1. Introduction
The atmosphere is primarily composed of N
2 and O
2. When the weld pool is exposed to the atmosphere, it tends to combine with O and N elements. The formation of oxides in the weld metal (WM) varies under different circumstances. The oxide layer on the metal surface may prevent the joining of the two workpieces together [
1]. Additionally, an excess of O in the WM may incur unexpected problems, such as enhanced porosity, reduced toughness, and depreciated hardenability [
2]. When N is absorbed by the molten metal, its presence exerts adverse impacts on the mechanical properties unless it combines with a strong nitride-forming element [
3].
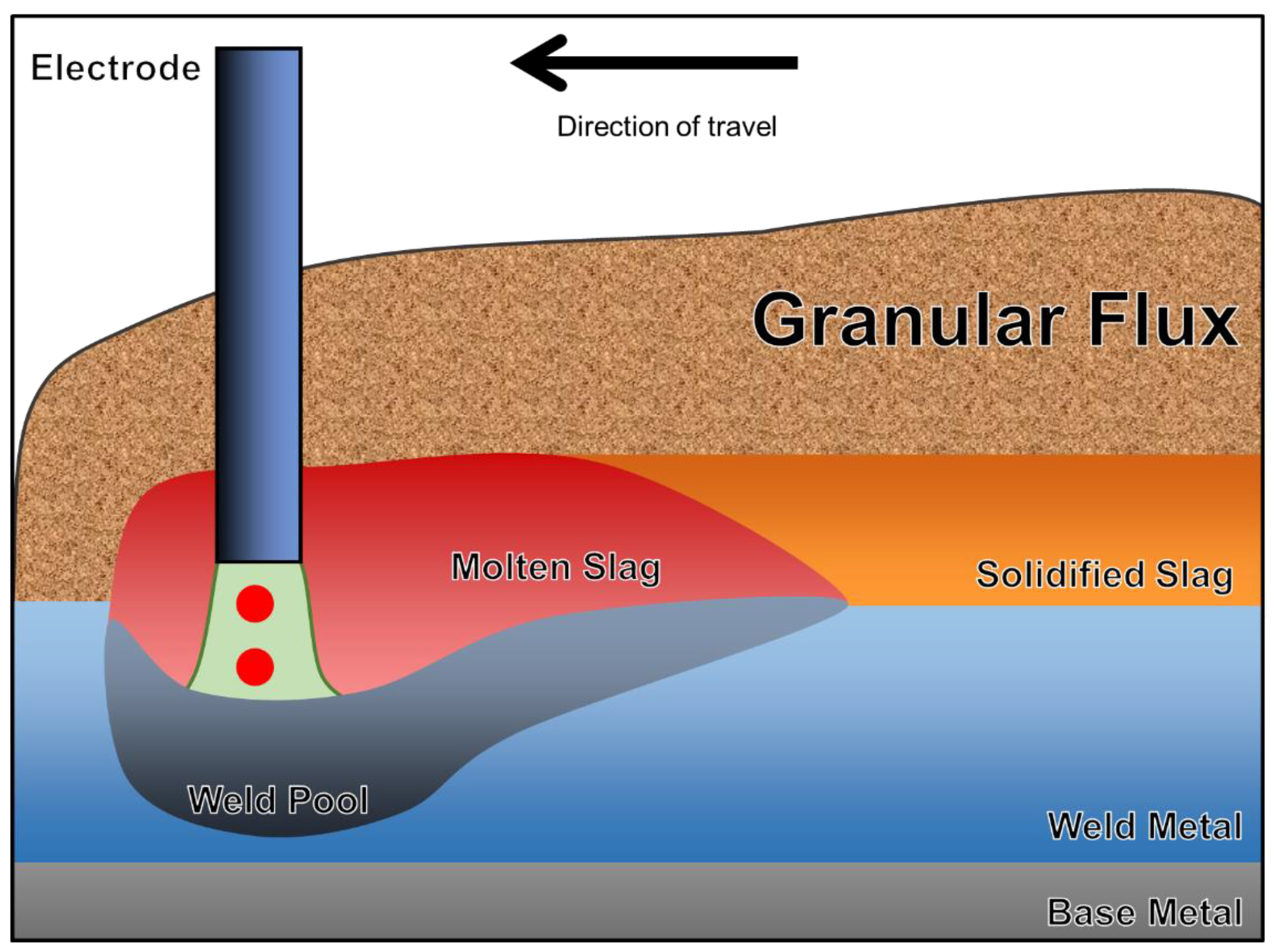
Submerged arc welding (SAW) is an automatic welding process particularly suitable for the joining of thick workpieces [
4]. Instead of permitting the air to exist between the base metal (BM) and the electrode, the arc cavity and weld pool are submerged under a layer of granular flux [
1].
Figure 1 illustrates the diagrammatic sketch of SAW. The SAW process is unique due to the permission of high welding currents in the absence of a violent arc [
5]. Therefore, high deposition rates and welding efficiencies are expected when SAW is applied [
6]. Flux is defined by the American Welding Society (AWS) as a material used for preventing, dissolving, or facilitating the removal of oxides and other undesirable surface substances [
7].
Flux serves essential functions in order to produce weldment with sound qualities. Most importantly, a flux must protect the metal from the atmosphere and stabilize the arc; other functions have been extensively documented, such as [
3,
4,
8]:
Provide a slag with a melting point lower than the metal.
Provide a slag with proper viscosity and low density.
Replenish the loss of metallic elements from the weld pool or micro-alloy WM via slag–metal reactions.
Perform metallurgical refining operations, such as deoxidation, desulphurization, and dephosphorization.
Reduce spatter and fumes during welding.
1.1. Research Gaps Based on Literature Review
The welding industry is constantly required to develop new fluxes or upgrade existing ones to meet the growing demands concerning SAW quality [
10]. To address such a challenge, an understanding of SAW, especially in terms of thermodynamics, is needed [
1]. Actually, the developments of flux design and thermodynamics have been promoting each other.
Some Review papers provide insight into the nature of fluxes subject to SAW. For example, Natalie et al. [
3] summarized the physical and chemical behavior of welding fluxes, including flux design criteria, compositional control for the WM, electrochemical reactions in arc welding, slag detachability, etc. Liu et al. [
2] documented the nature and behavior of fluxes used for fusion welding; they systematically generalized the function, classification, and possible application of steelmaking knowledge into welding metallurgy. Mitra et al. [
11], on the other hand, systematically evaluated the droplet–reaction time theories and slag–metal reaction theories subject to fusion welding, and studied the importance of chemical reactions in the compositional control for WM.
It is well known that an understanding of welding thermodynamics is essential for flux design and optimization [
4]. However, the development of welding thermodynamics and flux design has been pended for decades due to the lack of knowledge regarding thermodynamic knowledge under the high temperature of SAW, as high as 2000 °C. From the perspective of flux design for SAW, there have been few academic reviews concerning flux design since the 1990s. For instance, due to the lack of understanding of the physical and chemical properties of flux (slag), multicomponent fluxes are always designed on the basis of personal experience and random experiments [
12]. In the field of commercial flux, design achievements are mainly displayed in the form of patents, and the interconnection between flux design and thermodynamics is not fully clarified and reviewed.
1.2. Research Questions and Intended Contribution of the Study
Due to the incomplete understanding of flux’s thermodynamic properties, a definition of the basicity index (BI) drawn from steelmaking has been proposed to identify the flux’s O potential [
13,
14,
15,
16]. Then, based on the BI model, Chai et al. [
17,
18,
19] developed the slag–metal equilibrium model to predict the contents of Si and Mn in submerged arc welded metal. However, it should be pointed out that the BI and slag–metal equilibrium models are empirical in nature since there is a limited thermodynamic relationship between flux O potential and BI. The limitations of the BI and slag–metal equilibrium models have been discussed in our previous studies [
4,
13].
In recent years, a number of thermodynamic databases for multicomponent oxide and alloy systems have been developed by what has come to be known as the “Calphad technique” [
20,
21]. Although the effective temperature of SAW is as high as 2000 °C, under which the physical and chemical information of the flux (slag) remains scarce, the applicable thermodynamic models in the Calphad technique make it possible to obtain the thermodynamic data under high temperatures [
21]. The gas–slag–metal equilibrium model has been developed recently by employing the Calphad technique [
22]. It has been demonstrated that the gas–slag–metal equilibrium model is more feasible for aiding in the flux design and matching than the traditional BI and slag–metal equilibrium models [
6,
23]. Furthermore, one may get a better understanding of the SAW process by analyzing the thermodynamic data [
14,
24,
25,
26,
27].
Considering the research gaps mentioned above, the study addressed the following research questions:
How flux design and thermodynamics foster the development of each other.
Further the flux design criteria and specification from the perspective of thermodynamics.
The importance of Calphad technology and progressive thermodynamic databases in the flux design process.
1.3. Research Objectives
The present review has been undertaken to elaborate on the parallel development of flux design and welding thermodynamics subject to SAW. Typical fluxes designed at different stages are documented, and the corresponding design principle is evaluated from thermodynamic perspectives. Furthermore, the thermodynamic contributions of each flux to the understanding of the SAW process and welding metallurgy are evaluated. At last, the recent application of Calphad technology on flux design and matching is introduced.
2. Flux Classification
Flux is a granular material that primarily consists of oxides and CaF
2 [
4]. The manufacturing method plays an important role in shaping the flux features. In this section, the flux classification methods are documented to detail the manufacturing methods and to introduce an important definition widely applied in the field of flux design, namely, flux basicity.
2.1. Manufacturing Methods
2.1.1. Fused Flux
The fused flux is manufactured by melting the raw materials in a furnace at 1500 °C or higher. The melt is stirred for homogenization and then solidified by water or a metal plate. At last, the solidified fragments are screened to a proper size range [
2].
2.1.2. Bonded Flux
Raw materials for producing the bonded flux are dry mixed and then combined with an addition of potassium silicate or sodium silicate at approximately 300 °C or higher. The resulting mixture is then dried at a relatively low temperature and screened [
3].
2.1.3. Agglomerated Flux
The agglomerated flux is similar to the bonded flux except that it is bonded at a temperature higher than 760 °C.
Table 1 summarizes the advantages and disadvantages of fused flux, bonded flux, and agglomerated flux [
8].
2.2. Basicity Assessment of SAW Flux
The most widely accepted BI was proposed by Tuliani et al. [
16], as shown in Equation (1) (wt pct). The oxygen-free compound is CaF
2. The basic oxides include CaO, CaF
2, MgO, Na
2O, K
2O, MnO, and FeO, while the acid oxides include SiO
2, Al
2O
3, Cr
2O
3, TiO
2, and ZrO
2. Fluxes can be classified into three categories: acidic (BI < 1.0), neutral (1 ≤ BI < 1.2), and basic (BI ≥ 1.2) [
2]. Tuliania et al. [
16] have regressed the tendency of O level as a function of BI, as shown by Equation (1). Eagar [
28] then removed CaF
2 from Equation (1) since he assumed that CaF
2 should be considered as a neural component. Generally, the predicted O content decreases with increasing BI and then reaches a constant [
2]. From the empirical relationship between BI and WM O content, the flux’s O potential can be estimated, as shown in
Figure 2 [
16,
28,
29].
BI definition is a controversial subject. Much research has been performed in this area, but most of it has been empirical in nature [
3]. Numbers of controversy centers around the BI definition subject to SAW and how different components in a flux contribute to the flux behavior [
10]. Palm et al. [
30] also pointed out that there is a lack of fundamental basis regarding the correlation between BI and flux O potential.
2.3. Workflow Methodology
Submerged arc welding is a metallurgical process with a temperature as high as 2000 °C, which puts forward techniques and scientific challenges for flux design. An understanding of the physicochemical properties of flux and slag, especially under high temperatures, is essential to optimize the flux formula.
The overall flow diagram with respect to workflow methodology is plotted in
Figure 3 for better readership. As shown in
Figure 3, the development of the flux in submerged arc welding experiences three stages: the binary system, ternary system, and multicomponent system. Within this framework, the flux design stages have been documented and reviewed in detail. The design principles for fluxes are evaluated, and the limitations of each flux are elucidated. Furthermore, we explain and analyze the scientific significance of the designed fluxes upon the development of the welding metallurgy, especially in terms of the thermodynamic models developed to aid in the flux design. As last, the application of Calphad technology in welding metallurgy has been summarized.
3. Binary Flux
The early fluxes employed for SAW were acid in formulation and designed on the knowledge acquired in steelmaking [
31,
32]. During this period, designers primarily focused on the flux capability to perform the submerging function with high currents and facilitate high deposition rates.
3.1. Binary SiO2–MnO Flux
In the beginning, the manganese–silicate flux (flux with SiO
2 and MnO as major components) was designed from the MnO–SiO
2 phase diagram (plotted by Phase Diagram module in FactSage, similarly hereinafter [
20]) enjoying its low melting eutectic (38 wt pct SiO
2 at 1245 °C), as shown in
Figure 4 [
20].
SiO
2–MnO flux is regarded as acidic in nature due to the high level of SiO
2 incorporated. The WM produced by manganese-silicate flux always possess a high O content. SiO
2–MnO flux also favors the transfer of Mn and Si to the WM. Such flux is used for joining steels where no low-temperature toughness is needed. The thermodynamic analysis demonstrates that SiO
2 and MnO are the flux components with instabilities as they tend to decompose and release O
2 via Reactions (2) and (3), thereby improving the overall flux O potential [
28,
33,
34,
35,
36].
3.2. Binary CaO–SiO2 Flux
Redundant O causes a number of problems for the WM. In order to improve the mechanical properties of the weld, investigators were searching for effective methods to reduce the flux O potential. Binary CaO–SiO
2 flux is the prototype for calcium–silicate flux (flux with SiO
2 and CaO as major components). The flux design philosophy was poised to replace MnO with CaO (the oxide with lower O potential than MnO) by referring the knowledge from steelmaking [
31,
32].
As shown in
Figure 5, the liquid zone of CaO–SiO
2 flux is much narrower than that of SiO
2–MnO flux, so the design interval for CaO–SiO
2 flux is decreased [
20]. Nonetheless, binary CaO–SiO
2 flux fostered the development of research on thermodynamics for SAW; that is, through systematic welding experiments, investigators determined the equilibrium constants of Reaction (4) (
K1) and Reaction (5) (
K2) up to 2000 °C, which dominate the transfer of Si and Mn at the slag–metal interface [
37,
38]. Later on, Equations (6) and (7) provide the foundation for the development of the well-known slag–metal equilibrium model by the Massachusetts Institute of Technology group, which will be discussed in
Section 7 [
17,
18,
19].
4. Ternary Flux
The most imperative problems for binary flux are excessive O and Si transfer from flux due to the mass of SiO2 incorporated into flux. Benefiting from the development of thermodynamics, investigators started trying out the design of ternary fluxes.
4.1. Ternary Manganese-Silicate Flux
One major disadvantage of SiO
2–MnO flux is the excessive O uptake from the flux. Therefore, investigators started to replace SiO
2 and MnO with other components. By referring to ternary phase diagrams, Burck et al. [
39] designed manganese–silicate fluxes of FeO–SiO
2–MnO, CaO–SiO
2–MnO, and CaF
2–SiO
2–MnO systems; they checked the flux melting point from the isotherm projection graph (such as
Figure 6 for CaF
2–SiO
2–MnO system), measured the viscosity of the melts, performed bead-on-plate welding, and evaluated the transfer behaviors of O, Si, and Mn [
20].
Investigators revealed that FeO is a component with high O potential [
36,
40]. CaO, on the other hand, decreases the flux O potential by lowering the SiO
2 activity in slag [
6]. Within the study of Burck et al. [
39], it was revealed that CaF
2 could effectively reduce the flux O potential due to the fact that:
Subsequently, Chaveriat et al. [
41] investigated the mechanical properties of the WMs processed by FeO–SiO
2–MnO, CaO–SiO
2–MnO, and CaF
2–SiO
2–MnO fluxes (developed on the binary SiO
2–MnO fluxes), and revealed that CaF
2 helped to improve the impact toughness of the weldment by decreasing the flux O potential. Other fluxes of TiO
2–SiO
2–MnO, Al
2O
3–SiO
2–MnO, and MgO–SiO
2–MnO systems were also investigated, but none of them provide proper flux O potentials [
10].
4.2. Ternary Calcium–Silicate Flux
The typical commercial calcium–silicate flux in the early stage is SiO
2–CaO–MgO flux, particularly suitable for high-current SAW applications [
12]. Then, CaF
2 addition was found to be beneficial in improving the toughness [
42]. An important calcium–silicate flux is the CaF
2–SiO
2–CaO flux. Dallam et al. [
42] designed and manufactured CaF
2–SiO
2–CaO fluxes with 28 formulas based on the isotherm projection graph (see the yellow shaded area in
Figure 7) [
20]. It was concluded that such fluxes were capable of producing good-quality, high-strength, low-alloy (HSLA) steel weldments with O content spanning from 100 to 460 ppm. The optimum WM O was found to be from 200 to 300 ppm, corresponding to a microstructure of approximately 90 pct acicular ferrite (AF). Before that, it was believed that the higher the basicity, the cleaner the WM concerning nonmetallic inclusions, and thereby higher toughness [
16]. The conclusion drawn from the application of CaF
2–SiO
2–CaO flux revised the above hypothesis: A moderate O potential is anticipated for flux design since low O content means that there would be insufficient inclusions to promote the formation of AF [
4].
4.3. Ternary Alumina–Basic Flux
Si is an essential element in the WM that should be carefully controlled since excessive Si tends to reduce both elongation and toughness [
4,
19]. Despite the low O potential obtained in CaF
2–SiO
2–CaO flux, the SiO
2 favors the transfer of Si to the WM [
42]. With the increasing knowledge of slag structure, investigators realized that Al
2O
3, which may act as a slag network-builder, could substitute the SiO
2 in flux [
43,
44,
45]. Lau et al. [
33,
34] designed ternary alumina–basic fluxes of SiO
2–CaO–Al
2O
3 and Al
2O
3–CaO–MnO systems. The ensuing experimental results indicated that the level of Si in the WM was significantly decreased [
33,
34]. Nowadays, alumina–basic fluxes (the flux with CaO and Al
2O
3 as major components) are typical materials when high mechanical performances are required [
2].
Within the studies by Lau et al. [
33,
34], the source of O in SAW was also evaluated. Additionally, Lau et al. [
33,
34] tried to discuss the gas–slag–metal reactions when alumina–basic flux was applied. One important conclusion drawn by Lau et al. [
33,
34] was that the flux decomposition and O
2 pressure level within the arc should be considered as major sources of O contamination for the submerged arc-welded metal. Before that, only the relationship between slag and flux O potential was considered [
28]. Lau et al. [
33,
34], on the other hand, illustrated the importance of oxide decomposition on flux O potential.
5. Multicomponent Flux
5.1. Multicomponent TiO2–B2O3 Bearing Flux
Binary and ternary fluxes generally focused on the tuning of O, Si, and Mn contents subject to submerged arc welded metal. With the development of welding metallurgy and growing demand in the arctic regions for large, welded structures, investigators began trying to develop multicomponent fluxes due to the fact that:
Investigators revealed that the simultaneous addition of Ti and B into the submerged arc welded metal beneficially improved both strength and toughness. Recognizing the benefits of Ti and B for the WM, fluxes with the following characteristics were developed [
46]:
Good weldability, such as slag detachability and arc stability.
Obtain Ti and B in WM by the reduction of TiO2 and B2O3 in the flux.
Kohno et al. [
46] varied the levels of components until the best performance of the flux and WM was obtained. They reported the optimal flux system, with one being the TiO
2–BaO–CaO–CaF
2–Al
2O
3–SiO
2–B
2O
3 system and the other being the TiO
2–MgO–CaO–CaF
2–Al
2O
3–SiO
2 system. Kohno et al. [
46] have put forward serval recommendations for the design of multicomponent flux:
The precipitation of the chemical compound with a high melting point would deteriorate the slag detachability. For instance, when TiO2 was the main component, it was necessary to substitute BaO or MgO for CaO to eliminate the precipitation of perovskite (CaTiO3).
The arc stability is important for the reduction of N in the weld pool.
The transfer efficiency of Ti from flux (slag) to the WM is difficult to control.
5.2. Multicomponent Basic–Fluoride Flux
In recent decades, basic–fluoride flux has been developed due to its low O potential. In terms of basic–fluoride flux, CaF
2 acts as the major component, SiO
2 or Al
2O
3 acts as the network builder, with other oxides, such as MgO, CaO, TiO
2, etc., making up the remainder. In order to save energy, most basic–fluoride fluxes are manufactured via agglomeration. Despite possible moisture pickup when basic–fluoride flux is employed, the moisture can be removed via Reaction (9) during the welding process.
From the perspective of microalloying ability, a shortcoming of the basic–fluoride flux is the reduction of the alloying element from the formation of the fluoride gases [
22].
5.3. Recent Development Multicomponent Flux
As was concluded by Mitra et al. [
11,
47,
48], the transfer of alloy elements is governed by Reactions (10) and (11) at the slag–metal interface.
According to Reactions (10) and (11), the transfer of alloying element (M) is controlled by the activity of M
xO
y, O content, and the activity of FeO at the slag–metal interface [
11,
47,
48]. Indacochea et al. [
40] and Natalie et al. [
3] concluded that the level of FeO activity was proportional to the flux O potential. Considering that flux contributes a major source of O for the weld pool, it is concluded that the higher the flux O potential, the lower the element transfer of M from flux to the WM [
22].
Another essential factor dictating the transfer efficiency of alloying element from flux to the WM is the activity of the oxide in the flux (slag). Recently, Zhang et al. [
13,
22] and Coetsee et al. [
14,
49] demonstrated the chemical interactions of CaF
2 and oxides, especially when basic–fluoride flux is applied. Therefore, the reduction in the transfer efficiency of the alloying elements due to the formation of fluoride gases should be considered when multicomponent basic–fluoride fluxes are designed, as mentioned in
Section 5.2. Recently, a series of fluxes have been designed by Coetsee et al. [
24,
25,
26,
50] to improve the transfer efficiency of the alloying element from flux to the WM.
6. Flux Specification
Flux specification becomes more and more essential nowadays since it dictates how to match welding materials (BM, electrode, and flux) subject to SAW. Herein, two important technical parameters are introduced: Δ value and neutral point.
6.1. Δ Value
It is accepted that the WM composition is made up of the contributions from the electrode, flux, and BM [
2,
3]. The nominal composition can be calculated considering just the dilution effect of the electrode and BM, as shown in Equation (12).
The extent of loss or transfer of a specific element can be evaluated by a Δ quantity. A positive Δ indicates an elemental transfer from the flux to the WM, whereas a negative Δ suggests an elemental loss from the weld pool [
2,
3]. If the Δ changes rapidly with the flux formula, it will be difficult to maintain the WM composition with variations in the flux composition [
11,
47,
48]. A flux with a Δ that maintains a constant value can be attained without altering the desired WM composition by selecting the proper electrode/flux/BM combination.
6.2. Neutral Point
As is discussed in
Section 6.1, it is desirable to fine-tune the WM composition smoothly, especially the contents of the alloying elements. To describe the behavior of elemental transfer between flux (slag) and WM, Chai and Eagar et al. [
17,
18,
19] have proposed a “Neutral Point” (NP) definition to locate the flux formula when no transfer of a certain element between the flux and WM occurs [
5,
51]. Based on the slag–metal equilibrium consideration, Mitra et al. [
11,
47,
48,
52,
53] concluded that the NP is only affected by the flux formula. This conclusion is important since it is the basis for the Mitra kinetic model. However, by comparing the Δ values of CaF
2–SiO
2–CaO fluxes, a bias of NP has been captured when the heat input increases from 20 to 60 kJ/cm, as shown in
Figure 8 [
42,
51]. Therefore, the interconnection between NP and SAW parameters may be revised to make it applicable to high heat-input SAW, which is confirmed in another study [
54].
7. Application of Calphad Technology on Flux Design and Matching
SAW is a process with a temperature of as high as nearly 2000 °C. Due to incomplete understanding of flux thermodynamic properties, a high number of experiments are needed on flux design and matching, thereby consuming significant resources. FactSage is an integrated database of computing systems consisting of information, database, calculation, and manipulation modules that access various pure substances and solution databases [
20,
21]. Herein, the recent applications of FactSage on flux design are elucidated.
7.1. Viscosity Module
The viscosity of flux is viewed as a critical requirement for producing sound welds [
3]. For example, weld pool refining is facilitated by a fluid flux, as greater fluidity promotes a kinetic driving force for chemical reactions. Further, a flux must possess a high enough viscosity to protect the metal from the atmosphere. However, if the flux viscosity is too high, undercutting of the weld bead may occur. Jackson [
8] investigated the viscosity of the fluxes and suggested that the viscosities should be controlled from 0.2 to 0.7 Pa.s at 1400 °C. The temperature of SAW is as high as 2000 °C, under which the slag viscosity is unmeasurable. Therefore, the conclusion drawn by Jackson [
8] was rendered empirical since no fundamental formulations were developed between flux viscosity and the quality of the weld.
7.2. Phase Diagram Module
The Phase Diagram module is applied to plot types of phase diagrams for systems containing stoichiometric phases, solution phases, and any number of system components [
3]. By using this module, one can ensure that the flux melting point is lower than the metal to be joined [
55].
7.3. Equilib Module
Equilib calculates the equilibria for multicomponent systems [
3]. FactPS, FToxid, and FSstel are essential databases for thermodynamic simulation of the SAW process [
6,
13,
14,
24,
25]. The FactPS database contains data from standard compilations. The FToxid database contains data for pure oxides and oxide solutions. The oxyfluoride systems were also incorporated into the FToxid database to facilitate the equilibrium calculation when CaF
2-bearing fluxes are applied. The FSstel database is intended to provide a sound basis for calculations covering a wide range of steelmaking processes.
7.3.1. The Slag–Metal Equilibrium Model
The slag–metal equilibrium model was developed by Chai et al. [
17,
18,
19], with the following assumptions.
The effective temperature of chemical reactions in SAW is set as 2000 °C.
An empirical relationship between the BI and the O content of the WM is known to exist.
The activity data of steelmaking slag may be extrapolated to be from 1600 to 2000 °C by using the regular solution model.
The interaction items are neglected.
The primary reactions of interest are those involving Si, Mn, and O.
Recently, Zhang and Coetsee et al. [
22] performed a slag–metal equilibrium calculation by applying the Equilib module. It was revealed that the Equilib module is capable of compensating for the limitations of the slag–metal equilibrium model subject to the following aspects:
The activities of SiO2 and MnO are calculable at various temperatures.
The interaction items are fully considered.
Except for Si, Mn, and O, all contents of elements in the metal can be predicted.
7.3.2. The Gas–Slag–Metal Equilibrium Model
Previous investigations have demonstrated that the sole consideration of slag–metal reactions may increase the prediction error, especially when CaF
2-rich fluxes are applied [
13,
14,
22]. For instance, Lau et al. [
33,
34] concluded that the flux O potential (O content in submerged arc welded metal) is governed by the partial pressure of O
2 in the arc cavity. Chai et al. [
36] designed several binary CaF
2-oxide fluxes and found that the decomposition level of oxide dictates the O uptake from the flux.
Recently, the physical phenomena have been fully reviewed, based on which Zhang et al. [
56] established the model of gas–slag–metal interface subject to the SAW process [
1]. Then, the gas–slag–metal equilibrium model has been proposed to improve the prediction accuracy [
22]:
In further studies, we assessed the capabilities of the slag–metal and gas–slag–metal equilibrium models to predict the chemistries in submerged arc welded metal [
13,
22]. It was revealed that the slag–metal equilibrium model may underestimate the flux O potential since the impact of O
2 is not considered [
13,
14,
22]. Additionally, the contents of alloying elements in the WM may be overestimated because the loss of elements from the gas formation is not considered in the slag–metal equilibrium model [
13,
14,
22].
7.3.3. Matching of Flux, BM, and the Electrode
Each country processes a number of standards (most of them are different in detail) on the matching strategies of the flux, BM, and electrode subject to SAW. The significance of SAW metallurgy is to model these complex matching standards in another way in lieu of referring to the various standards in different countries. As mentioned previously, the nominal composition is defined to determine the compositional contribution from BM and the electrode, and the Δ value is defined to quantify the compositional contribution from the flux. Then, a Neutral Point definition is proposed to describe the flux formula where no transfer of alloying element between the flux and WM occurs. As such, the above parameters could be employed to establish the matching strategy of flux, electrode, and BM.
Recently, Zhang et al. [
55] proposed a thermodynamic method to facilitate flux design and to aid in the development of the matching strategy instead of performing experiments.
8. Concluding Remarks and Further Research
The review above has demonstrated that thermodynamics is vital for the development of the flux design, while the ongoing flux design requirement further fosters the research in thermodynamics subject to high temperatures. From the perspective of technology, the measurement of thermodynamic data under 2000 °C is impossible [
4]. As such, one needs the application of certain models, such as the Modified Quasichemical Model, Cell Model, and Associate Model, to obtain thermodynamic data even if the temperature is higher than for steelmaking [
21]. Therefore, to further improve the thermodynamic calculation accuracy, the development of the thermodynamic data under high temperatures is anticipated.
Then, a significant feature of SAW concerns the chemical interaction between plasma and the alloy element. For instance, it has been extensively established that the element loss of Mn is favored during the SAW process, and the sole consideration of thermodynamic equilibrium is insufficient to explain such a loss. Therefore, a deeper understanding of the interconnection between plasma and welding consumables is required.
It should also be pointed out that, as was concluded by Mitra et al. [
11,
47,
48], the welding parameters are kinetic factors for SAW in nature and are subject to flux/electrode combinations with comprehensive issues, including both thermodynamic and kinetic knowledge. We will review the kinetic subjects on SAW in our coming work.
The development of new fluxes for submerged arc welding is ongoing. The present study provides a revealing insight into the flux design at various stages from the thermodynamic perspective: The flux design philosophy regarding binary, ternary, and multicomponent systems has been systematically reviewed. The contribution of each flux system to the development of welding thermodynamics and technology is documented. The Calphad technology is introduced to facilitate the flux design, flux selection, and the matching of welding consumables. Based on the critical outcome, the following conclusions can be drawn:
Binary fluxes were mainly applied in the early trials of SAW, primarily focused on the flux capability of performing the submerging function with high currents and facilitating high deposition rates. Despite several limitations, the binary fluxes promote the development of welding thermodynamics, especially in terms of the transfer of Si and Mn mechanisms at the slag–metal interface.
Ternary fluxes were developed based on binary fluxes benefiting from deeper understanding of thermodynamics. Within these stages, various types of fluxes, such as manganese–silicate fluxes, calcium–silicate fluxes, and alumina–basic fluxes, have been developed to fulfill different SAW conditions. With the development of welding metallurgy and growing demand in the arctic regions for large, welded structures, investigators began trying to develop multicomponent fluxes.
The Calphad technology and progressive thermodynamic databases facilitate the flux design process and strengthen the understanding of the SAW process. By using Calphad technology, the gas–slag–metal equilibrium model has been developed, which processes stronger universality than the traditional BI slag–metal equilibrium models.
Then, the Viscosity module, Phase Diagram module, and Equilib module are able to aid in the flux design so that some random experiments regarding flux design can be replaced, thereby saving human and material resources.
Author Contributions
Conceptualization, J.Z. and D.Z.; funding acquisition, D.Z., L.W., J.F. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Natural Science Founda-tion of China (Nos. 12101441, 50474085), Initial Fund of Suqian University (No. 2022XRC040).
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding—A Review. Weld. J. 2019, 98, 283–313. [Google Scholar] [CrossRef]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding; ASM Handbook; ASM International: Almere, The Netherlands, 1993; Volume 6, pp. 55–63. [Google Scholar] [CrossRef]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Cong, W.; Zhang, J. Fine-tuning Weld Metal Compositions via Flux Optimization in Submerged Arc Welding: An Overview. Acta Metall. Sin. 2022, 57, 1126–1140. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2-MnO Fluxes under High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 885–890. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Coetsee, T. Thermodynamic Evaluation of Element Transfer Behaviors for Fused CaO-SiO2-MnO Fluxes Subjected to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2021, 52, 1937–1944. [Google Scholar] [CrossRef]
- A 3.0 M/A3.0; Standard Welding Terms and Definitions. AWS: Bellevue, WA, USA, 2010.
- Jackson, C. Flux and Slag in Welding. Weld Res. Bull 1973, 190. [Google Scholar]
- Sidhu, A.S.; Singh, S.; Kumar, R.; Pimenov, D.Y.; Giasin, K. Prioritizing Energy-Intensive Machining Operations and Gauging the Influence of Electric Parameters: An Industrial Case Study. Energies 2021, 14, 4761. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 3rd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part I. Evaluation and Reassessment of Existing Theories. Metall. Trans. B 1991, 22, 65–71. [Google Scholar] [CrossRef]
- Indacochea, J.; Olson, D. Relationship of Weld-metal Microstructure and Penetration to Weld-metal Oxygen Content. J. Mater. Energy Syst. 1983, 5, 139–148. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Coetsee, T. Assessment of Weld Metal Compositional Prediction Models Geared Towards Submerged Arc Welding: Case Studies Involving CaF2-SiO2-MnO and CaO-SiO2-MnO Fluxes. Mater. Trans. B 2021, 52, 2404–2415. [Google Scholar] [CrossRef]
- Coetsee, T.; Mostert, R.J.; Pistorius, P.G.H.; Pistorius, P.C. The Effect of Flux Chemistry on Element Transfer in Submerged Arc Welding: Application of Thermochemical Modelling. J. Mater. Res. Technol. 2021, 11, 2021–2036. [Google Scholar] [CrossRef]
- Fox, A.; Eakes, M.; Franke, G. The Effect of Small Changes in Flux Basicity on the Acicular Ferrite Content and Mechanical Properties of Submerged Arc Weld Metal of Navy HY-100 Steel. Weld. J. 1996, 75, 330–333. [Google Scholar]
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld Metal. Weld. Met. Fabr. 1969, 37, 327–339. [Google Scholar]
- Chai, C.; Eagar, T. Prediction of weld-metal composition during flux-shielded welding. J. Mater. Energy Syst. 1983, 5, 160–164. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag-metal Equilibrium during Submerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Chai, C.-S. Slag-metal Reactions during Flux Shielded Arc Welding. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, UK, 1980. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Jung, I.-H. Overview of the Applications of Thermodynamic Databases to Steelmaking Processes. Calphad 2010, 34, 332–362. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Basu, S.; Wang, C. Impact of Gas Formation on the Transfer of Ti and O From TiO2-bearing Basic-fluoride Fluxes to Submerged Arc Welded Metals: A Thermodynamic Approach. Calphad 2020, 71, 102195. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Liu, H.; Wang, Z.; Wang, C. Addressing Weld Metal Compositional Variations in EH36 Shipbuilding Steel Processed by CaF2-SiO2-CaO-TiO2 Fluxes. Metall. Mater. Trans. B 2022, 53, 1329–1334. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F. Aluminium Assisted Nickel Alloying in Submerged Arc Welding of Carbon Steel: Application of Unconstrained Metal Powders. Appl. Sci. 2022, 12, 5392. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F. Aluminium-Assisted Alloying of Carbon Steel in Submerged Arc Welding: Application of Al-Cr-Ti-Cu Unconstrained Metal Powders. Processes 2022, 10, 452. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F. Application of Copper as Stabiliser in Aluminium Assisted Transfer of Titanium in Submerged Arc Welding of Carbon Steel. Processes 2021, 9, 1763. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F. Chemical Interaction of Cr-Al-Cu Metal Powders in Aluminum-Assisted Transfer of Chromium in Submerged Arc Welding of Carbon Steel. Processes 2022, 10, 296. [Google Scholar] [CrossRef]
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80. [Google Scholar]
- Shao, G.; Liu, Z.; Fan, J.; Guo, Y.; Xu, Q.; Zhang, J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals 2022, 12, 1530. [Google Scholar] [CrossRef]
- Palm, J. How fluxes determine the metallurgical properties of submerged arc welds. Weld. J. 1972, 51, 358. [Google Scholar]
- Lewis, W.; Faulkner, G.; Rieppel, P. Flux and Filler Wire Developments for Submerged Arc Welding HY80 Steel. Weld. J. 1961, 40, 337s–340s. [Google Scholar]
- Lewis, W.; Faulkner, G.; Martin, D.; Rieppel, P. Submerged Arc Welding HY-80 Steel. Weld. J. 1960, 6, 39–42. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. Gas/metal/slag Reactions in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1986, 65, 31–38. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding using CaO-Al2O3 Based Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Zhang, J.; Coetsee, T.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2 Fluxes Subject to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 16–21. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag Metal Reactions in Binary CaF2-Metal Oxide Welding Fluxes. Weld. J. 1982, 61, 229–232. [Google Scholar]
- Belton, G.; Moore, T.; Tankins, E. Slag-metal Reactions in Submerged Arc Welding. Weld. J. 1963, 42, 289s–297s. [Google Scholar]
- Christensen, N.; Chipman, J. Slag-metal interaction in arc welding. Weld. J. 1953, 15, 1–14. [Google Scholar]
- Burck, P.; Indacochea, J.; Olson, D. Effects of Welding Flux Additions on 4340 Steel Weld Metal Composition. Weld. J. 1990, 3, 115–122. [Google Scholar]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions During Submerged Arc Welding with FeO-MnO-SiO2 Fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Chaveriat, P.; Kim, G.; Shah, S.; Indacochea, J. Low Carbon Steel Weld Metal Microstructures: The Role of Oxygen and Manganese. J. Mater. Eng. 1987, 9, 253–267. [Google Scholar] [CrossRef]
- Dallam, C.; Liu, S.; Olson, D. Flux Composition Dependence of Microstructure and Toughness of Submerged Arc HSLA Weldments. Weld. J. 1985, 64, 140–151. [Google Scholar]
- Kim, G.-H.; Sohn, I. Effect of Al2O3 on the Viscosity and Structure of Calcium Silicate-based Melts Containing Na2O and CaF2. J. Non-Cryst. Solids 2012, 358, 1530–1537. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Z.; Zhao, T.; Wang, C. A Raman and Multinuclear 29Si, 27Al, and 19F NMR Study on the Structural Roles of CaF2 in SiO2–CaO–Al2O3-based Welding Fluxes. Metall. Mater. Trans. B 2022, 53, 232–241. [Google Scholar] [CrossRef]
- Park, J.H. Solidification Structure of CaO–SiO2–MgO–Al2O3(–CaF2) Systems and Computational Phase Equilibria: Crystallization of MgAl2O4 Spinel. Calphad 2007, 31, 428–437. [Google Scholar] [CrossRef]
- Kohno, R.; Takami, T.; Mori, N.; Nagano, K. New Fluxes of Improved Weld Metal Toughness for HSLA Steels. Weld. J. 1982, 61, 373–378. [Google Scholar]
- Mitra, U.; Eagar, T. Slag-metal Reactions During Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part III. Verification of the Theory. Metall. Trans. B 1991, 22, 83–100. [Google Scholar] [CrossRef]
- Coetsee, T. Phase Chemistry of Submerged Arc Welding (SAW) Fluoride Based Slags. J. Mater. Res. Technol. 2020, 9, 9766–9776. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F.J. Improved Titanium Transfer in Submerged Arc Welding of Carbon Steel through Aluminum Addition. Miner. Process. Extr. Metall. Rev. 2022, 43, 771–774. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Fine-Tuned Element Transfer Strategies for Ternary CaF2-SiO2-CaO Fluxes in Submerged Arc Welding: An Environmentally Friendly Approach. Metall. Mater. Trans. B 2020, 51, 1350–1354. [Google Scholar] [CrossRef]
- Mitra, U. Kinetics of Slag Metal Reactions during Submerged Arc Welding of Steel. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, UK, 1984. [Google Scholar]
- Mitra, U.; Eagar, T. Slag Metal Reactions during Submerged Arc Welding of Alloy Steels. Metall. Trans. A 1984, 15, 217–227. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, G.; Zhang, J.; Liu, Z. On the Moving of Neutral Point for Mn Subject to Submerged Arc Welding under Various Heat Inputs: Case Study into CaF2-SiO2-Na2O-MnO Agglomerated Fluxes. Processes 2022, 10, 1888. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, G.; Guo, Y.; Xu, Q.; Liu, Z. Facilitating Flux Design Process Geared Towards Submerged Arc Welding via Thermodynamic Approach: Case Study into CaF2–SiO2–Na2O–Al2O3–TiO2 Agglomerated Flux. Calphad 2022, 79, 102483. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Elucidating the Roles of SiO2 and MnO upon Decarburization During Submerged Arc Welding: A Thermodynamic Study into EH36 Shipbuilding Steel. Metall. Mater. Trans. B 2020, 51, 1805–1812. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).