Abstract
Determining residual oil saturation by the single-well chemical tracer test (SWCTT) is of key importance for assessing the potential of enhanced oil recovery (EOR) and developing EOR pilot projects. However, the test trials conducted since the first injections of tracer compositions until now have not resulted in a detailed analysis of the selection of candidates for single-well tracers and their limits of applicability in various reservoir conditions. The purpose of this study was to consider the influence of the structure on the kinetic and thermodynamic components of tracers to assess their application’s operating intervals. It is shown that the rate of single-phase and two-phase hydrolysis of the primary partitioning tracer makes it possible to predict the shut-in time by calculating when the tracer is injected at the reservoir temperature. The influence of the tracer structure during the extraction process with an increase in the hydrocarbon chain of the ester in a different range of brine salinity and temperature has been studied. As a result, this work provides a method for evaluating the thermodynamic and kinetic behavior of primary tracers to establish minimum and maximum threshold K-values at various values of residual oil saturation, temperature, and brine salinity, taking into account the optimal time of the well shut-in to carry out at least 1/2 hydrolysis of esters.
1. Introduction
Before applying enhanced oil recovery (EOR) methods, it is crucial to evaluate the effectiveness of the technologies used, which is especially important for late-stage fields of development [1]. It requires measuring and comparing residual oil saturation before and after applying technology to EOR. The difference between these values serves as an indicator of the effectiveness of improved oil recovery technologies. In practice, the oil saturation of the reservoir is usually determined from the data of geophysical (penetration depth ~80 cm) well surveys (well logging) and based on core studies [2,3]. Compared to expensive well logging operations or complex core studies, an alternative way to determine the oil saturation used in the field is through single-well tracer studies (having a larger exploration radius ~3–7 m) [4,5]. The single-well chemical tracer test (SWCTT) technology, used to evaluate the efficiency of EOR, makes it possible to measure oil saturation by injecting substances (usual esters) partitioned between phases (oil-water) into the reservoir through a production well, which undergo a hydrolysis reaction to form a secondary tracer (alcohol) and acid during a well shut-in time [2,4,5,6]. The duration of the well shut-in depends on the reactivity of the primary tracer in reservoir conditions. After the shut-in, the well is returned to production. During reverse production, fluid samples are taken through a wellhead sampler, preserved if necessary, and then analyzed for tracer content. On average, the interval for sampling wellhead production is 10–20 min [7]. The sampling frequency determines the accuracy of data interpretation when calculating the extremum of the tracer production profile.
Since the secondary tracer, alcohol, does not dissolve in oil, it is extracted before the ester, which is predominantly in oil. Based on the difference in the arrival time to the wellhead between the two tracers and the K-value, the residual oil saturation Sor is calculated [5,6]:
where K-value is the partitioning coefficient (see experimental section) of primary tracer, Sor is the residual oil saturation, and β is the delay coefficient of the secondary tracer, which is determined by Equation (2):
where V1, V2—is the cumulative volume of fluid production containing the maximum value of the primary and secondary tracer concentration.
In recent years, the SWCTT method has become widespread as interest in the quantitative measurement of residual oil saturation has increased. Some experts [4,5] prefer the SWCTT study because of its accuracy and larger study radius. More than 600 tests have been performed to determine the residual oil saturation using the SWCTT method in the USA, Canada, the Middle East, Europe, South America, and the South Asian region [8,9,10,11]. The technique has been widely used to assess the effect of polymer, surfactant, and alkaline–surfactant–polymer (ASP) flooding and to study the impact of “Smart Water” flooding [8,12,13,14].
There are practically no universal substances that would become an effective SWCTT indicator for working in an extensive temperature range. Some recent studies [15] have explored the possibilities of new aromatic esters with good kinetic and thermodynamic properties in field tests. Aliphatic esters do not have such broad properties, due to which it is necessary to select candidates for tracers for specific the reservoir conditions among them.
For each tracer type, there are some criteria by which it can initially determine its suitability for SWCTT technology. To prepare aqueous solutions at the wellhead, esters with a low water solubility index, which should be at least 1% and preferably a high boiling point, are limited [16,17]. When choosing an ester, remember that the secondary tracer formed by it should have a high degree of solubility in water with the value of a measure of hydrophobicity logKow (logarithm of the molecular 1-octanol–water partition coefficient at T = 20 °C) value not exceeding 0.3 (i.e., have a minimum partitioning in the oil phase). Table 1 shows data on the physical and chemical characteristics of esters and alcohols. If, for the secondary tracer, logKow is negative or close to 0, then, for the use of several alcohols as partitioning and non-partitioning cover tracers, logKow may be higher. Esters such as methyl formate can also be excluded since such an ester has a negative logKow = −0.21, and it will not be partitioned in the oil.

Table 1.
Physico-chemical properties of tracer candidates [18,19,20,21,22,23].
In addition, the success of the performed test directly depends on the optimal choice of the tracer composition, which will contribute to the qualitative determination of the parameters required in the calculation of the residual oil saturation of the formation. The reservoir temperature determines the type of partitioning tracer utilized, which is a parameter that affects the degree of hydrolysis of the primary tracer. Formic acid esters hydrolyze 50-times faster than acetic acid esters and can be used at low reservoir temperatures in the range from 21 to 57 °C. Above this temperature, esters of acetic acid should be preferred, as they react more slowly and are mainly used in the temperature range from 54 to 121 °C [24,25].
It is convenient to use the dynamic value of the 1/2 hydrolysis (τ1/2) to analyze the shut-in time well—the time during which half of the original primary tracer will react (i.e., H = 50%). To enable the detection of tracers (primary and secondary) and the interpretation of the obtained data at the following stages at the wellhead during reverse production of the study, hydrolysis should undergo from 10 to 50% of the primary tracer–ester [4,5,25].
The primary tracer is hydrolyzed to acid and alcohol exclusively in the aqueous phase, while the rate of the processes occurring is primarily due to formation (reservoir) temperature, and completeness depends on the well shut-in time. The optimal time of the well shut-in (usually 2–15 days [5,26,27,28]) is determined by the rate of the hydrolysis of the selected tracer at the specified formation temperature.
Thus, in order to establish the boundary conditions for the applicability of the tracer in the field, this paper considers the kinetic and thermodynamic features of primary tracers that can be used in various reservoir conditions. The estimated kinetic parameters were evaluated depending on the temperature and partitioning coefficient in the water–oil phase, and a method for selecting the working interval of the primary tracer was presented for a given residual oil saturation, produced brine salinity, and reservoir temperature, as described in the study results.
2. Experimental Section
2.1. Materials
Ethyl acetate (Sigma Aldrich, Burlington, MA, USA), ethyl formate (Acros organics, Geel, Belgium), and ethyl propionate (Sigma Aldrich, Burlington, MA, USA) with a mass fraction of the main substance >98% were chosen as primary tracers. Oil (dehydrated, without mechanical impurities, density is 825 kg·m−3) from Western Siberia deposits was used for research. Different electrolytes (sodium chloride, calcium chloride, magnesium chloride, Sigma–Aldrich, 99%) dissolved in distilled water were used to prepare the brine model.
For obtaining graphs, a solution was prepared with a different known concentration of the primary tracer in the brine model range from 0.5 to 2.5% mass. Calibration solutions were designed in the vials with disposable fluoroplastic screw caps. Gas chromatographic analysis was used to determine the concentration. The measurements were performed on an Agilent 7820 A gas chromatograph with a flame ionization detector (FID). The GC oven is equipped with a chromatographic column designed to quantify tracers in the aqueous phase. The compounds were identified by individual quenching of the pure substances. The OpenLAB CDS ChemStation Edition software version C.01.07 SR3 was used to process the results.
2.2. Experimental Apparatus
A piston-cylinder (Amcore, Tyumen, Russia) with a volume of 50 mL was used to determine the tracer’s K-value and degree of hydrolysis (Figure 1). These measurements of K-value were made under static conditions. A brine model of different concentrations was prepared by dissolving the salt in distilled water. Next, 24 mL of brine model, 24 mL of oil, and 0.5 mL of tracer were injected into the piston cylinder. Such volumes were chosen to maximize the filling of the piston cylinder and reduce the gas cap during sampling. The high-pressure piston pump created pressure in the cylinder up to 7 MPa. Samples were placed in an oven. The temperature was set at an accuracy of ±1 °C and kept for the time required for primary tracer separation in the organic and water phases and for the hydrolysis reaction to proceed (24 h). After the expiration of time, samples of the liquid phase through the backpressure regulator were taken through the cooling line into vials with disposable screw caps made of fluoroplastic (T = −5 °C). After sampling, samples were analyzed on a gas chromatograph to quantify the tracer content in the aqueous phase.
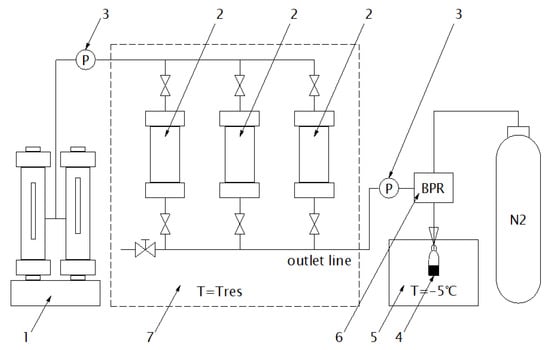
Figure 1.
Scheme of equipment for tracer testing under reservoir conditions in water-organic phases. 1. High-pressure plunger pump; 2. Piston cylinder (3 pcs.) for tracer solution in oil/water phases; 3. Pressure sensors; 4. Vial for effluent collection; 5. Cryostat (thermostat); 6. Backpressure regulator; 7. Thermo-regulated oven (heating up to reservoir temperature—Tres).
2.3. K-Value and Degree of Hydrolysis Calculation
The equation for calculating the K-value [29] for the water phase after equilibrium for a closed system with equal volumes of water and oil phases (Vw = V0) can be represented by:
where —concentration of ester in the oil phase at the current moment in time; —concentration of ester in the aqueous phase at the current moment in time; —the amount of ester substance in the oil phase at the current moment in time; —the amount of ester substance in the aqueous phase at the current moment in time; —the mass of ester in the oil phase at the current moment in time; —the mass of ester in the aqueous phase at the current moment in time.
Taking into account the weight loss of ester for hydrolysis and transition to the oil phase, K-value can be represented through the concentrations of tracers in the water phase as follows:
where —the mass of ester in the aqueous phase at the initial moment; —the mass of the aqueous phase; —a mass fraction of ester in the aqueous phase at the current moment in time; —a mass fraction of alcohol in the aqueous phase at the current moment in time; —the molar mass of ester; —the molar mass of alcohol.
The last contribution to the numerator the mass of ester consumed for the hydrolysis reaction. For the case of a slow hydrolysis reaction rate (i.e., the hydrolysis reaction is much slower than the partitioning tracer) and for tracers used in PITT technology (which lacks hydrolysis), this contribution is zero. The ratio of the weight of the ester consumed for hydrolysis to the initial weight of the ester is the degree of hydrolysis H:
3. Results and Discussion
To estimate the 1/2 hydrolysis of the primary tracer, consider the case of hydrolysis in a single-phase system (i.e., K-value = 0). It should be noted that the degree of hydrolysis in the reaction study in single-phase (water) and two-phase (water/oil) systems are different. This is explained by the progress of two competing processes (interfacial partitioning of the ester and its hydrolysis reaction in the aqueous phase). Table 2 shows the literature data on the rate constants and the energy of activation of the neutral hydrolysis reaction of the primary tracers.

Table 2.
The rate constant (kN, days−1), 1/2 hydrolysis (τ1/2, days), and activation energy (EA, kJ∙mol−1) for neutral ester hydrolysis.
The reaction rate constants at other temperatures for the hydrolysis of some esters can be recalculated using the Arrhenius equation (Equation (6)):
where kT2 and kT1—hydrolysis rate constants at two different temperatures (days−1); EA—activation energy (kJ/mol); R—universal gas constant (J/mol∙K).
From the calculated data obtained for the rate constant at different temperatures, we estimated the 1/2 hydrolysis for the esters shown in Figure 2. It is shown that a significant increase in hydrolysis time (well shut-in time) is caused by the replacement of the functional group from H- to CH3- in the acid-moiety ester. Data from the literature on the reaction rate of neutral hydrolysis for esters with a chain longer than CH2-CH3- in acid-moiety are limited. However, as can be seen from the kinetics of formate and acetate, an increase in the length of the hydrocarbon radical acid-moiety esters in the hydrolysis rate leads to an increase in the 1/2 hydrolysis (Figure 2, right offset). To a lesser extent, the hydrolysis time changes as the hydrocarbon substituent increases in alcohol moiety. Accordingly, varying the acid and alcohol moiety structure in the ester is key in selecting the partitioning tracer candidate for reservoirs with different reservoir temperatures.
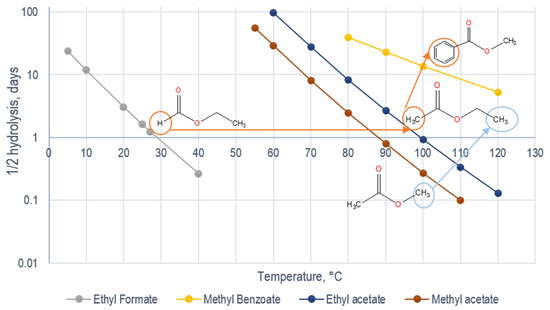
Figure 2.
One-half hydrolysis (neutral) of esters at various temperatures (log scale).
However, it should be remembered that, after the injection of ester in the aqueous phase into the formation together with the hydrolysis reaction, an extraction process takes place, i.e., the partitioning of ester between two immiscible phases (water, oil). The kinetics of ester hydrolysis in a two-phase system is considered similarly according to the pseudo-first order reaction with the rate constant k(2phase), which is 1 + β (β = K∙(Sor/1 − Sor)) times less than the reaction rate constant in a single-phase system k(1phase) [34]:
Thus, it follows from Equation (7) that, for the shut-in time (ts, the 1/2 hydrolysis is used), a given K-value can be calculated after converting the reaction rate constant over the half-life from the ratio:
Analyzing the change in the 1/2 hydrolysis of the esters with different reaction rates, in Figure 3, the satisfying area of the applicability of an individual tracer can always be distinguished depending on the temperature of the reservoir at a minimum and maximum K-value. Figure 3 illustrates the effect of the K-value on the hydrolysis time, and the value of the 1/2 hydrolysis time that is required to achieve the same chosen temperature, albeit with a different K-value (depending on the type of oil, the K-value may vary). The example of the tracers of ethyl format and ethyl acetate, using Equation (8), shows how the reaction time changes with an increase in the K-value (Figure 3); here, the value of the residual oil saturation of 0.5 was used, and the value of 1 + β in this case was equal to 1 + K. Red dots in Figure 3 indicate the intervals for using these tracers if the well shut-in time does not exceed fifteen days (blue shade, Figure 3).
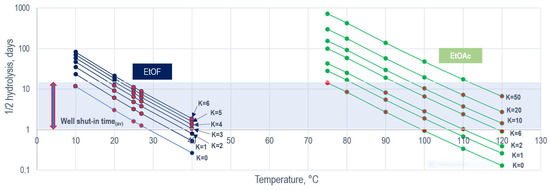
Figure 3.
One-half hydrolysis (neutral) of ethyl formate and ethyl acetate in the temperature range at various K-value and Sor = 0.5 (log scale).
In this case, the non-catalytic hydrolysis of esters (pH~7) in the temperature range at various K-values was evaluated. The effect of pH of a particular reservoir can affect the reduction in the hydrolysis shut-in time. Under reservoir conditions, the pH is difficult to control, and one reason for this is that the product of the hydrolysis reaction is acid. Thus, by establishing the hydrolysis kinetics of the primary tracer in a system of produced brine with known mineral composition and oil, it is possible to estimate as closely as possible the limits of use of such in a real single-well test.
When entering a porous medium containing residual oil, the primary tracer will pass from the mobile aqueous phase, from which it was initially injected, to an immobile oil phase until phase equilibrium is reached, as shown in Figure 4. Its total concentration in the aqueous solution decreases in proportion to the amount of residual oil. In this case, the interphase equilibrium of the primary tracer (ester) is determined by its K-value. The circulation sign in this figure explains that, since the partition coefficient must be a constant value, in order to maintain this constancy of K, the ester balances between two phases (water and oil), replenishing the lost ester during hydrolysis.
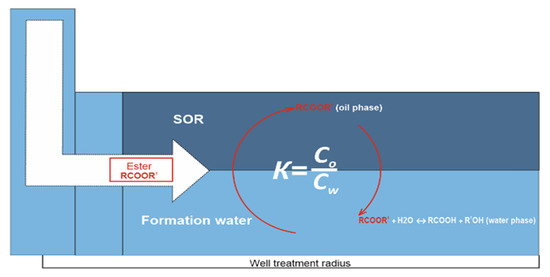
Figure 4.
Schematic representation of the reactive-extraction process of primary tracer.
A too-small K-value results in insufficient separation between the tracer’s production curves, increasing the error in the determination of Sor or even making it impossible. On the other hand, when the K-value is too high, the delay unnecessarily prolongs the test, leading to increased tailing of primary and sharp secondary tracer production history. The optimal range of K-values for a specific field depends on the anticipated oil saturation and has been proposed by Deans [17]:
Deans’ coefficients (β = 0.5; β = 1.5) represent the optimal range of the delay coefficient of the secondary tracer β showing the K-value range taking into account the Sor of the formation. If residual oil saturation varies from 0.2 to 0.5, for example, the K-value used in SWCTT should be within the range of 0.5–6 based on Equation (9). If the residual oil saturation is expected to be high, a tracer with a low K-value can be selected, and the test can be terminated earlier. If the residual oil saturation is low, a common K-value tracer will not exhibit sufficient retardation for a unique estimate of residual oil [35]. However, this interval will be longer for the high salinity/high temperature (HS/HT) reservoir conditions, and the K-value can range from 6 to 50 [25]. For the high-temperature (HT) reservoir, due to the high hydrolysis rate constant, it is desirable to reduce the residence time of the primary tracer in the water phase when closing the well, which is achieved by increasing its concentration in the oil phase. It is worth noting that high K-values will simultaneously lead to an increase in retention time and, as a result, to the flattening of the primary tracer production history, which requires the injection of an additional tracer as a cover tracer (partitioning or/and non-partitioning). The main element in this case is associated with a change in the structure of esters used as primary tracers in SWCTT, in which not only a change in the rate of hydrolysis occurs, but also a change in the hydrophobic properties of the ether. For this, the work studied the effect of low and high temperatures in a different salinity range on the K-value of esters with a different hydrocarbon chain (ethyl formate → ethyl acetate → ethyl propionate) in a two-phase water–oil system.
For HT reservoirs, ethyl propionate can be assumed as the preferred tracer for SWCTT due to the slow rate of hydrolysis wherein it cannot be used at moderate to low reservoir temperatures. Nevertheless, the extraction tests in the water–oil system of ethyl propionate showed equally high K-values at temperatures of 60 and 90 °C in brines with investigated salinities. Therefore, the use of ethyl propionate for field tests in the HT of reservoir condition will be inefficient and will lead to a significant increase in retention time and, as a result, to the flattening of the primary tracer produced profile and accordingly the loss of the reliability of the test results. Concurrently, for ethyl acetate having less hydrophobic properties, the obtained K-value in the water–oil system has values from 4.5 up to 17 in the range of salinity from 0 to 200 g/L at 110 °C. The K-value for HT reservoirs over a wide range of salinity confirms the applicability of EtOAc with the possible use of a cover tracer to determine retention time for a reasonable shut-in time.
For an ester with a moderate rate of hydrolysis as ethyl formate, a maximum experimental K-value in the range of 4–5 at HS/LT conditions (Table 3) was found. A slight change in K-value (3.4 and 4.5) for ethyl formate at a temperature rise from 25 to 45 °C in HS region (200 g/L) suggests that it is not possible to achieve a greater partition in the oil phase. It follows from this that the injection of ethyl formate in determining the residual oil saturation is effective in reservoirs with low and high salinity with a temperature limit of 20–45 °C. At higher temperatures, 1/2 hydrolysis of ethyl formate is already below 1 day (Figure 3) and will not become higher if the K-value remains at 5. Thus, the growth of the tracer carbon skeleton in a two-phase system leads to an increase in the constant of the hydrolysis reaction rate and simultaneous growth of the K-value (decrease in affinity for the water phase), showing the boundary conditions for the use of the primary tracer for different reservoirs conditions.

Table 3.
Partition coefficients (K-value) of ethyl formate, ethyl acetate, and ethyl propionate in a two-phase oil–water system (1:1) at various temperatures (T, °C) and water salinity (S, g/L).
An accurate K-value for certain oil type, water salinity, and reservoir temperature will always take precedence in understanding the tracer’s potential for use in SWCTT. Combining the K-values obtained for ethyl formate and ethyl acetate (Table 3) with the kinetic parameters, the intervals of the partitioning coefficient of these tracers were calculated using Equation (8) when used in formations with different temperatures and oil saturation. For ethyl propionate, it was difficult to obtain similar data due to the scarcity of kinetic parameters for the hydrolysis of this ester reported in the literature. The found intervals (Figure 5, marked in color) indicate the minimum and maximum K-value thresholds at which the hydrolysis rate at a given temperature corresponds to the well shut-in time from 1 to 15 days for technological exposure at various residual oil saturation, given that the hydrolysis of the ester will leak by at least 50%.
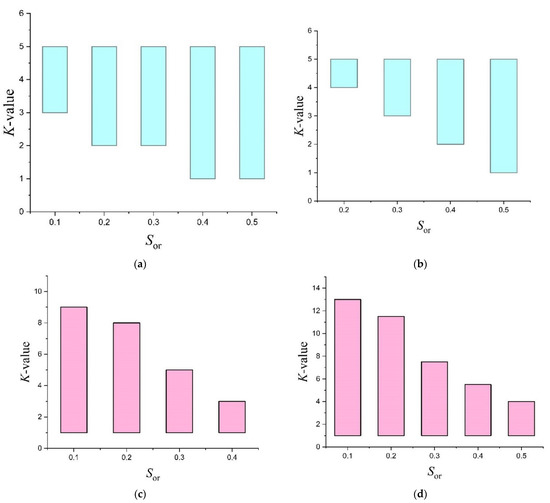
Figure 5.
Range of K-values at different values of residual oil saturation Sor and formation water salinity, leading to the optimal well shut-in time for ethyl formate: (a) T = 35 °C; (b) T = 45 °C and ethyl acetate: (c) T = 70 °C; (d) T = 90 °C.
4. Conclusions
This article focuses on the main criteria for using tracer compositions in SWCTT technology. For this, the initial requirements for primary tracers were analyzed, which should be taken into account when implementing injection in the field. Interpretation of data on the kinetics of hydrolysis of the partitioning tracer indicates the structural effect of the acid and alcohol parts of the ester with a change in its energy properties, which mainly determine the reaction rate in reservoir conditions. The existing relationship between the 1/2 hydrolysis of single-phase (water) and two-phase (oil) hydrolysis of the primary tracer at various K-values is shown, which makes it possible to calculate the optimal shut-in time.
The effect of salinity and temperature on the K-value of aliphatic esters of ethyl formate, ethyl acetate, and ethyl propionate has been studied. It has been shown that an increase in the hydrocarbon radical in the acid part of the ester dramatically changes the extraction process from the aqueous phase into the oil phase and affects the reactivity of the primary tracer in the aqueous phase, which, together, must be taken into account to optimize the composition of the tracer in certain reservoir conditions for the implementation of SWCTT projects. A method for the analytical solution of the choice of optimal primary tracers with an assessment of the boundary conditions for their applicability has been developed. This shows the required minimum and maximum threshold values of K-value at various values of Sor, temperature, and brine salinity, taking into account the optimal well shut-in time.
Author Contributions
Conceptualization, O.V.A. and A.V.B.; methodology, O.V.A., A.V.B. and A.R.M.; writing-original draft preparation, O.V.A., A.R.M. and A.V.B.; visualization, O.V.A. and A.R.M.; supervision, A.V.B. and M.A.V.; project administration, M.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the subsidy allocated to Kazan Federal University for the state as-signment in the sphere of scientific activities (Project NO 0671-2020-0048 of State Assignment № 075-00216-20-05 of 04.06.2020 (Part II Section I)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
No conflicts of interest associated with this publication. As corresponding author, I confirm that the manuscript had been read and approved for submission by the authors.
Abbreviations
| kN | Neutral hydrolysis rate constant |
| k(1phase) | Single-phase hydrolysis rate constant |
| k(2phase) | Two-phase hydrolysis rate constant |
| K | Partitioning coefficient |
| Vo | Oil volume |
| Vw | Water volume |
| τ1/2 | Reaction half-life (1/2 hydrolysis) |
| ts | Shut-in time |
| logKow | Octanol/water partition coefficient |
| S | Salinity |
| EtOF | Ethyl formate |
| EtOAc | Ethyl acetate |
| EtOPr | Ethyl propionate |
| MeOAc | Methyl acetate |
| LSW | Low salinity water |
| HSW | High salinity water |
| LT | Low temperature |
| HT | High temperature |
References
- Babadagli, T. Development of mature oil fields—A review. J. Pet. Sci. Eng. 2007, 57, 221–246. [Google Scholar] [CrossRef]
- Chang, M.M.; Maerefat, N.L.; Tomutsa, L.; Honarpour, M.M. Evaluation and Comparison of Residual Oil Saturation Determination Techniques. SPE Form. Eval. 1988, 3, 251–262. [Google Scholar] [CrossRef]
- Teklu, T.W.; Brown, J.S.; Kazemi, H.; Graves, R.M.; Al Sumaiti, A.M. A Critical Literature Review of Laboratory and Field Scale Determination of Residual Oil Saturation. SPE-164483-MS. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 23–26 March 2013. [Google Scholar] [CrossRef]
- Deans, H.A. The Single-Well Chemical Tracer Method for Measuring Residual Oil Saturation; Final Report, Contract No. US DOE/BC/20006-18; U.S. Department of Energy Office of Scientific and Technical Information: Washington, DC, USA, 1980. [Google Scholar]
- Doorwar, S.; Tagavifar, M.; Dwarakanath, V. A 1D Analytical Solution to Determine Residual Oil Saturations from Single-well Chemical Tracer Test Copyright. Chevron ETC. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020. [Google Scholar] [CrossRef]
- Mechergui, A.; Agenet, N.; Romero, C.; Nguyen, M.; Batias, J. Design, Operation, and Laboratory Work for Single-Well Tracer Test Campaign in Handil Field Indonesia. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013. [Google Scholar] [CrossRef]
- Al Abbad, M.; Balasubramanian, S.; Sanni, M.; Kokal, S.; Zefzafy, I.; Adam, F.; Al Hajji, A. Single-Well Chemical Tracer Test for Residual Oil Measurement: Field Trial and Case Study. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 25–28 April 2016. [Google Scholar] [CrossRef]
- Al Murayri, M.T.; Hassan, A.A.; Rahim, A.A.; Decroux, B.; Negre, A.; Salaun, M. Surfactant-Polymer Flooding: Single Well Chemical Tracer Test Design and Implementation in a Major Sandstone Kuwaiti Reservoir. In Proceedings of the SPE Kuwait Oil & Gas Show and Conference, Mishref, Kuwait, 13–16 October 2019. [Google Scholar] [CrossRef]
- Zoshchenko, O.; Aleshchenko, A.; Trushin, Y. Assessment of the Potential of Low-Salinity Water Injection Technology to Increase the Oil Recovery of the Carbonate Reservoir of the Kharyaga Field. In Proceedings of the SPE Russian Petroleum Technology Conference, Moscow, Russia, 22–24 October 2019. [Google Scholar] [CrossRef]
- Zecheru, M.; Goran, N. The use of chemical tracers to water injection processes applied on Romanian reservoirs. EPJ Web Conf. 2013, 50, 2005. [Google Scholar] [CrossRef][Green Version]
- Karimi, M. Single Well Tracer Test for Residual Oil Estimation; The Technical University of Crete, School of Mineral Resources Engineering, Petroleum Engineering, MSc Course: Chania, Greece, 2018. [Google Scholar]
- Hernández, C.; Chacón, L.; Anselmi, L.; Angulo, R.; Manrique, E.; Romero, E.; de Audemard, N.; Carlisle, C. Single Well Chemical Tracer Test to Determine ASP Injection Efficiency at Lagomar VLA-6/9/21 Area, C4 Member, Lake Maracaibo, Venezuela. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 13–17 April 2002. [Google Scholar] [CrossRef]
- Oyemade, S.N.; Al Harthy, S.A.; Jaspers, H.F.; Van Wunnik, J.; de Kruijf, A.; Stoll, M. Alkaline-Surfactant-Polymer Flood (ASP): Single Well Chemical Tracer Tests-Design, Implementation and Performance. In Proceedings of the SPE EOR Conference at Oil & Gas West Asia, Muscat, Oman, 11–13 April 2010. [Google Scholar] [CrossRef]
- Callegaro, C.; Masserano, F.; Bartosek, M.; Buscaglia, R.; Visintin, R.; Hartvig, S.K.; Huseby, O. Single Well Chemical Tracer Tests to Assess Low Salinity Water and Surfactant EOR Processes in West Africa. In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 10–12 December 2014. [Google Scholar] [CrossRef]
- Huseby, O.; Galdiga1, C.; Hartvig, S.; Zarruk, G.; Dugstad, O. A New Generation of Single Well Chemical Tracer Tests—Tracers and Methodologies. In Proceedings of the IOR—20th European Symposium on Improved Oil Recovery, Pau, France, 8–11 April 2019. [Google Scholar] [CrossRef]
- Bursaux, R.; Peltier, S.; Nguyen, M.; Romero, C.; Morel, D.D. Single Well Tracer Test Results in a High Temperature, High Salinity Offshore Carbonate Reservoir for Chemical EOR Pilot Evaluation. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar] [CrossRef]
- Deans, H.A.; Ghosh, R. pH and Reaction Rate Changes During Single-Well Chemical Tracer Tests. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 17–20 April 1994; pp. 203–212. [Google Scholar] [CrossRef]
- Vogel, A.I. 130. Physical properties and chemical constitution. Part XIII. Aliphatic carboxylic esters. J. Chem. Soc. 1948, 2, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Wilhoit, R.C.; Zwolinski, B.J. Physical and Thermodynamic Properties of Aliphatic Alcohols. J. Phys. Chem. Ref. Data 1973, 2, 420. [Google Scholar]
- Barton, A.F.M. Alcohols with Water Solubility Data Series; Pergamon Press: New York, NY, USA, 2013; p. 458. [Google Scholar]
- Getzen, F.; Maczynski, A.; Hefter, G.T. Esters with Water. Part I: Esters 2-C to 6-C; Pergamon Press: New York, NY, USA, 1992; Volume 48, p. 372. [Google Scholar] [CrossRef]
- Cumming, H.; Rücker, C. Octanol–Water Partition Coefficient Measurement by a Simple 1H NMR Method. ACS Omega 2017, 2, 6244–6249. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.S.; Zaramello, L.; Kuhnen, C.A.; Junkes, B.D.S.; Yunes, R.A.; Heinzen, V.E.F. Estimating the Octanol/Water Partition Coefficient for Aliphatic Organic Compounds Using Semi-Empirical Electrotopological Index. Int. J. Mol. Sci. 2011, 12, 7250–7264. [Google Scholar] [CrossRef] [PubMed]
- Galeev, R.I.; Bolotov, A.V.; Varfolomeev, M.A.; Mukhutdinova, A.R.; Smirnov, A.E.; Kornilov, A.V.; Kruglov, D.S.; Zhirov, A.V.; Sansiev, G.V.; Fedorchenko, G.D. New and simple methods of determination partition coefficient and degree hydrolysis of tracer for estimating residual oil saturation by SWCTT technologies. Pet. Sci. Technol. 2021, 39, 1043–1059. [Google Scholar] [CrossRef]
- Mechergui, A.; Romero, C.; Ganzo, A. Design of SWTT at High Salinity and High Temperature: Where are the Limits? In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar] [CrossRef]
- Pedersen, T. A Single Well Chemical Tracer model that accounts for temperature gradients, pH changes and buffering. J. Pet. Sci. Eng. 2021, 201, 108500. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, P.-X. Determination of Residual Oil Saturation in A Carbonate Reservoir. SPE-72111-MS. In Proceedings of the SPE Asia Pacific Improved Oil Recovery Conference, Kuala Lumpur, Malaysia, 8–9 October 2001. [Google Scholar] [CrossRef]
- Tomich, J.F.; Dalton, R.L.; Deans, H.A.; Shallenberger, L.K. Single-Well Tracer Method to Measure Residual Oil Saturation. J. Pet. Technol. 1973, 25, 211–218. [Google Scholar] [CrossRef]
- Wang, S.; Shiau, B.; Harwell, J.H. Effect of Reservoirs Conditions on Designing Single-Well Chemical Tracer Tests Under Extreme Brine Conditions. Transp. Porous Media 2018, 121, 1–13. [Google Scholar] [CrossRef]
- Segreda, J.F.M. Spontaneous hydrolysis of ethyl formate: Isobaric activation parameters. Int. J. Chem. Kinet. 2000, 32, 67–71. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Weinberg, N.; Wolfe, S. The neutral hydrolysis of methyl acetate—Part 1. Kinetic experiments. Can. J. Chem. 2009, 87, 539–543. [Google Scholar] [CrossRef]
- Comisar, C.M.; Hunter, S.E.; Walton, A.A.; Savage, P.E. Effect of pH on Ether, Ester, and Carbonate Hydrolysis in High-Temperature Water. Ind. Eng. Chem. Res. 2007, 47, 577–584. [Google Scholar] [CrossRef]
- Cooper, G.D.; Williams, B. Hydrolysis of Simple Aromatic Esters and Carbonates. J. Org. Chem. 1962, 27, 3717–3720. [Google Scholar] [CrossRef]
- Tang, J.S.; Harker, B. Mass Balance Method to Determine Residual Oil Saturation from Single Well Tracer Test Data. J. Can. Pet. Technol. 1990, 29, 115–124. [Google Scholar] [CrossRef]
- Bu, P.X.; Al Sofi, A.M.; Liu, J.; Benedek, L.; Han, M. Simulation of single well tracer tests for surfactant–polymer flooding. J. Pet. Explor. Prod. Technol. 2014, 5, 339–351. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).