Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications
Abstract
1. Introduction
2. Chemical Composition
2.1. Macronutrients
2.2. Vitamins
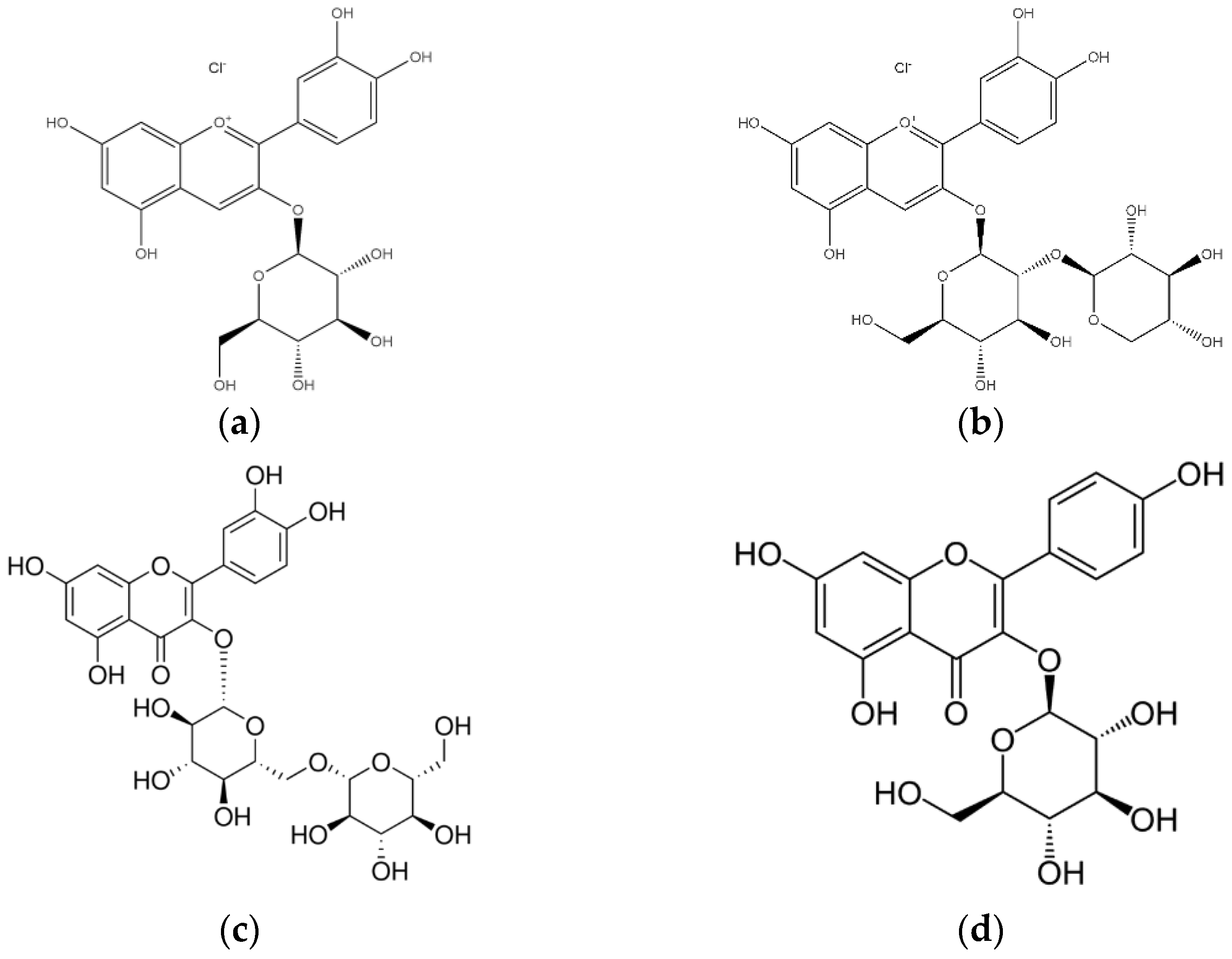
2.3. Phenolic Compounds
2.4. Cyanogenic Glycosides
3. Elderberries in Food Products
4. Health Benefits
4.1. Antioxidant Capacity
4.2. Antibacterial Properties
4.3. Antiviral Properties
4.4. Antidiabetic Properties
5. Extraction of Bioactive Compounds
5.1. Conventional Solvent Extractions
5.2. Soxhlet Extraction
5.3. Pressurized Liquid Extraction
5.4. Microwave-Assisted Extraction
5.5. Ultrasound-Assisted Extraction
5.6. Supercritical Fluid Extraction
5.7. Pulsed Electric Field Extraction
5.8. High Hydrostatic Pressure Extraction
5.9. Enzymatic Extraction
6. Separation and Purification
6.1. Membrane Based Process
6.2. Electrodialysis with Ultrafiltration Membrane
6.3. Precipitation of Anthocyanins
6.4. Countercurrent Chromatography
7. Extract Characterization
7.1. Determination of the Total Phenolic Content (TPC)
7.2. Determination of the Total Flavonoid Content (TFC)
7.3. Determination of Total Anthocyanin Content (TAC)
7.4. Determination of Phenolic Profile/Phytochemical Composition
7.5. Determination of the Antioxidant Activity (AA)
7.5.1. ABTS—2,2-Azino-Bis(3-ethylbenzothiazoline-6-sulfonic Acid)
7.5.2. DPPH—(2,2′-Diphenyl-picrylhydrazyl) Radical-Scavenging Activity
7.5.3. ORAC Assay
7.5.4. FRAP Assay
7.5.5. Cellular Antioxidant Activity Assay
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vallès, J.; Bonet, M.A.; Agelet, A. Ethnobotany of Sambucus nigra L in Catalonia (Iberian Peninsula): The Integral Exploitation of a Natural Resource in Mountain Regions. Econ. Bot. 2004, 58, 456–469. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Moerman, D.E. Native American Ethnobotany; Timber Press: Portland, OR, USA, 2002. [Google Scholar]
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Porter, R.S.; Bode, R.F. A review of the antiviral properties of black elder (Sambucus nigra L.) products. Phytother. Res. 2017, 31, 533–554. [Google Scholar] [CrossRef]
- Zielinska—Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczynski, R. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef]
- Lorenzo, J.M.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.F.J.; Putnik, P.; Kovačević, D.B.D.B.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Milena, V.; Tatjana, M.; Gökhan, Z.; Ivana, B.; Aleksandra, C.; Mohammad, M.F.; Marija, R. Advantages of contemporary extraction techniques for the extraction of bioactive constituents from black elderberry (Sambucus nigra L.) flowers. Ind. Crops Prod. 2019, 136, 93–101. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 2016, 97, 2623–2632. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Microencapsulation of polyphenols—The specific case of the microencapsulation of Sambucus nigra L. extracts. Trends Foods Sci. Technol. 2020, 105, 457–467. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; de Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Mlynarczyk, K.; Walkowiak-Tomczak, D.; Lysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Kiprovski, B.; Malencic, D.; Ljubojevic, M.; Ognjanov, V.; Veberic, R.; Hudina, M.; Mikulic-Petkovsek, M. Quality parameters change during ripening in leaves and fruits of wild growing and cultivated elderberry (Sambucus nigra) genotypes. Sci. Hortic. 2021, 277, 109792. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127–266. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Vulić, J.; Ljubo, J.; Vračar, O.; Šumić, Z.M. Chemical characteristics of cultivated elderberry fruit. Acta Periodica Technol. 2008, 39, 85–90. [Google Scholar] [CrossRef]
- Ho, G.T.; Zou, Y.F.; Aslaksen, T.H.; Wangensteen, G.; Barsett, H. Structural characterization of bioactive pectic polysaccharides from elderflowers (Sambuci flos). Carbohydr. Polym. 2016, 135, 128–137. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef]
- Thomas, A.L.; Byers, P.L.; Gu, S.; Avery, J.D.; Kaps, M.; Datta, A.; Rottinghaus, G.E. Occurrence of polyphenols, organic acids, and sugars among diverse elderberry genotypes grown in three Missouri (USA) locations. Acta Hortic. 2015, 1061, 147–154. [Google Scholar] [CrossRef]
- Gleńsk, M.; Czapińska, E.; Woźniak, M.; Ceremuga, I.; Włodarczyk, M.; Terlecki, P.; Ziółkowski, P.; Seweryn, E. Triterpenoid acids as important antiproliferative constituents of European elderberry fruits. Nutr. Cancer 2017, 69, 643–651. [Google Scholar] [CrossRef]
- Salvador, Â.C.; Silvestre, A.J.; Rocha, S.M. Unveiling elderflowers (Sambucus nigra L.) volatile terpenic and norisoprenoids profile: Effects of different postharvest conditions. Food Chem. 2017, 229, 276–285. [Google Scholar] [CrossRef]
- Kitrytė, V.; Laurinavičienė, A.; Syrpas, M.; Pukalskas, A.; Venskutonis, P.R. Modeling and optimization of supercritical carbon dioxide extraction for isolation of valuable lipophilic constituents from elderberry (Sambucus nigra L.) pomace. J. CO2 Utiliz. 2020, 35, 225–235. [Google Scholar] [CrossRef]
- Dulf, F.V.; Oroian, I.; Vodnar, D.C.; Socaciu, C.; Pintea, A. Lipid classes and fatty acid regiodistribution in triacylglycerols of seed oils of two Sambucus species (S. nigra L. and S. ebulus L.). Molecules 2013, 18, 11768–11782. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.P.; Plastina, J.; Meijerink, R.F.; Witkamp, B.G. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef]
- Kołodziej, B.; Maksymiec, N.; Drozdzal, K.; Antonkiewicz, J. Effect of traffic pollution on chemical composition of raw elderberry (Sambucus nigra L.). J. Elementol. 2012, 17, 67–78. [Google Scholar] [CrossRef]
- Czech, A.; Rusinek, E.; Merska, M. Zawartość wybranych biopierwiastków w owocach i sokach owoców jagodowych. Probl. Hig. Epidemiol. 2011, 92, 836–839. [Google Scholar]
- Jabłońska-Ryś, E.; Zalewska-Korona, M.; Kalbarczyk, J. Antioxidant capacity, ascorbic acid and phenolics content in wild edible fruits. J. Fruit Ornam. Plant Res. 2009, 17, 115–120. [Google Scholar]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Stéger-Máté, M.; Abrankó, L. Evaluation of colouring ability of main European elderberry (Sambucus nigra L.) varieties as potential resources of natural food colourants. Int. J. Food Sci. Technol. 2015, 50, 1317–1323. [Google Scholar] [CrossRef]
- Kaack, K. Aroma composition and sensory quality of fruit juices processed from cultivars of elderberry (Sambucus nigra L.). Eur. Food Res. Technol. 2008, 227, 45–56. [Google Scholar] [CrossRef]
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Stănciuc, N.; Oancea, A.M.; Aprodu, I.; Turturică, M.; Barbu, V.; Ioniţă, E.; Râpeanu, G.; Bahrim, G. Investigations on binding mechanism of bioactives from elderberry (Sambucus nigra L.) by whey proteins for efficient microencapsulation. J. Food Eng. 2018, 223, 197–207. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, J.; Tanaka, I.; Seo, S.; Yamazaki, M.; Saito, K. LC/ PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. J. Biomed. Biotechnol. 2004, 5, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Gu, L.W.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Brønnum-Hansen, K.; Hansen, S.H. High performance liquid chromatographic separation of anthocyanins of Sambucus nigra L. J. Chromatograph. 1983, 262, 385–392. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of Anthocyanins in Elderberry and Chokeberry Dietary Supplements. Phytother. Res. 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Fiorini, M. Preparative high-performance liquid chromatography for the purification of natural anthocyanins. J. Chromatogr. A. 1995, 692, 213–219. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef] [PubMed]
- Kaack, K.; Austed, T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum. Nutr. 1998, 52, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts) LWT. Food Sci. Technol. 2006, 39, 308–315. [Google Scholar]
- Ulbricht, C.; Basch, E.; Cheung, L.; Goldberg, H.; Hammerness, P.; Isaac, R.; Khalsa, K.P.S.; Romm, A.; Rychlik, I.; Varghese, M.; et al. An evidence-based systematic review of elderberry and elderflower (Sambucus nigra) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2014, 11, 80–120. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Ivancic, A.; Todorovic, B.; Veberic, R.; Stampar, F. Fruit phenolic composition of different elderberry species and hybrids. J. Food Sci. 2015, 80, C2180–C2190. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.; Driver, S.; Baxter, K. Stockley’s Herbal Medicine Interactions: A Guide to the Interactions of Herbal Medicines, Dietary Supplements and Nutraceuticals with Conventional Medicines; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Bolarinwa, I.F.; Oke, M.O.; Olaniyan, S.A.; Ajala, A.S. A Review of Cyanogenic Glycosides in Edible Plants. In Toxicology, 2nd ed.; Soloneski, S., Larramendy, M.L., Eds.; IntechOpen: Rijeka, UK, 2016; Chapter 8; pp. 180–191. [Google Scholar] [CrossRef]
- Krüger, S.; Mirgos, M.; Morlock, G.E. Effect-directed analysis of fresh and dried elderberry (Sambucus nigra L.) via hyphenated planar chromatography. J. Chromatogr. A 2015, 1426, 209–219. [Google Scholar] [CrossRef]
- Jiménez, P.; Cabrero, P.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Garrosa, M.; Girbés, T. Lectin digestibility and stability of elderberry antioxidants to heat treatment in vitro. Molecules 2017, 22, 95. [Google Scholar] [CrossRef]
- Senica, M.F.; Stampar, R.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT-Food Sci. Technol. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Wang, W.D.; Xu, S.Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Akande, K.E.; Fabiyi, E.F. Effect of processing methods on some antinutritional factors in legume seeds for poultry feeding. Int. J. Poult. Sci. 2010, 10, 996–1001. [Google Scholar] [CrossRef]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M.I. Metabolic differentiations of dwarf elder by NMR-based metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Mulero, J.; Pardo, F.; Zafrilla, P. Effect of principal polyphenolic components in relation to antioxidant activity in conventional and organic red wines during storage. Eur. Food Res. Technol. 2009, 229, 807–812. [Google Scholar] [CrossRef]
- Chung, H.Y.; Son, J.H.; Park, E.Y.; Kim, E.J.; Lim, S.T. Effect of vibration and storage on some physicochemical properties of a commercial red wine. J. Food Compos. Anal. 2008, 21, 655–659. [Google Scholar] [CrossRef]
- Coarfă, E.; Popa, M.E. Some relevant quality indicators of red wine from three grapes cultivars—A minireview. Sci. Bull. Ser. F Biotechnol. 2018, 22, 70–80. [Google Scholar]
- Ivanova, V.; Stefova, M.; Vojnovski, B. Assay of the phenolic profile of merlot wines from Macedonia: Effect of maceration time, storage, SO2 and temperature of storage. Maced. J. Chem. Chem. Eng. 2009, 8, 141–149. [Google Scholar] [CrossRef]
- Antoce, A.O.; Stockley, C. An overview of the implications of wine on human health, with special consideration of the wine-derived phenolic compounds. AgroLife Sci. J. 2019, 8, 21–34. [Google Scholar]
- Cordeiro, T.; Fernandes, I.; Pinho, O.; Calhau, C.; Mateus, N.; Faria, A. Anthocyanin content in raspberry and elderberry: The impact of cooking and recipe composition. Int. J. Gastron. Food Sci. 2021, 24, 100316. [Google Scholar] [CrossRef]
- Fernandes, A.; Ivanova, G.; Bras, N.F.; Mateus, N.; Ramos, M.J.; Rangel, M.; de Freitas, V. Structural characterization of inclusion complexes between cyanidin-3-O-glucoside and β-cyclodextrin. Carbohydr. Polym. 2014, 102, 269–277. [Google Scholar] [CrossRef]
- Stahl, L.; Miller, K.B.; Apgar, J.; Sweigart, D.S.; Stuart, D.A.; McHale, N.; Ou, B.; Kondo, M.; Hurst, W.J. Preservation of cocoa antioxidant activity, total polyphenols, flavan-3-ols, and procyanidin content in foods prepared with cocoa powder. J. Food Sci. 2009, 74, C456–C461. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D.; Jin, D.; Waterhouse, G.I.N. Effect of adding elderberry juice concentrate on the quality attributes, polyphenol contents and antioxidant activity of three fibre-enriched pastas. Food Res. Int. 2013, 54, 781–789. [Google Scholar] [CrossRef]
- da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin Profile of Elderberry Juice: A Natural-Based Bioactive Colouring Ingredient with Potential Food Application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef] [PubMed]
- Rozylo, R.; Wójcik, M.; Dziki, D.; Biernacka, B.; Cacak-Pietrzak, G.; Gawlowski, S.; Zdybel, A. Freeze-dried elderberry and chokeberry as natural colorants for gluten-free wafer sheets. Int. Agrophys. 2019, 33, 217–225. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Walkowiak-Tomczak, D. Consumer-perception, nutritional, and functional studies of a yogurt with restructured elderberry juice. J. Dairy Sci. 2021, 104, 1318–1335. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Liszka, K.; Tabaszewska, M.; Domagała, J. Probiotic Yoghurts with Sea Buckthorn, Elderberry, and Sloe Fruit Purees. Molecules 2021, 26, 2345. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Myracle, A.D. Development and evaluation of kefir products made with aronia or elderberry juice: Sensory and phytochemical characteristics. Int. Food Res. J. 2018, 25, 1373–1383. [Google Scholar]
- Jin, S.K.; Kim, G.D.; Jeong, J.Y. Evaluation of the Effect of Inhibiting Lipid Oxidation of Natural Plant Sources in a Meat Model System. J. Food Qual. 2021, 2021, 6636335. [Google Scholar] [CrossRef]
- Cordeiro, T.; Viegas, O.; Silva, M.; Martins, Z.E.; Fernandes, I.; Ferreira, I.M.L.P.V.O.; Pinho, O.; Mateus, N.; Calhau, C. Inhibitory effect of vinegars on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Meat Sci. 2020, 167, 108083. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Paini, M.; Alberto, A. Effect of encapsulating agent on physical-chemical characteristics of olive pomace polyphenols-rich extracts. Chem. Eng. Trans. 2015, 43, 97–102. [Google Scholar]
- Balanc, B.; Trifkovic, K. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of natural antioxidants for food application—The specific case of coffee antioxidants—A review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Wahab, N.A.; Rahman, R.A.; Ismail, A.; Mustafa, S.; Hashim, P. Assessment of antioxidant capacity, anti-collagenase and anti-elastase assays of Malaysian unfermented cocoa bean for cosmetic application. Nat. Prod. Chem. Res. 2014, 2, 1–6. [Google Scholar]
- Ghimeray, A.; Jung, U.; Lee, H.; Kim, Y.; Ryu, E.; Chang, M. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba fruits extract. Clin. Cosmet. Investig. Dermatol. 2015, 8, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Perše, M. Oxidative stress in the pathogenesis of colorectal cancer: Cause or consequence? BioMed Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Olejnik, A.; Olkowicz, M.; Kowalska, K.; Rychlik, J.; Dembczynski, R.; Myszka, K.; Juzwa, W.; Białas, W.; Moyer, M.P. Gastrointestinal digested Sambucus nigra L. fruit extract protects in vitro cultured human colon cells against oxidative stress. Food Chem. 2016, 197, 648–657. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2014, 18, 941–958. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacteria growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Lila, M.A.; Ribnicky, D.M.; Rojo, L.E.; Rojas-Silva, P.; Oren, A.; Havenaar, R.; Janle, E.M.; Raskin, I.; Yuself, G.G.; Grace, M.H. Complementary approaches to gauge the bioavailability and distribution of ingested berry polyphenolics. J. Agric. Food Chem. 2012, 60, 5763–5771. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Kowalska, K.; Olkowicz, M.; Rychlik, J.; Juzwa, W.; Myszka, K.; Dembczynski, R.; Białas, W. Anti-inflammatory effects of gastrointestinal digested Sambucus nigra L. fruit extract analysed in co-cultured intestinal epithelial cells and lipopolysaccharide-stimulated macrophages. J. Funct. Foods 2015, 19, 649–660. [Google Scholar] [CrossRef]
- Debnath, T.; Kim, D.H.; Lim, B.O. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules 2013, 18, 7253–7270. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Baenas, N.; García-Viguera, C.; Villaño, D. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Folmer, F.; Basavaraju, U.; Jaspars, M.; Hold, G.; El-Omar, E.; Dicato, M.; Diederich, M. Anticancer effects of bioactive berry compounds. Phytochem. Rev. 2014, 13, 295–322. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Frøkiær, H.; Henningsen, L.; Metzdorff, S.B.; Weiss, G.; Roller, M.; Flanagan, J.; Fromentin, E.; Ibarra, A. Astragalus root and elderberry fruit extracts enhance the IFN-β stimulatory effects of Lactobacillus acidophilus in murine-derived dendritic cells. PLoS ONE 2012, 7, e47878. [Google Scholar] [CrossRef]
- Pliszka, B.; Waźbińska, J.; Puczel, U.; Huszcza-Ciolkowska, G. Biologicznie czynne związki polifenolowe zawarte w owocach różnych odmian hodowlanych i dziko rosnącego bzu czarnego. Zeszyty Problemowe Postępów Nauk Rolniczych 2005, 507, 443–449. [Google Scholar]
- Boroduske, A.; Jekabsons, K.; Riekstina, U.; Muceniece, R.; Rostoks, N.; Nakurte, I. Wild Sambucus nigra L. from north-east edge of the species range: A valuable germplasm with inhibitory capacity against SARS-CoV2 S-protein RBD and hACE2 binding in vitro. Ind. Crops Prod. 2021, 165, 113438. [Google Scholar] [CrossRef]
- Gleńsk, M.; Glinski, J.A.; Wlodarczyk, M.; Stefanowicz, P. Determination of ursolic and oleanolic acid in Sambucus fruits. Chem. Biodiver. 2014, 11, 1939–1944. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Nowak, H.; Kujawa, R.; Zadernowski, B.; Roczniak Kozłowska, H. Antioxidative and bactericidal properties of phenolic compounds in rapeseeds. Lipid/Fett 1992, 94, 149–152. [Google Scholar] [CrossRef]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plant Res. 2010, 4, 1805–1809. [Google Scholar]
- Chatterjee, A.; Yasmin, T.; Bagchi, D.; Stohs, S.J. Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromyc. Mol. Cell. Biochem. 2004, 265, 19–26. [Google Scholar] [CrossRef]
- Krawitz, C.; Mraheil, M.A.; Stein, M.; Imirzalioglu, C.; Domann, E.; Pleschka, S.; Hain, T. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complement. Altern. Med. 2011, 11, 16–21. [Google Scholar] [CrossRef]
- Chen, C.; Zuckerman, D.M.; Brantley, S.; Sharpe, M.; Childress, K.; Hoiczyk, E.; Pendleton, A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Roschek, B., Jr.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef]
- Tiralongo, E.; Wee, S.S.; Lea, R.A. Elderberry supplementation reduces cold duration and symptoms in air-travellers: A randomized, double-blind placebo-controlled clinical trial. Nutrients 2016, 8, 182. [Google Scholar] [CrossRef]
- Zakay-Rones, Z.; Varsano, N.; Zlotnik, M.; Manor, O.; Regev, L.; Schlesinger, M.; Mumcuoglu, M. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an Elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. J. Altern. Complement. Med. 1995, 1, 361–369. [Google Scholar] [CrossRef]
- Kinoshita, E.; Hayashi, K.; Katayama, H.; Hayashi, T.; Obata, A. Anti-Influenza virus effects of elderberry juice and its fractions. Biosci. Biotechnol. Biochem. 2012, 76, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Hidari, K.I.P.J.; Abe, T.; Suzuki, T. Crabohydrate-related inhibitors of dengue virus entry. Viruses 2013, 5, 605–618. [Google Scholar] [CrossRef]
- McCutcheon, A.R.; Roberts, T.E.; Gibbons, E.; Ellis, S.M.; Babiuk, L.A.; Hancock, R.E.W.; Towers, G.H.N. Antiviral screening of British Columbian medicinal plants. J. Ethnopharmacol. 1995, 49, 101–110. [Google Scholar] [CrossRef]
- Van der Meer, F.J.U.M.; de Haan, C.A.M.; Schuurman, N.M.P.; Haijema, B.J.; Verheije, M.H.; Bosch, B.J.; Balzarini, J.; Egberink, H.F. The carbohydrate-binding plant lectins and the non-peptidic antibiotic pradimicin A target the glycans of the coronavirus envelope glycoproteins. J. Antimicrob. Chemother. 2007, 60, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.R.M.; Chertova, E.; Hilburn, J.M.; Arthur, L.O.; Hildreth, J.E.K. Cholesterol depletion of human immunodeficiency virus type 1 and Simian Immunodeficiency virus with β-Cyclodextrin inactivates and permeabilizes the virions: Evidence for virion-associated lipid rafts. J. Virol. 2003, 77, 8237–8248. [Google Scholar] [CrossRef] [PubMed]
- Bartak, M.; Lange, A.; Słońska, A.; Cymerys, J. Antiviral and healing potential of Sambucus nigra extracts. Bionatura 2020, 5, 1264–1270. [Google Scholar] [CrossRef]
- Rechenchoski, D.Z.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Herpesvirus: An underestimated virus. Folia Microbiol. 2017, 62, 151–156. [Google Scholar] [CrossRef]
- Harnett, J.; Oakes, K.; Carè, J.; Leach, M.; Brown, D.; Cramer, H.; Pinder, T.A.; Steel, A.; Anheyer, D. The effects of Sambucus nigra berry on acute respiratory viral infections: A rapid review of clinical studies. Adv. Integr. Med. 2020, 7, 240–246. [Google Scholar] [CrossRef]
- Kronbichler, A.; Effenberger, M.; Eisenhut, M.; Lee, K.H.; Shin, J.I. Seven recommendations to rescue the patients and reduce the mortality from COVID-19 infection: An immunological point of view. Autoimmun. Rev. 2020, 19, 102570. [Google Scholar] [CrossRef]
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhaes, N.P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharmacol. 2020, 11, 1479. [Google Scholar] [CrossRef]
- Daryani, A.; Ebrahimzadeh, M.A.; Sharif, M.; Ahmadpour, E.; Edalatian, S.; Esboei, B.R.; Sarvi, S. Actividad anti-Toxoplasma de extractos metanólicos de frutos y hojas de Sambucus nigra (Caprifoliaceae). Rev. Biol. Trop. 2015, 63, 7–12. Available online: https://hdl.handle.net/10669/27457 (accessed on 2 February 2022). [CrossRef]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic elderberry extracts, anthocyanins, procyanidins, and metabolites influence glucose and fatty acid uptake in human skeletal muscle cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Farrella, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Salvador, Â.C.; Król, E.; Lemos, V.C.; Santos, S.A.; Bento, F.P.; Costa, C.P.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of elderberry (Sambucus nigra L.) extract supplementation in STZ-induced diabetic rats fed with a high-fat diet. Int. J. Molec. Sci. 2017, 18, 13. [Google Scholar] [CrossRef]
- Farrell, N.; Norris, G.; Lee, S.G.; Porter, C.M.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Opriș, R.; Tatomirn, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, A.; Decea, N.; Kiss, M.L.; Filip, G.A. The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids Surf. B Biointerfaces 2017, 150, 192–200. [Google Scholar] [CrossRef]
- Karthick, V.; Kumar, V.G.; Dhas, T.S.; Singaravelu, G.; Sadiq, A.M.; Govindaraju, K. Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats-An in vivo approach. Colloids Surf. B Biointerfaces 2014, 122, 505–511. [Google Scholar] [CrossRef]
- Bădescu, L.; Bădulescu, O.; Bădescu, M.; Ciocoiu, M. Mechanism by Sambucus nigra extract improves bone mineral density in experimental diabetes. Evid. Based Complement. Alternat. Med. 2012, 2012, 848269. [Google Scholar] [CrossRef]
- Yeh, G.Y.; Eisenberg, D.M.; Kaptchuk, T.J.; Phillips, R.S. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 2003, 26, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Cutrim, C.S.; Barros, R.F.D.; Costa, M.P.D.; Franco, R.M.; Conte-Junior, C.A.; Cortez, M.A.S. Survival of Escherichia coli O157:H7 during manufacture and storage of traditional and low lactose yogurt. LWT-Food Sci. Technol. 2016, 70, 178–184. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. UNIT F1.2 Characterization and measurement of anthocyanins by UV-Visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 00, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Turek, S.; Cisowski, W. Free and chemically bonded phenolic acids in barks of Viburnum opulus L. and Sambucus nigra L. Acta. Pol. Pharm. 2007, 64, 377–383. [Google Scholar] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Jovanović, A.; Petrović, P.; Đorđević, V.; Zdunić, G.; Šavikin, K.; Bugarski, B. Polyphenols extraction from plant sources. Lek. Sirovine 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Miron, T.; Plaza, M.; Bahrim, G.; Ibanez, E.; Herrero, M. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants. J. Chromatogr. A 2011, 1218, 4918–4927. [Google Scholar] [CrossRef]
- Suwal, S.; Marciniak, A. Technologies for the Extraction, Separation and Purification of polyphenols—A Review. Nepal J. Biotechnol. 2019, 6, 74–91. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Vatai, T.; Škerget, M.; Knez, Ž. Extraction of phenolic compounds from elderberry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. [Google Scholar] [CrossRef]
- Salamon, I.; Mariychuk, R.; Grulova, D. Optimal Extraction of Pure Anthocyanins from Fruits of Sambucus nigra. Acta Hortic. 2015, 1061, 73–78. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Moreno, D.A.; Garcia-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed]
- Denery, J.R.; Dragull, K.; Tang, C.S.; Li, Q.X. Pressurized fluid extraction of carotenoids from Haematococcus pluvialis and Dunaliella salina and kavalactones from Piper methysticum. Anal. Chim. Acta. 2004, 501, 175–181. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta. 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta. 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Ong, E.S.; Cheong, J.S.H.; Goh, D. Pressurized hot water extraction of bioactive or marker compounds in botanicals and medicinal plant materials. J. Chromatogr. 2006, 1112, 92–102. [Google Scholar] [CrossRef]
- Ong, E.S.; Woo, S.O.; Yong, Y.L. Pressurized liquid extraction of berberine and aristolochic acids in medicinal plants. J. Chromatogr. 2000, 904, 57–64. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharma. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Jia, C.; Zhang, X.; Xia, W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Dragović-Uzelac, V.; Režek, J.A.; Jukić, M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). J. Food Eng. 2013, 117, 437–442. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Azari, B.; Siami, A.; Ebrahimzadeh, M.; Khan, B. Antioxidants activity of extracts from Sambucus nigra, efficiency of different extraction methods. Lat. Am. Appl. Res. 2015, 45, 139–144. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Olech, M.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Wójtowicz, A. Extraction methods, LC-ESI-MS/MS analysis of phenolic compounds and antiradical properties of functional food enriched with elderberry flowers or fruits. Arab. J. Chem. 2016, 12, 4719–4730. [Google Scholar] [CrossRef]
- dos Santos Nascimento, L.B.; Gori, A.; Degano, I.; Mandoli, A.; Ferrini, F.; Brunetti, C. Comparison between Fermentation and Ultrasound-Assisted Extraction: Which Is the Most Efficient Method to Obtain Antioxidant Polyphenols from Sambucus nigra and Punica granatum Fruits? Horticulturae 2021, 7, 386. [Google Scholar] [CrossRef]
- Henriques, A.M.; Marçalo, A.J.; Ntungwe, E.N.; Pereira, P.; João, C.M.; Bernardo-Gil, M.G.; Molpeceres, J.; Rijo, P.; Silveira, V.A.; Ascensão, L.; et al. Green extraction of Sambucus nigra L. for potential application in skin nanocarriers. Green Mater. 2020, 8, 181–193. [Google Scholar]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of Pulsed Electric Field Treatments for the Enhancement of Mass Transfer from Vegetable Tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Liu, D.; Vorobiev, E.; Savoire, R.; Lanoisellé, J.L. Extraction of polyphenols from grape seeds by unconventional methods and extract concentration through polymeric membrane. In Proceedings of the 11th International Congress on Engineering and Food (ICEF 11), Athens, Greece, 22–26 May 2011; pp. 1939–1940. [Google Scholar]
- Nawaz, H.; Shi, J.; Mittal, G.S.; Kakuda, Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep. Purif. Technol. 2006, 48, 176–181. [Google Scholar] [CrossRef]
- Susanto, H.; Feng, Y.; Ulbricht, M. Fouling behavior of aqueous solutions of polyphenolic compounds during ultrafiltration. J. Food Eng. 2009, 91, 333–340. [Google Scholar] [CrossRef]
- Bazinet, L.; DeGrandpré, Y.; Porter, A. Electromigration of tobacco polyphenols. Sep. Purif. Technol. 2005, 41, 101–107. [Google Scholar] [CrossRef]
- Schwarz, M.; Hillebrand, S.; Habben, S.; Degenhardt, A.; Winterhalter, P. Application of high-speed countercurrent chromatography to the large-scale isolation of anthocyanins. Biochem. Eng. J. 2003, 14, 179–189. [Google Scholar] [CrossRef]
- Jarzycka, A.; Lewińska, A.; Gancarz, R.; Wilk, K.A. Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J. Photochem. Photobiol. B Biol. 2013, 128, 50–57. [Google Scholar] [CrossRef]
- Goud, N.S.; Prasad, G. Antioxidant, antimicrobial activity and total phenol and flavonoids analysis of Sambucus nigra (elderberry). Int. J. Curr. Pharm. Res. 2020, 12, 35–37. [Google Scholar] [CrossRef]
- Idham, Z.; Muhamad, I.D.A.I.; Sarmidi, M.R. Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from Hibiscus sabdariffa L. J. Food Process. Eng. 2010, 35, 522–542. [Google Scholar] [CrossRef]
- Huang, H.-S.; Yu, H.-S.; Yen, C.-H.; Liaw, E.-T. HPLC-DAD-ESI-MS Analysis for Simultaneous Quantitation of Phenolics in Taiwan Elderberry and Its Anti-Glycation Activity. Molecules 2019, 24, 3861. [Google Scholar] [CrossRef] [PubMed]
- Vankova, D.V.; Todorova, M.N.; Kisselova-Kaneva, Y.D.; Galunska, B.T. Development of new and robust LC-MS method for simultaneous quantification of polyphenols from Sambucus ebulus fruits. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 408–416. [Google Scholar] [CrossRef]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.P.; de Sousa, H.C. Fractioned high pressure extraction of anthocyanins from elderberry (Sambucus nigra L.) pomace. Food Bioproc. Tech. 2010, 3, 674–683. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Sicari, V.; Ursino, C.; Manfredi, I.; Conidi, C.; Figoli, A.; Cassano, A. Concentration of Bioactive Compounds from Elderberry (Sambucus nigra L.) Juice by Nanofiltration Membranes. Plant Foods Hum. Nutr. 2018, 73, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agri. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Giordano, A.; Morales-Tapia, P.; Moncada-Basualto, M.; Pozo-Martínez, J.; Olea-Azar, C.; Nesic, A.; Cabrera-Barjas, G. Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots. Molecules 2022, 27, 610. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D. Comparision of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascariu, O.-E.; Israel-Roming, F. Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications. Processes 2022, 10, 2288. https://doi.org/10.3390/pr10112288
Pascariu O-E, Israel-Roming F. Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications. Processes. 2022; 10(11):2288. https://doi.org/10.3390/pr10112288
Chicago/Turabian StylePascariu, Oana-Elena, and Florentina Israel-Roming. 2022. "Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications" Processes 10, no. 11: 2288. https://doi.org/10.3390/pr10112288
APA StylePascariu, O.-E., & Israel-Roming, F. (2022). Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications. Processes, 10(11), 2288. https://doi.org/10.3390/pr10112288








