Field Survey on Generation Patterns of Airborne Fungi in a Livestock Manure Composting Plant in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject
2.2. Measurement
- Cf: CFU (Colony Forming Unit)/m³
- F: Number of colonies counted on agar plates
- Av: Air volume (m³)
2.3. Data Analysis
3. Results and Discussion
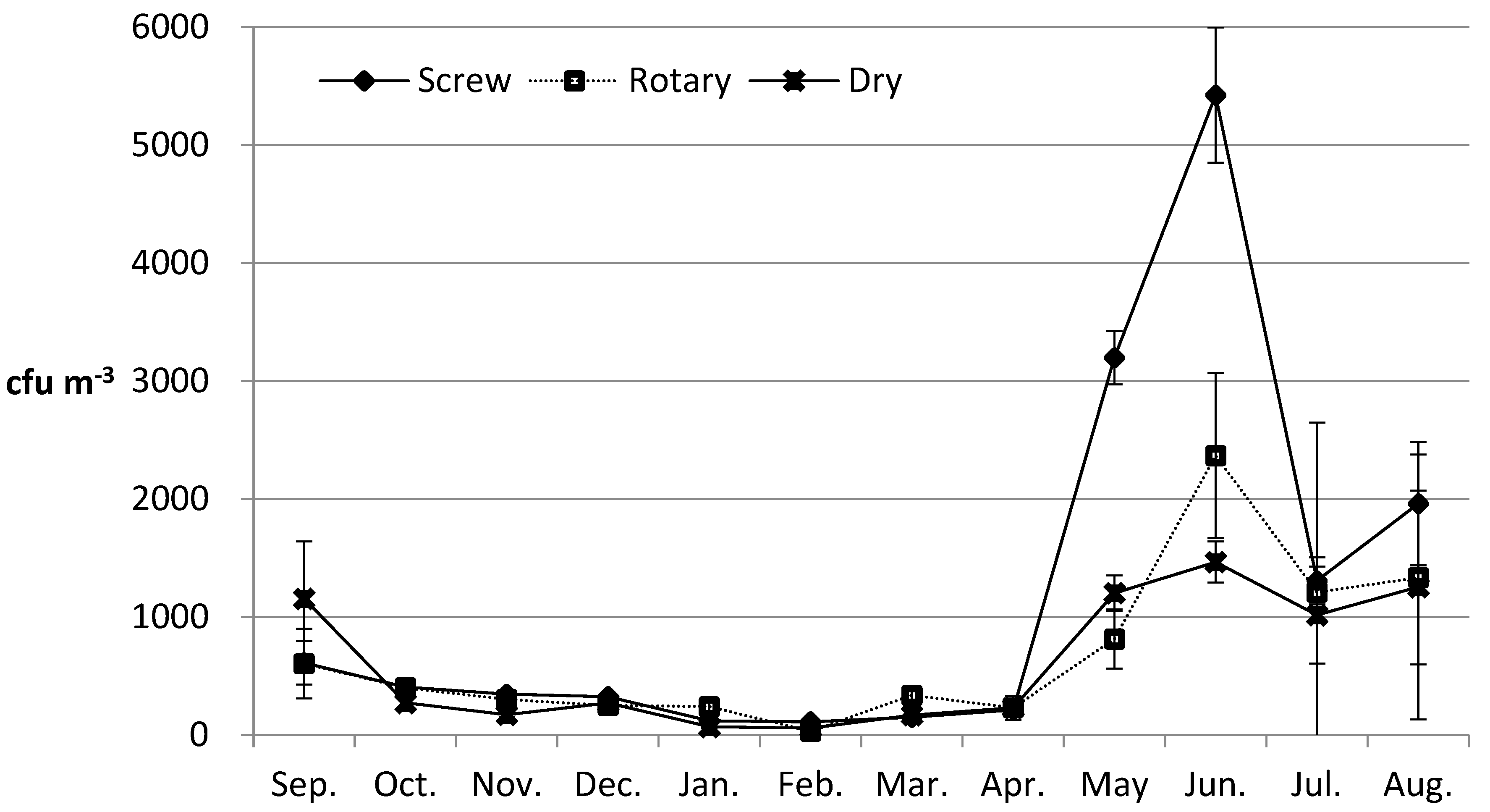
3.1. Monthly Concentration Distribution of Airborne Fungi According to the Type of Livestock Manure Composting Plant
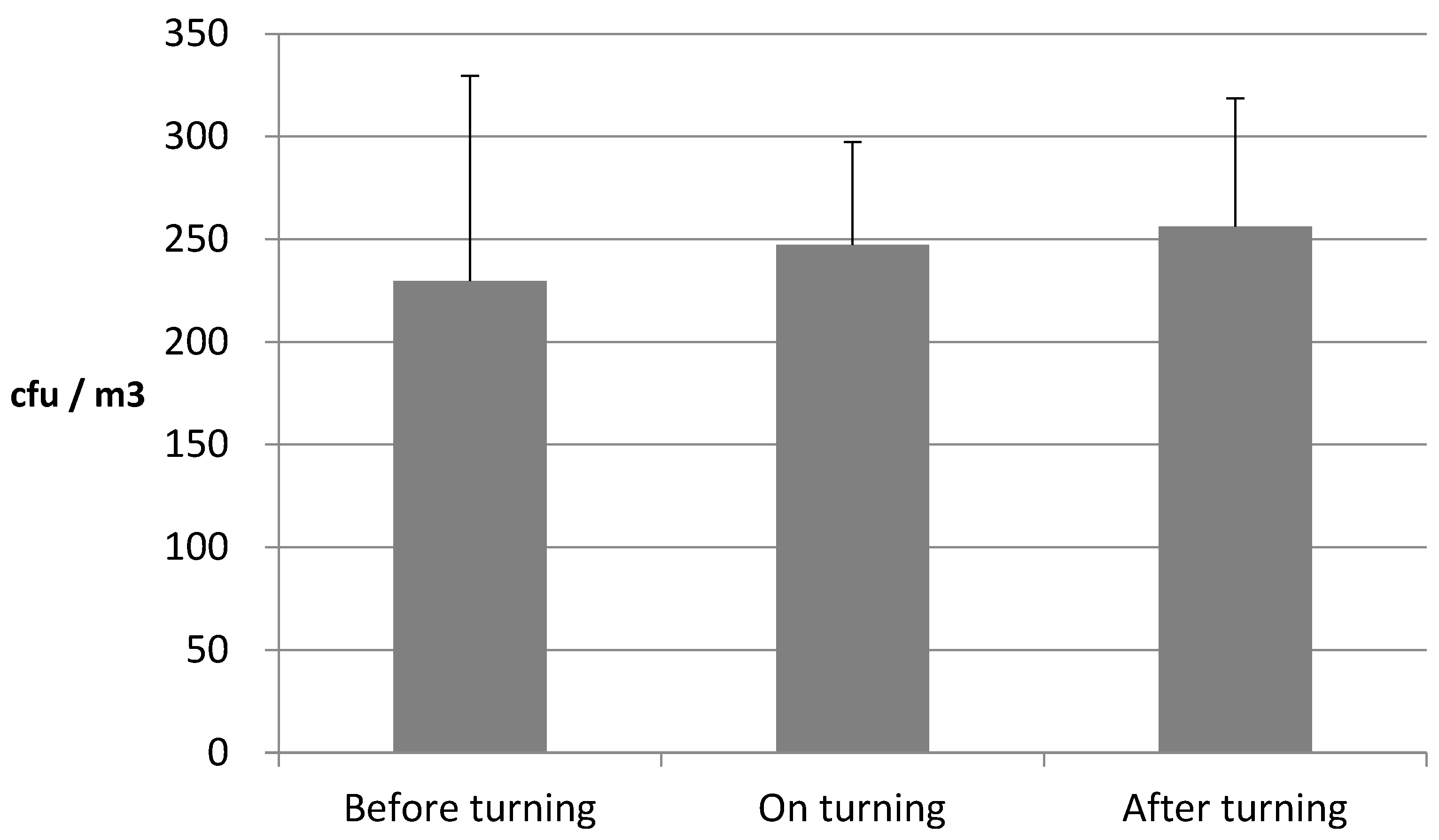
3.2. Comparison of Airborne Fungi Emitted from Livestock Manure Composting Plant According to Agitation Time of Compost Pile
3.3. Size Distribution Characteristics of Airborne Fungi According to the Type of Livestock Manure Composting Plant
3.4. Association between Airborne Fungi and Environmental Factors in Livestock Manure Composting Plant
3.5. Qualitative Analysis of Airborne Fungi According to the Type of Livestock Manure Composting Plant
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karra, S.; Katsivela, E. Microorganisms in bioaerosol emissions from wastewater treatment plants during summer at a Mediterranean site. Water Res. 2007, 41, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Bunger, J.; Antlau-Lammers, M.; Schulz, T.G.; Westphal, G.; Muller, M.; Ruhnau, P.; Hallier, E. Health complaints and immunological markers of exposure to bioaerosols among biowaste collectors and compost workers. Occup. Environ. Med. 2000, 57, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.E.W.; zur Nieden, A.; Jankofsky, M.; Stilianakis, N.I.; Boedeker, R.H.; Eikmann, T.F. Effects of bioaerosols polluted outdoor air on airways of residents: A cross sectional study. Occup. Environ. Med. 2003, 60, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Wounter, I.; Dubbeld, H.; van Zwieten, L.; Steerenberg, P.; Doekes, G.; Heederik, D. Upper airway inflammation assessed by nasal lavage in compost workers: A relation with bio-aerosol exposure. Am. J. Ind. Med. 2000, 37, 459–468. [Google Scholar] [CrossRef]
- Wouters, I.M.; Hilhorst, S.K.M.; Kleppe, P.; Doekes, G.; Douwes, J.; Peretz, C.; Heederik, D. Upper airway inflammation and respiratory symptoms in domestic waste collectors. Occup. Environ. Med. 2002, 59, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar]
- Heldal, K.K.; Halstensen, A.S.; Thorn, J.; Djupesland, P.; Wouters, I.; Eduard, W.; Halstensen, T.S. Upper airway inflammation in waste handlers exposed to bioaerosols. Occup. Environ. Med. 2003, 60, 444–450. [Google Scholar] [CrossRef]
- Heldal, K.K.; Halstensen, A.S.; Thorn, J.; Eduard, W.; Halstensen, T.S. Airway inflammation in waste handlers exposed to bioaerosols assessed by induced sputum. Eur. Respir. J. 2003, 21, 641–645. [Google Scholar] [CrossRef]
- Directive, E.C. EU Council Directive 2000/54/EC on the protection of workers from risks related to exposure to biological agents at work. Off. J. Eur. Communities 2000, 12, 21–45. [Google Scholar]
- Carducci, A.; Tozzi, E.; Rubulotta, E.; Casini, B.; Cantiani, L.; Rovini, E.; Muscillo, M.; Pacini, R. Assessing airborne biological hazard from urban wastewater treatment. Water Res. 2000, 34, 1173–1178. [Google Scholar] [CrossRef]
- Fischer, G.; Muller, T.; Ostrowski, R.; Dott, W. Mycotoxins of Aspergillus Fumigatus in pure culture and in native bioaerosols from compost facilties. Chemosphere 1999, 38, 1745–1755. [Google Scholar] [CrossRef]
- Hryhorczuk, D.; Curtis, L.; Schleff, P.; Chung, J.; Rizzo, M.; Lewis, C.; Keys, N.; Moomey, M. Bioaerosols emission from a suburban yard waste composting facility. Ann. Agric. Environ. Med. 2001, 8, 177–185. [Google Scholar] [PubMed]
- Sachez-Monedero, M.A.; Stentiford, E.I. Generation and dispersion of airborne microorganisms from composting facilities. Process Saf. Environ. 2003, 81, 166–170. [Google Scholar] [CrossRef]
- Fracchia, L.; Pietronave, S.; Rinaldi, M.; Martinotti, M.G. The assessment of airborne bacterial contamination in three composting plants revealed site-related biological hazard and seasonal variations. J. Appl. Microbiol. 2006, 100, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Grisoli, P.; Rodolfi, M.; Villani, S.; Grignani, E.; Cottica, D.; Berri, A.; Picco, A.M.; Dacarro, C. Assessment of airborne microorganism contamination in an industrial area characterized by an open composting facility and wastewater treatment. Environ. Res. 2009, 109, 135–142. [Google Scholar] [CrossRef]
- Tong, Y.; Lighthart, B. Solar radiation has a lethal effect on natural populations of culturable outdoor atmospheric bacteria. Atmos. Environ. 1997, 31, 897–900. [Google Scholar] [CrossRef]
- Folmsbee, M.; Strevett, K. Bioaerosol concentration at an outdoor composting center. J. Air. Waste Manag. Assoc. 1999, 49, 554–561. [Google Scholar] [CrossRef]
- Jones, A.M.; Harrison, R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total Environ. 2004, 326, 151–180. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, C.N. Airborne microbiological characteristics in the public buildings of Korea. Build. Environ. 2007, 42, 2188–2196. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, Y.S.; Kim, D. Distribution characteristics of airborne bacteria and fungi in the general hospitals of Korea. Ind. Health 2010, 48, 236–243. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, H.T.; Kim, D.; Nakajima, J.; Takashi, H. Distribution characteristics of airborne bacteria and fungi in the feedstuff-manufacturing factories. J. Hazard. Mater. 2009, 169, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Marthi, B.; Lighthart, B. Effects of betaine on the enumeration of airborne bacteria. Appl. Envrion. Microbiol. 1990, 56, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.V.; Marthi, B.; Fieland, V.P.; Ganio, L.M. Effect of aerosolization on subsequent bacterial survival. Appl. Environ. Microbiol. 1990, 56, 3468–3472. [Google Scholar] [CrossRef]
- Macher, J.M.; Huang, F.Y.; Flores, M. A two-year study of microbiological indoor air quality in a new apartment. Arch. Environ. Health 1991, 46, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Hsu, L.Y. Home dampness and childhood respiratory symptoms in a subtropical climate. Arch. Environ. Health 1996, 51, 42–46. [Google Scholar] [CrossRef]
- DeKoster, J.A.; Thorne, P.S. Bioaerosol concentrations in noncomplaint, complaint and intervention homes in the Midwest. Am. Ind. Hyg. Assoc. J. 1995, 56, 576–580. [Google Scholar] [CrossRef]
- Gorny, R.L.; Dutkiewicz, J.; Krysinska-Traczyk, E. Size distribution of bacterial and fungal bioaerosols in indoor air. Ann. Agric. Environ. Med. 1999, 6, 105–113. [Google Scholar]
- Pastuszka, J.S.; Paw, U.K.T.; Lis, D.O.; Wlazlo, A.; Ulfig, K. Bacterial and fungal aerosol in indoor environment in Upper Silesia, Poland. Atmos. Environ. 2000, 34, 3833–3842. [Google Scholar] [CrossRef]
- Gorny, R.L.; Dutkiewicz, J. Bacterial and fungal aerosols in indoor environment in central and eastern European countries. Ann. Agric. Environ. Med. 2002, 9, 17–23. [Google Scholar]

| Site | Reactor Type | Turning Mode | Treatment Capacity | Location |
|---|---|---|---|---|
| 1 | Cross | Screw | 10 (7.5) * ton/day | Jeju |
| 2 | Cross | Rotary | 5 (1) ton/day | |
| 3 | Pile | Natural dry | 3 (1.5) ton/day |
| Airborne Fungi | Temp. | RH | TSP | PM10 | PM2.5 | PM1 | Odor | |
|---|---|---|---|---|---|---|---|---|
| Airborne fungi | 0.708 ** | −0.085 | 0.011 | −0.071 | −0.298 | −0.130 | 0.035 | |
| Temp. | −0.367 | 0.078 | −0.266 | −0.776 ** | −0.419 | 0.150 | ||
| RH | 0.470 * | 0.615 ** | 0.383 | 0.127 | 0.043 | |||
| TSP | 0.752 ** | −0.303 | −0.302 | −0.017 | ||||
| PM10 | 0.150 | −0.178 | 0.009 | |||||
| PM20.5 | 0.722 ** | 0.069 | ||||||
| PM1 | 0.144 | |||||||
| Odor |
| Rotary Type | Screw Type | Dry Type | |
|---|---|---|---|
| Aspergillus spp. Chrysosporium spp. Cladosporium spp. Fusarium spp. Mucor spp. Penicillium spp. Ulocladium spp. Yeasts Unknown Total | 26.2 3.8 24.2 3.6 7.2 19.8 2.1 6.2 6.9 100.0 | 29.3 8.2 18.5 4.1 6.4 20.2 1.0 4.3 8.0 100.0 | 35.3 2.4 19.2 5.6 5.8 17.6 3.7 1.2 9.2 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.-S.; Kim, D.-H.; Kim, K.-Y. Field Survey on Generation Patterns of Airborne Fungi in a Livestock Manure Composting Plant in South Korea. Processes 2022, 10, 2231. https://doi.org/10.3390/pr10112231
Ahn J-S, Kim D-H, Kim K-Y. Field Survey on Generation Patterns of Airborne Fungi in a Livestock Manure Composting Plant in South Korea. Processes. 2022; 10(11):2231. https://doi.org/10.3390/pr10112231
Chicago/Turabian StyleAhn, Jin-Soo, Doo-Hwan Kim, and Ki-Youn Kim. 2022. "Field Survey on Generation Patterns of Airborne Fungi in a Livestock Manure Composting Plant in South Korea" Processes 10, no. 11: 2231. https://doi.org/10.3390/pr10112231
APA StyleAhn, J.-S., Kim, D.-H., & Kim, K.-Y. (2022). Field Survey on Generation Patterns of Airborne Fungi in a Livestock Manure Composting Plant in South Korea. Processes, 10(11), 2231. https://doi.org/10.3390/pr10112231






