into liquid.
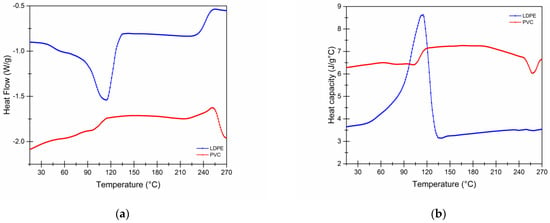
Figure 6.
(a) Representative heat flow thermogram (W/g). (b) Specific heat (J/g °C) of PVC and LDPE films.
As the heating cycle progresses, the behavioral tendency of the LDPE film has a greater change corresponding to its fusion [37], reaching 114.93 °C with an enthalpy of 101.9 J/g, where a structural rearrangement of its molecules is promoted. For PVC, two fewer visible transitions were observed at 96.93 and 253.75 °C with a melting enthalpy of only 12.21 and 17.16 J/g, respectively.
The crystallinity of the films has an important relationship with respect to the permeability to water vapor, because the transport of water is limited in the most crystalline regions by the ordering of the intermolecular bonds [38]; therefore, in LDPE, having a lower melting temperature, it has a low crystallinity, and its amorphous fraction allows a better passage of water molecules that reduce its permeability capacity.
Under the established interval condition, Figure 6b shows that there is no effect on the films in the low-temperature zone (simulating the freezing and storage process); the behavioral trend of Cp is similar in both cases, so that during crystallization the inter- and intramolecular changes in the chains do not affect the behavior as a container in storage.
Some of the transitions are shown during this analysis interval (Table 3), in which a minimum energy requirement is observed for the two containers. Therefore, materials subjected to low temperatures are not exposed to a condition such that they can generate molecular transitions or rearrangements influencing the melting points.

Table 3.
Film transitions from −45 to 20 °C.
According to Table 4, the maximum glass transition temperature (Tg) of PVC is higher due to its composition in additives that provide a free volume to the polymer by separating the chains and even blocking intermolecular interactions, providing greater thermal stability. LDPE, coming from the family of polyolefins, characterized by poor thermal properties and a low specific heat capacity, have a much lower maximum transition temperature [39]. Therefore, the energy required to change physical state is less for PVC than for LDPE.

Table 4.
Experimental glass temperature (Tg) of the films.
3.5.2. Kinetic and Structural Behavior of Ultrapure Water and from Meat Frost and Container Surfaces
Since water is not always pure but consists of a solution of soluble salts, sugars, and proteins that form a complex of molecules that are in colloidal suspension, there is a decrease in its freezing point. Therefore, in most cases, the free water is frozen first and then the water that interacts with solutes freezes; otherwise, when the process is contrary and melts, the water that interacts with the solutes is the first to thaw and, for that to happen, a greater heat flow is required. Figure 7a shows the displacement of the initial temperatures for the melting of the samples due to the change in the composition of the frost compared to that of ultrapure water.
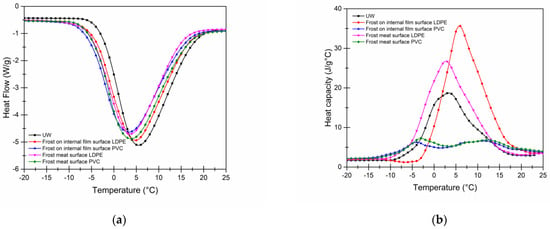
Figure 7.
(a) Heat flow (W/g). (b) Specific heat (J/g °C) of frost water from the surface of the packages and meat.
Data from Table 5 indicate that the frost water on the surface of the PVC container is the first to start melting at −5.60 °C followed by the frost water on the surface of the LDPE-packaged meat at −5.25 °C; however, it requires less enthalpy to melt than the latter, indicating that the temperature between the surface of the PVC container, in terms of the IR spectrum of frost water on the surface of the containers, suggests that there is no significant contamination, as it is close to the behavior of UW water.

Table 5.
Starting temperatures-maximum melting point and enthalpies of frost water.
On the other hand, as expected, the water with the lowest impurities that had the highest temperature at melting and that also required the maximum energy was the UW. This is because since there are no more molecules attached to it; it takes longer to melt, which produces a higher-energy expenditure, which can also be seen in Figure 7b where this water has the highest Cp.
For fusion to occur, the intermolecular forces that hold the structure of a compound together need to be broken; for this reason, the presence of impurities, even in small quantities, causes a decrease in the melting point, usually accompanied by an increase in the melting interval, when observing the amplitude of the curves in Figure 7b for the melting points that demand less energy. Melting corresponds to meat frost, indicating low interaction or contamination by the components of the packaging.
Specific heat can be understood as the thermal capacity that involves absorbing, yielding, or storing heat; Figure 7b shows that there is no significant energy storage in PVC frost water, as it is for LDPE water, since there are important structural changes in the Cp delta to reach the maximum melting points.
Taking as a reference the UW of (14.83 J/g °C) with respect to the delta of frost on the surface of the container from 30.37 J/g °C in LDPE to a minimum of 2.14 J/g °C PVC while on the surface of the meat 19.65 J/g °C to 3.348 J/g °C, respectively, it relates to transitions, although not exactly fusion transitions for the PVC surface; however, they are discarded, as they do not show noticeable changes in the total heat flow. Regarding components immersed in the frost water, LDPE is found mostly in the surface frost of meat and packaging, which also causes the Cp values to be raised.
4. Conclusions
Although it was not considered for this study, one way to have quantitative control over the amount of frost that forms inside the container could be by recording the initial and final weight of the product after the days of storage, an activity that makes experimentation difficult.
There is an important difference between frost from metal plates, since there is no molecular interaction between water and metal, as in the case of food, where interactions with proteins and carbohydrates are primarily created.
In this sense, it is important to mention that the differences between LDPE and PVC are relevant, since PVC showed better characteristics and stability in terms of permeability and frost formation, both on the surface and inside with a meat sample, that is, due to its chemical nature and molecular affinity with ambient humidity outside and relative humidity inside the container.
Among the future perspectives, it is considered that this information can be expanded so that there is a normative proposal that regulates the use of plasticizers used in the production of films, laminations, and containers that will be in direct contact with foods so that they reduce or control the permissible degree of toxicity, since the magnitude of the residues can migrate and deposit on the surface of the food, both from the molecules of the environment or from the container itself, resulting in risks of contamination and effects on the final quality of the food.
Author Contributions
Conceptualization, R.M.-P., Y.R.-H. and J.C.-H.; methodology, R.M.-P. and Y.R.-H.; validation, J.L.A.-R. and J.C.-H.; formal analysis, R.M.-P. and J.C.-H.; investigation, Y.R.-H.; resources, J.L.A.-R., R.M.-P. and A.M.-A.; data curation, R.M.-P. and Y.R.-H.; writing—original draft preparation, R.M.-P.; writing—review and editing, J.C.-H. and A.M.-A.; supervision, J.L.A.-R. and R.M.-P.; project administration, R.M.-P. and J.C.-H.; funding acquisition, R.M.-P., J.L.A.-R. and A.M.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by CONACYT grant number 299804 and UNAM-DGAPA, projects PAPIIT IT200622, PIAPI 2006, and CI2235.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moraga, N.O.; Jauriat, L.O.; Lemus-Mondaca, R.A. Heat and mass transfer in conjugate food freezing/air natural convection. Int. J. Refrig. 2012, 35, 880–889. [Google Scholar] [CrossRef]
- Kim, K.H.; Ko, H.; Kim, K.; Kim, Y.; Cho, K.J. Analysis of heat transfer and frost layer formation on a cryogenic tank wall exposed to the humid atmospheric air. Appl. Therm. Eng. 2009, 29, 2072–2079. [Google Scholar] [CrossRef]
- Lee, Y.; Ro, S. Analysis of the frost growth on a flat plate by simple models of saturation and supersaturation. Exp. Therm. Fluid Sci. 2005, 29, 685–696. [Google Scholar] [CrossRef]
- Kandula, M. Frost growth and densification in laminar flow over flat surfaces. Int. J. Heat Mass Transf. 2011, 54, 3719–3731. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Jia, W.; Wang, K. Study on the heat transfer characteristics of an ambient air vaporizer with multi-component fluids. Processes 2022, 10, 851. [Google Scholar] [CrossRef]
- Li, S.H.; Yang, K.S.; Wang, C.C. A Semi-empirical model for predicting frost properties. Processes 2021, 9, 412. [Google Scholar] [CrossRef]
- Laguerre, O.; Flick, D. Frost formation on frozen products preserved in domestic freezers. J. Food Eng. 2007, 79, 124–136. [Google Scholar] [CrossRef]
- Campañone, L.A.; Salvatori, V.O.; Mascheroni, R.H. Weight loss during freezing and storage of unpackaged foods. J. Food Eng. 2001, 47, 69–79. [Google Scholar] [CrossRef]
- Do, G.S.; Sagara, Y.; Tabata, M.; Kudoh, K.; Higuchi, T. Three-dimensional measurement of ice crystals in frozen beef with a micro-slicer image processing system. Int. J. Refrig. 2004, 27, 184–190. [Google Scholar] [CrossRef]
- Bertram, H.; Andersen, R.; Andersen, H. Development in myofibrillar water distribution of two pork qualities during 10-month freezer storage. Meat Sci. 2007, 75, 128–133. [Google Scholar] [CrossRef]
- Sawyer, J.Y.; Baublits, R.T.; Apple, J.K.; Meullent, J.F.; Johnson, Z.B.; Alpers, T.K. Lateral and longitudinal characterization of color stability, instrumental tenderness, and sensory characteristics in the beef semimembranosus. Meat Sci. 2007, 75, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Choi, Y.M.; Lee, S.H.; Choe, J.H.; Hong, K.C.; Park, H.C.; Kim, B.C. Correlations of trained panel sensory values of cooked pork with fatty acid composition, muscle fiber type, and pork quality characteristics in berkshire pigs. Meat Sci. 2010, 86, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.J.; Won-Lee, J.W. How Does the Freezer Burn Our Food? J. Food Sci. Educ. 2009, 8, 45–52. [Google Scholar] [CrossRef]
- Campañone, L.A.; Salvadori, V.O.; Mascheroni, R.H. Food freezing with simultaneous surface dehydration: Approximate prediction of freezing time. Int. J. Heat Mass Transf. 2005, 48, 1205–1213. [Google Scholar] [CrossRef]
- Urquiola, A.; Álvarez, G.; Flick, D. Frost formation modeling during the storage of frozen vegetables exposed to temperature fluctuations. J. Food Eng. 2017, 214, 16–28. [Google Scholar] [CrossRef]
- Navid, P.; Niroomand, S.; Simonson, C. An analytical model for predicting frosting limit in membranes. Int. J. Refrig. 2018, 99, 316–326. [Google Scholar] [CrossRef]
- Rafati, N.M.; Fauchoux, F.; Besant, R.; Simonson, R. A review of frosting in air-to-air energy exchangers. Renew. Sustain. Energy Rev. 2014, 30, 538–554. [Google Scholar] [CrossRef]
- Nompumelelo, S.; Thandeka, N.M.; Pieter, A.G.; Louwrens, C.H. The influence of normal and high ultimate muscle pH on the microbiology and colour stability of previously frozen black wildebeest (Connochaetes gnou) meat. Meat Sci. 2018, 135, 14–19. [Google Scholar]
- Medic, H.; Djurkin, I.; Pleadinc, J.; Kozacinskid, L.; Njarid, B. The impact of frozen storage duration on physical, chemical and microbiological properties of pork. Meat Sci. 2018, 140, 119–127. [Google Scholar] [CrossRef]
- Eskin, M. Food shelf-life stability. In Chemical, Biochemical, and Microbiological Changes; CRC Press: Washington, DC, USA, 2001. [Google Scholar]
- Braña, D.; Ramírez, E.; Rubio, M.S.; Sánchez, A. Manual de Análisis de Calidad en la Carne; Centro Nacional de Investigación Disciplinaria en Fisiología y Mejoramiento Animal: Querétaro, Mexico, 2011. [Google Scholar]
- Schultz, J. Polymer crystallization. In The Development of Crystalline Order in Thermoplastic Polymers; Oxford University Press: New York, NY, USA, 2001; pp. 21–30. [Google Scholar]
- Arrieta, M.P.; Samper, M.D.; Jiménez-López, M.; Aldas, M.; López, J. Combined effect of linseed oil and gum rosin as natural additives for PVC. Ind. Crops Prod. 2017, 99, 196–204. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Water Vapor Transmission of Materials. Standard Designations: E96-95. In Annual Book of American Society for Testing Materials; ASTM International: West Conshocken, PA, USA, 1995. [Google Scholar]
- Meléndez, P.R.; Rosas, M.M.E.; Mercado, M.C.; Velázquez, C.R.; Arjona, R.J.L. Comparison of melting frost layers after 2 frozen methods in pork cuts (Longissimus dorsi). Procedia Food Sci. 2011, 1, 363–369. [Google Scholar] [CrossRef][Green Version]
- Abdel, M.A.; Aly, S.S.; Elshaer, Y.H. Effect of gamma radiation on low density polyethylene (LDPE) films: Optical, dielectric and FTIR. Spectrochim. Acta Part A 2012, 93, 203–207. [Google Scholar]
- Bertuzzi, M.A.; Armanda, M.; Gottifredi, J.C.; Aparicio, A.R.; Jiménez, P. Estudio de la permeabilidad al vapor de agua de films comestibles para recubrir alimentos. In Congreso Regional de Ciencia y Tecnología; Universidad de Catamarca: Buenos Aires, Argentina, 2002. [Google Scholar]
- Thomas, S.; Wilson, W.; Kumar, A.S.; Soney, C. Transport Properties of Polymeric Membranes; Elsevier: Amsterdam, The Netherlands; Bookaid International: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Araki, Y.; Kobayashi, Y.; Kawaguchib, T.; Kanekob, T.; Arai, N. Water permeation in polymeric membranes: Mechanism and synthetic strategy for water-inhibiting functional polymers. J. Membr. Sci. 2018, 564, 184–192. [Google Scholar] [CrossRef]
- Quintana, P.J. Análisis y Diseño de Empaques Flexibles, Laminados Para Envasar Alimentos. Bachelor’s Thesis, Tesis de Licenciatura, Facultad de Ingeniería en Mecánica y Ciencias de la Producción, Guayaquil, Ecuador, 2007. [Google Scholar]
- Leoni, A.; Mondot, M.; Durier, F.; Revellin, R.; Haberschill, P. Frost formation and development on flat plate: Experimental investigation and comparison to predictive methods. Exp. Therm. Fluid Sci. 2017, 88, 220–233. [Google Scholar] [CrossRef]
- Qu, K.; Komori, S.; Jiang, Y. Local variation of frost layer thickness and morphology. Int. J. Therm. Sci. 2006, 45, 116–123. [Google Scholar] [CrossRef]
- Colom, X.; Arias, M.; Valldeperas, J.; Carrillo, F. Caracterización Mediante Espectrofotometría FT-IR del PVC Sometido a Degradación Térmica en Medio Ácido Oxidante; Polytechnic University of Catalonia: Barcelona, Spain, 2003. [Google Scholar]
- Inca, F.; Quiroz, I.; Aldás, M. Recuperación de Policloruro de Vinilo (PVC) apartir de tarjetas de identificación para la obtención de materiales plastificados. Esc. Politécnica Nac. 2016, 37, 46. [Google Scholar]
- Dogana, F.; Sirinb, K.; Kolcua, F.; Kayaa, I. Conducting polymer composites based on LDPE doped with poly (aminonaphthol sulfonic acid). J. Electrostat. 2018, 94, 85–93. [Google Scholar] [CrossRef]
- Mondragón, C.P. Espectroscopia de Infrarrojo Para Todos…y 51 Espectros de Alimentos Consumidos en México; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. Unidad de Tecnología Alimentaria: Zapopan, Jalisco, Mexico, 2017. [Google Scholar]
- Gáscue, B.; Figueroa, A. Caracterización de polietilenos obtenidos a partir de diferentes sistemas catalíticos de coordinación. Rev. Lat. Metal. Mat. 2003, 23, 9–15. [Google Scholar]
- Ozturk, K.O.; Singh, P.T. Water transport in starchy foods: Experimental and mathematical aspects. Trends Food Sci. Technol. 2018, 78, 11–24. [Google Scholar] [CrossRef]
- Agwuncha, S.; Ibrahim, I.; Sadiku, E. Improving the thermal and flame resistance properties of polyolefins. In Polylefin Fibres, 2nd ed.; Woodhead Publishing: Thorston, UK, 2017; pp. 421–448. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).