Investigation of Hydroxyl Radical Yield in an Impact-Jet Hydraulic Cavitator
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Process Parameters
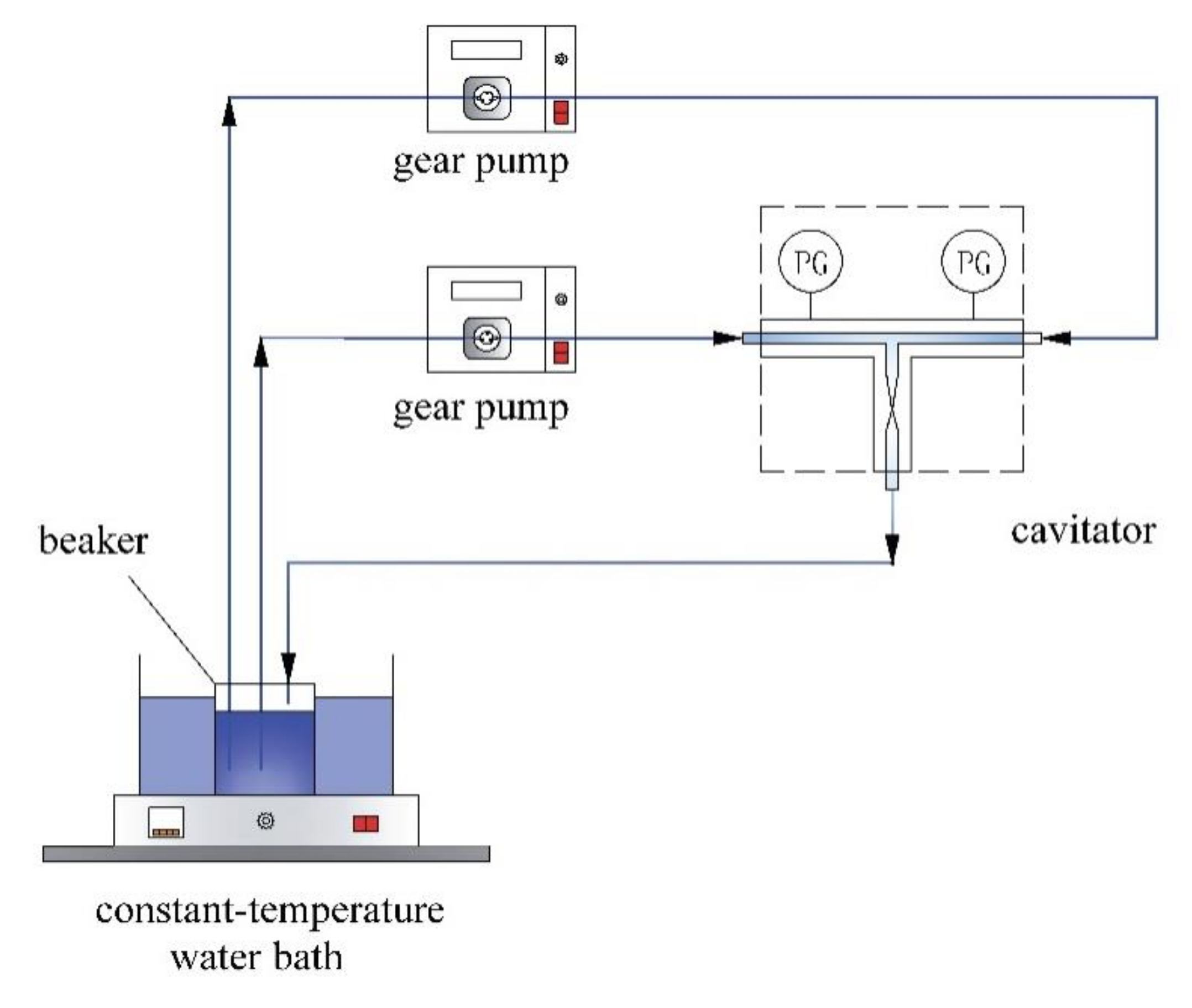
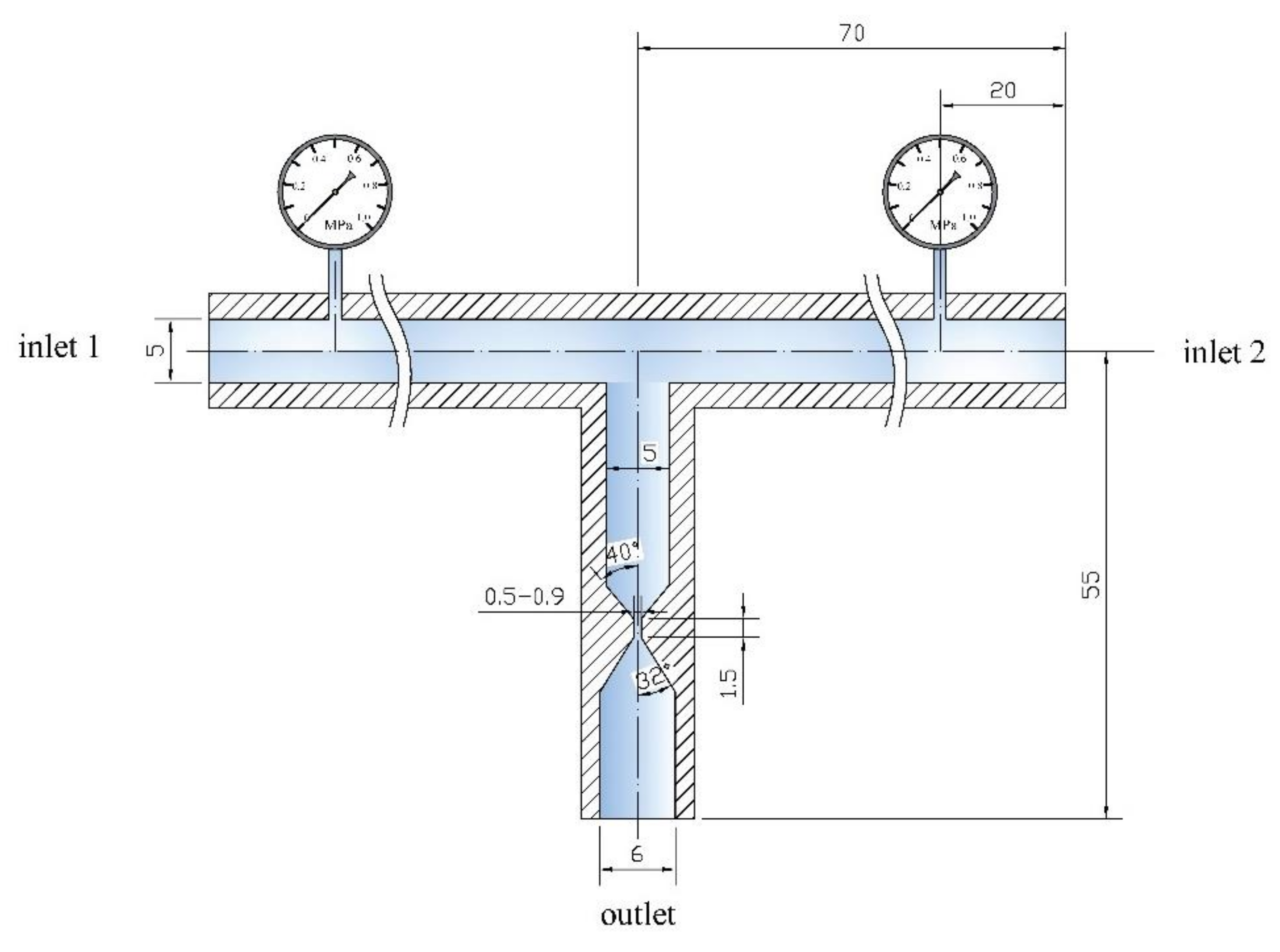
2.3. Experimental Setup
2.4. Experimental Analysis
3. Results and Discussion
3.1. ·OH Yield in MB Solution
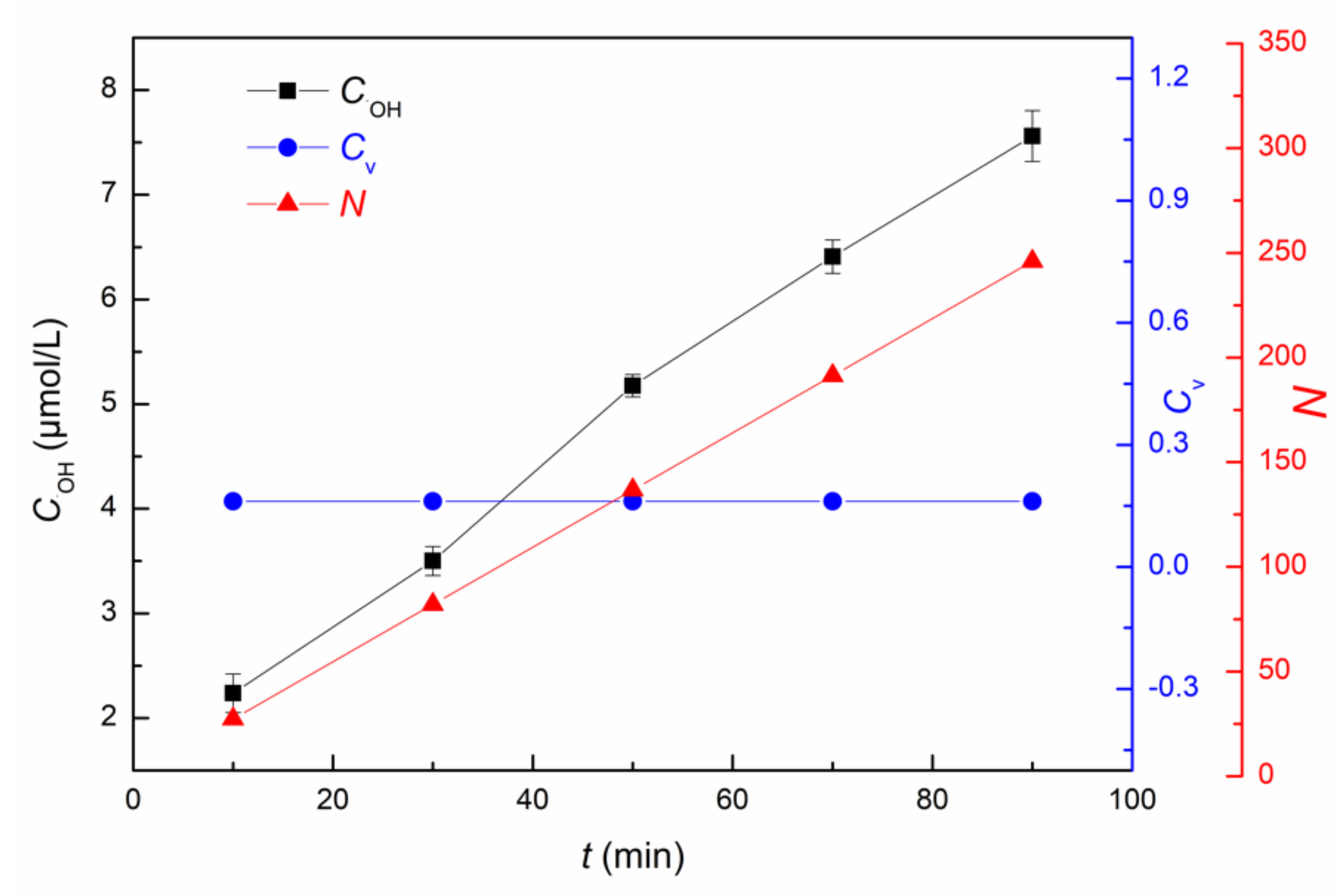
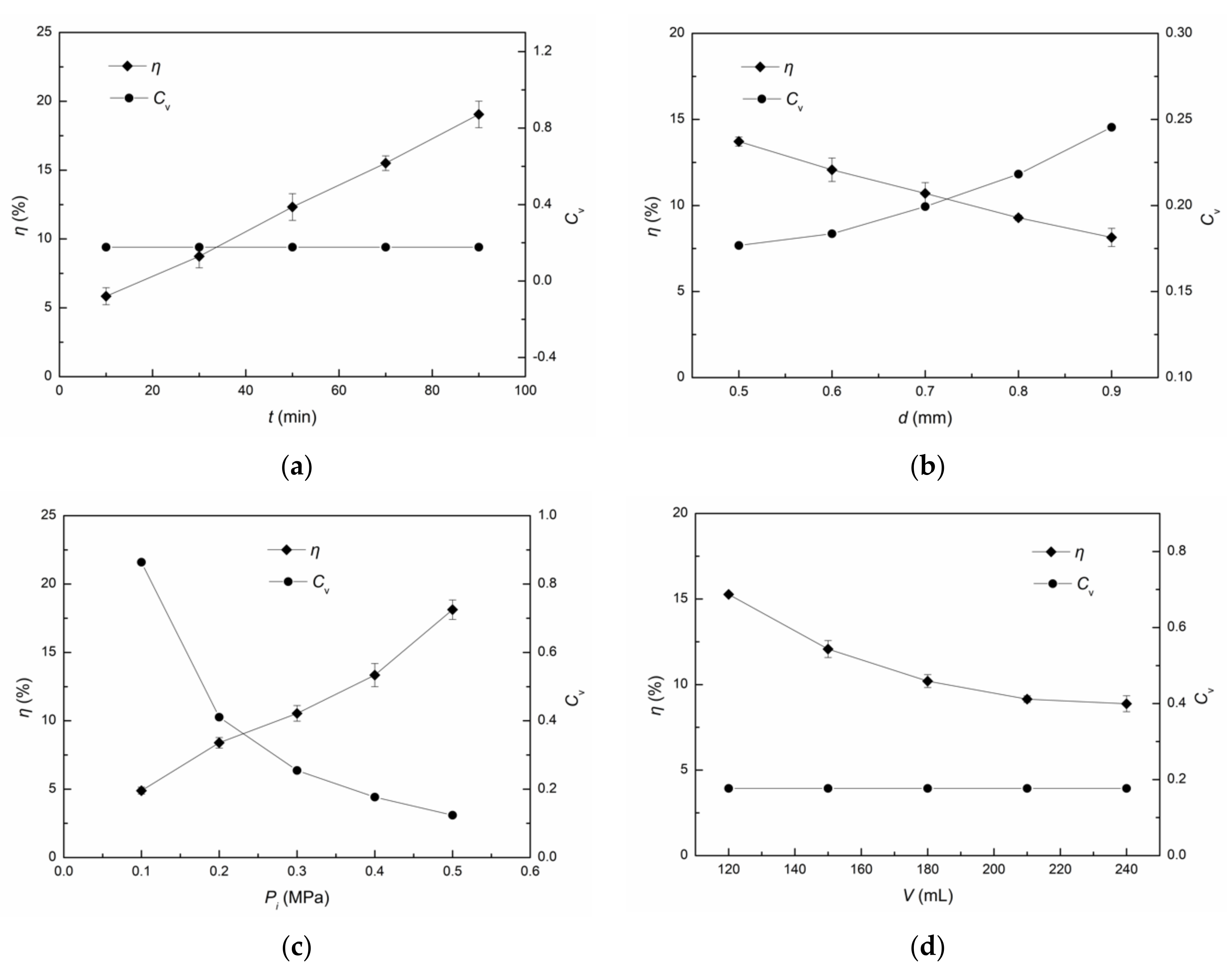
3.1.1. Influence of Cavitation Time on ·OH Yield
3.1.2. Influence of Throat Diameter on ·OH Yield
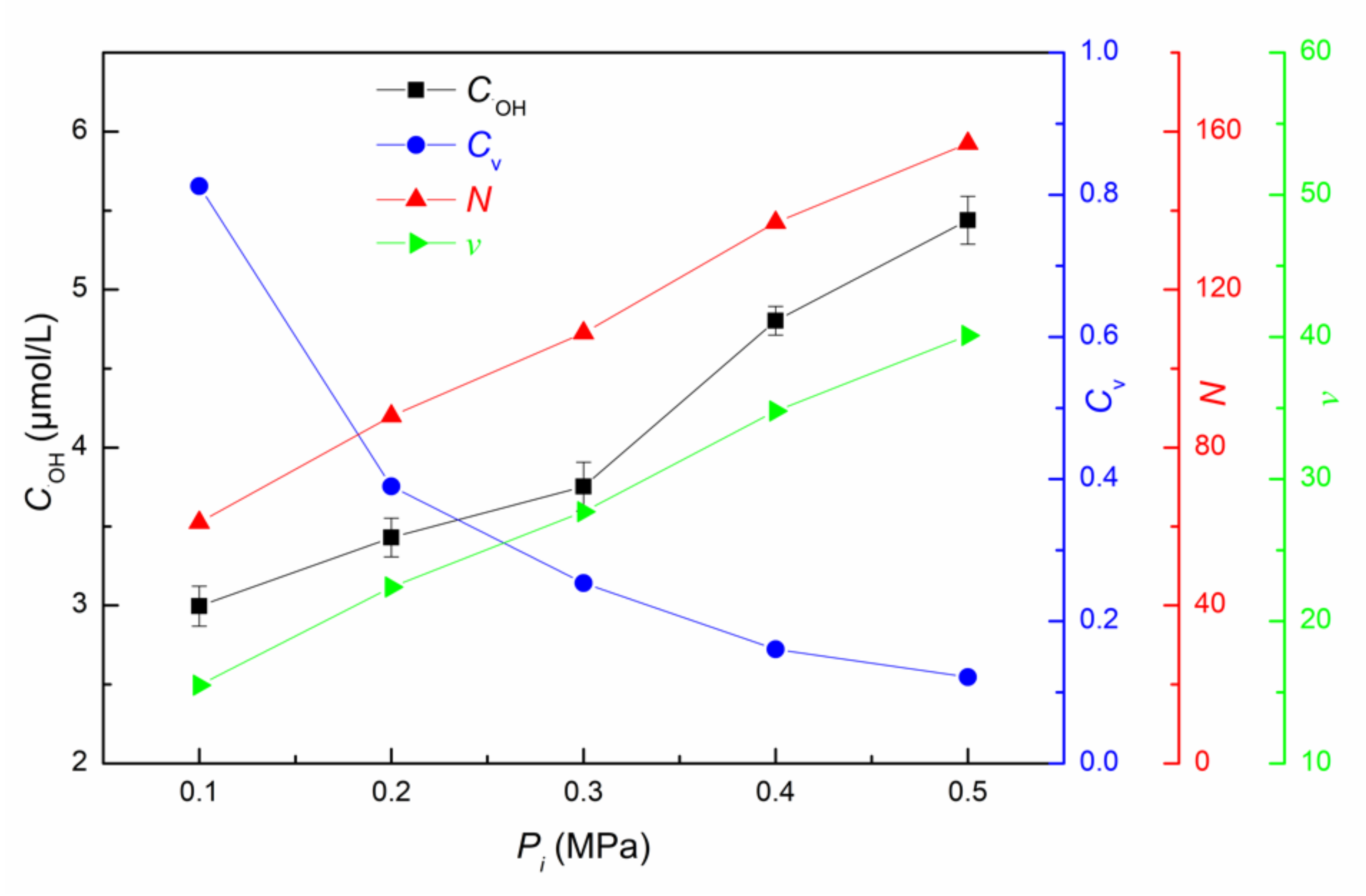
3.1.3. Influence of Inlet Pressure on ·OH Yield
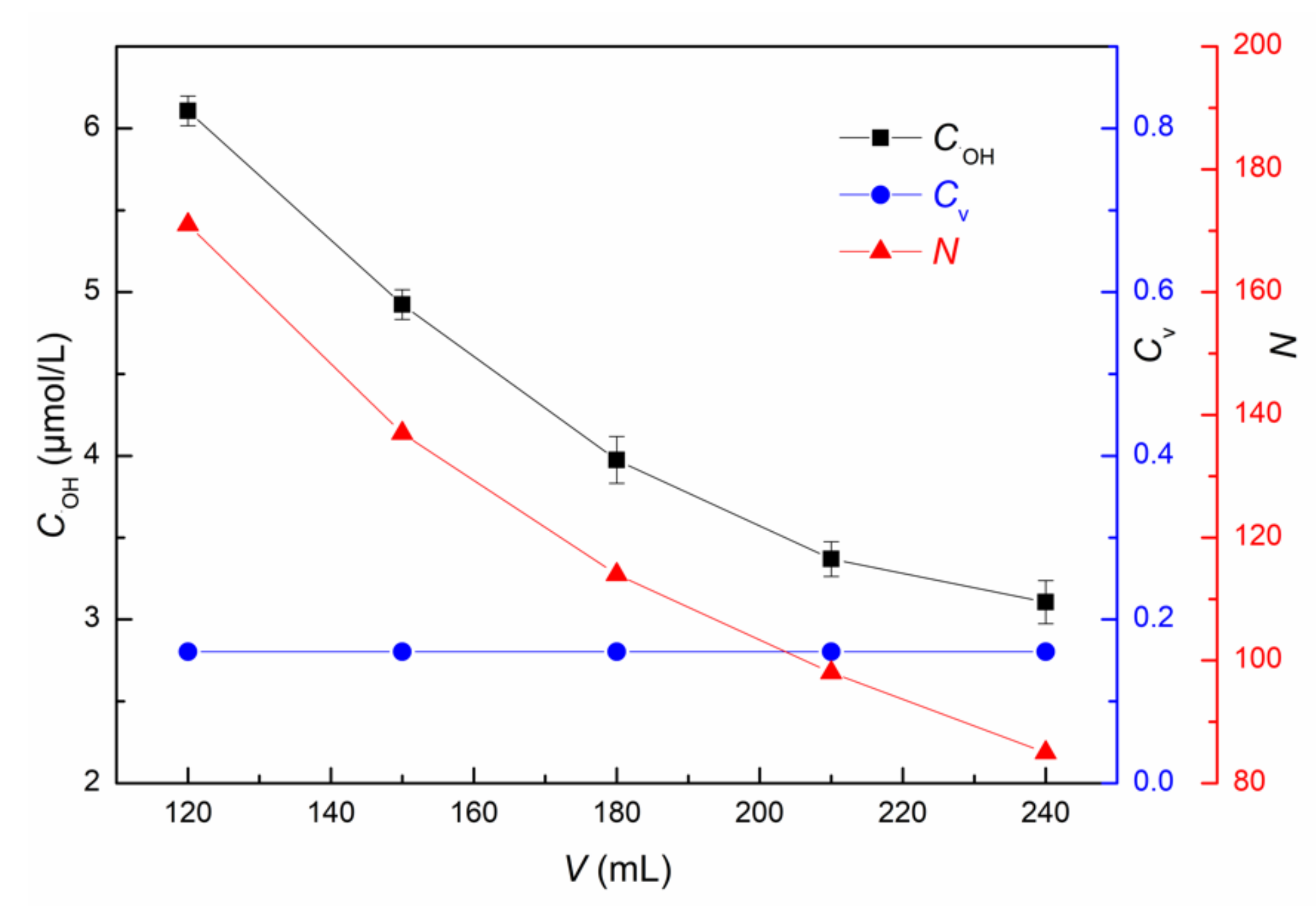
3.1.4. Influence of Solution Volume on ·OH Yield
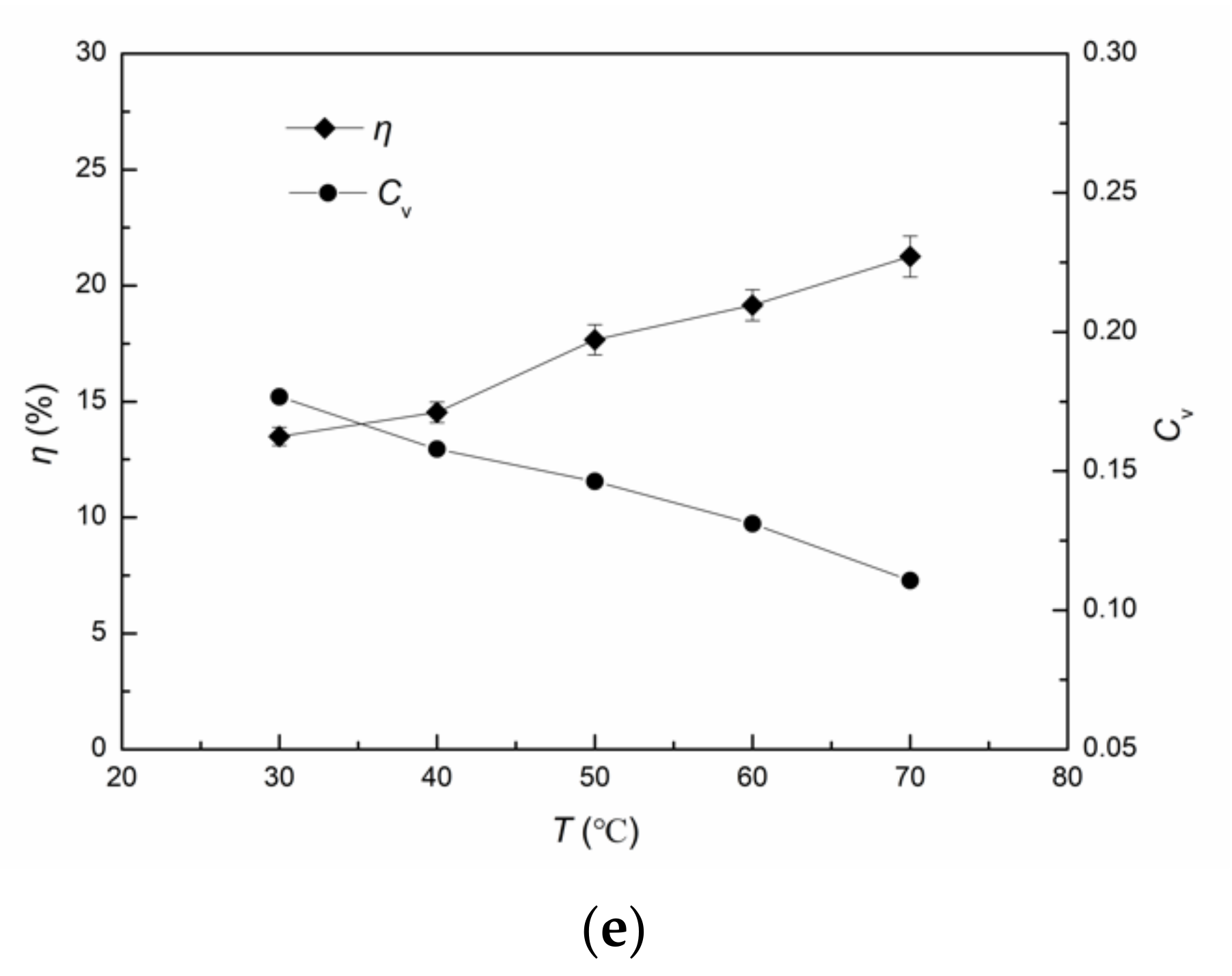
3.1.5. Influence of Temperature on ·OH Yield
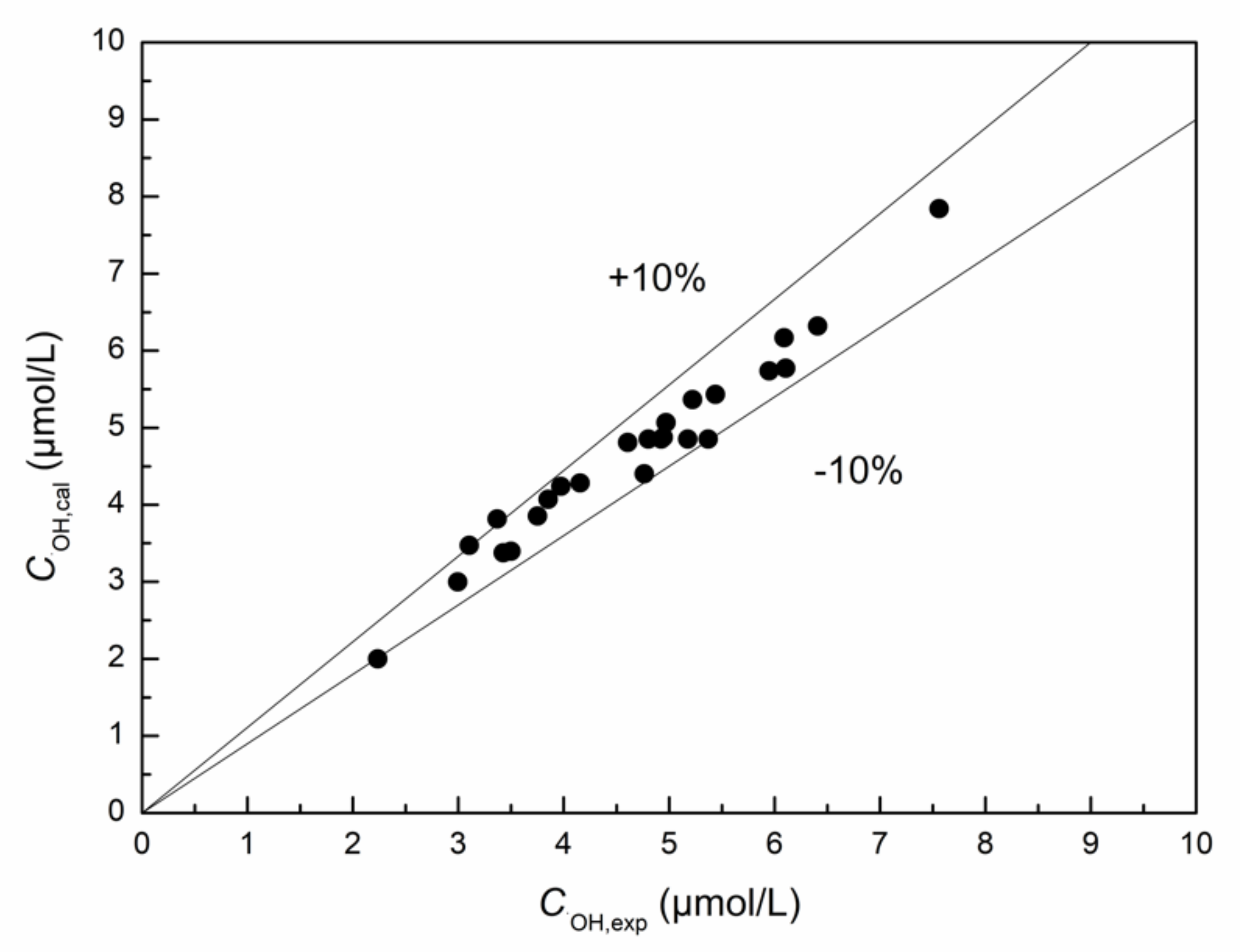
3.1.6. Prediction Model of ·OH Yield in MB Solution
3.2. ·OH Yield in CS Solution
3.2.1. Influence of Various Factors on the Intrinsic Viscosity Reduction Rate of CS
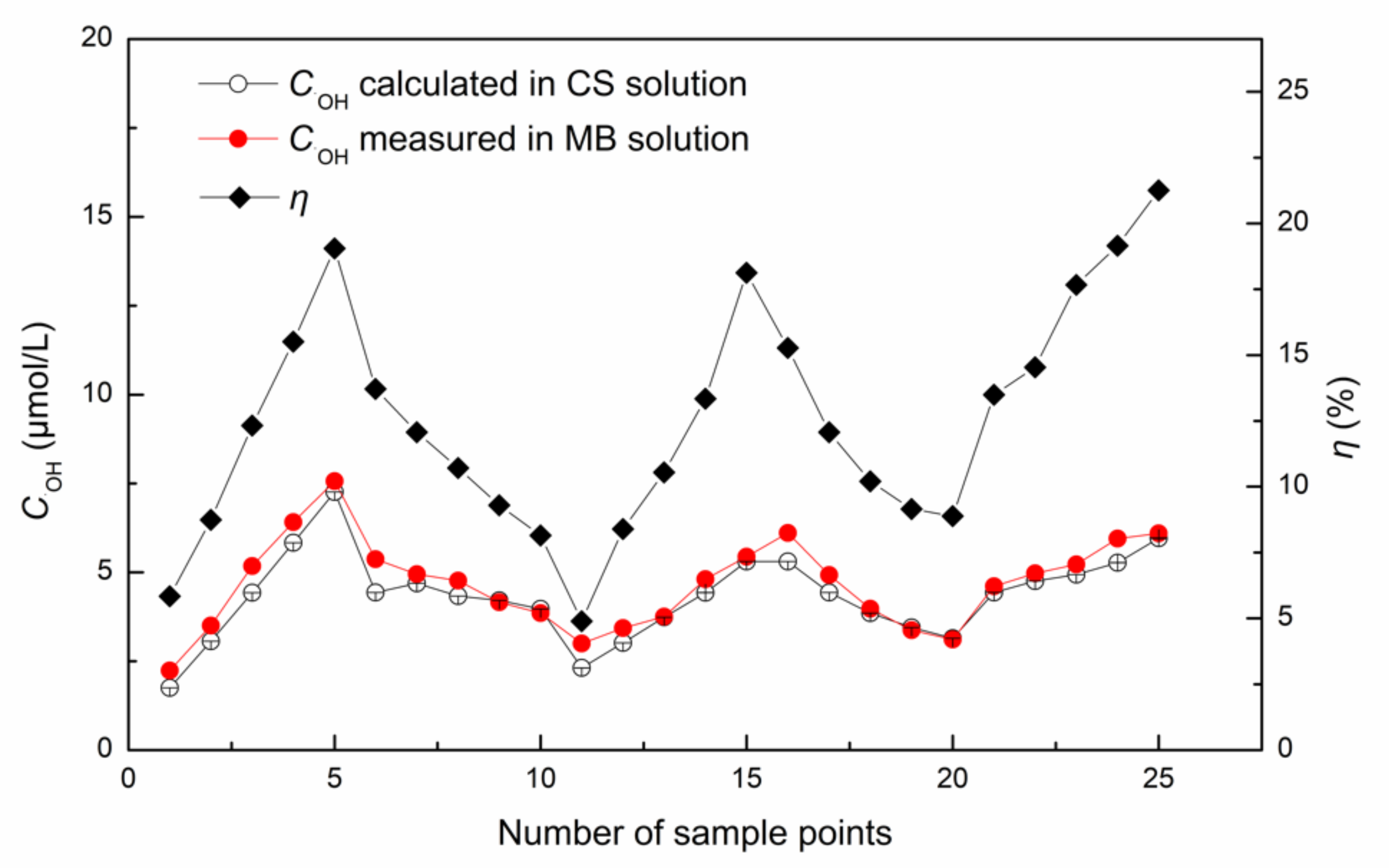
3.2.2. Prediction of ·OH Yield in CS Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | absorbance |
| c | concentration of CS solution, g/L |
| CMB | concentration of MB solution, μmol/L |
| C·OH | concentration of ·OH, μmol/L |
| Cv | Cavitation number |
| d | throat diameter, m |
| D | inner diameter of pipe, m |
| Eu | Euler number |
| n1–5 | correction factor |
| N | number of passes |
| p | pressure, MPa |
| Pr | fully recovered downstream pressure, MPa |
| Pv | saturated vapor pressure, · |
| Q | total flow rate of fluid, m3/s |
| Re | Reynolds number |
| t | cavitation time, s |
| t′ | residence time of the solution in the bath before next cycle, s |
| tp | efflux time of acetic acid/sodium acetate buffer solution measured by the Ubbelohde viscometer, s |
| ts | efflux time of CS solution measured by the Ubbelohde viscometer, s |
| T | temperature, ℃ |
| v | velocity of fluid, m/s |
| V | volume of fluid, m3 |
| We | Weber number |
| α | geometrical parameter, 1/m |
| γ | dimensionless parameter |
| ρ | density, kg/m3 |
| η | intrinsic viscosity reduction rate, % |
| [η] | intrinsic viscosity, L/g |
| [η]0 | intrinsic viscosity of the initial CS solution, L/g |
| [η]1 | intrinsic viscosity of the solution after CS degradation, L/g |
| ηr | relative viscosity |
| ηsp | specific viscosity |
| μ | viscosity, Pa·s |
| σ | interfacial tension, N/m |
| Subscripts | |
| 0 | before cavitation |
| 1 | after cavitation |
References
- Panda, D.; Saharan, V.K.; Manickam, S. Controlled hydrodynamic cavitation: A review of recent advances and perspectives for greener processing. Processes 2020, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Asaithambi, N.; Singha, P.; Dwivedi, M.; Singh, S.K. Hydrodynamic cavitation and its application in food and beverage industry: A review. J. Food Process Eng. 2019, 42, e13144. [Google Scholar] [CrossRef]
- Wang, B.W.; Su, H.J.; Zhang, B. Hydrodynamic cavitation as a promising route for wastewater treatment-A review. Chem. Eng. J. 2021, 412, 128685. [Google Scholar] [CrossRef]
- Bargole, S.; George, S.; Saharan, V.K. Improved rate of transesterification reaction in biodiesel synthesis using hydrodynamic cavitating devices of high throat perimeter to flow area ratios. Chem. Eng. Process. Process Intensif. 2019, 139, 1–13. [Google Scholar] [CrossRef]
- Carpenter, J.; George, S.; Saharan, V.K. Low pressure hydrodynamic cavitating device for producing highly stable oil in water emulsion: Effect of geometry and cavitation number. Chem. Eng. Process. Process Intensif. 2017, 116, 97–104. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.W.; Ren, X.E.; Huang, Y.C. Removal of Fe3+ from ammonium dihydrogen phosphate solution in an impact-jet hydraulic cavitation extractor. Arab. J. Chem. 2022, 15, 103637. [Google Scholar] [CrossRef]
- Benito, Y.; Arrojo, S.; Hauke, G.; Vidal, P. Hydrodynamic cavitation as a low-cost AOP for wastewater treatment: Preliminary results and a new design approach. WIT Trans. Ecol. Environ. 2005, 80, 495–503. [Google Scholar]
- Panda, D.; Manickam, S. Sonochemical degradation of endocrine-disrupting organochlorine pesticide Dicofol: Investigations on the transformation pathways of dechlorination and the influencing operating parameters. Chemosphere 2018, 204, 101–108. [Google Scholar]
- Carpenter, J.; Badve, M.; Rajoriya, S.; George, S.; Saharan, V.K.; Pandit, A.B. Hydrodynamic cavitation: An emerging technology for the intensification of various chemical and physical processes in a chemical process industry. Rev. Chem. Eng. 2017, 33, 433–468. [Google Scholar] [CrossRef]
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandekar, P. A systematic review of physical techniques for chitosan degradation. Carbohyd. Polym. Technol. Appl. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Han, J.L.; Hsieh, K.H. Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleaux formation. Carbohyd. Res. 2011, 346, 94–102. [Google Scholar] [CrossRef]
- Shi, Z.H.; Mahdavian, Y.; Mahdavian, Y.; Mahdigholizad, S.; Irani, P.; Karimian, M.; Abbasi, N.; Ghaneialvar, H.; Zangeneh, A.; Zangeneh, M.M. Cu immobilized on chitosan-modified iron oxide magnetic nanoparticles: Preparation, characterization and investigation of its anti-lung cancer effects. Arab. J. Chem. 2021, 14, 103224. [Google Scholar] [CrossRef]
- Mei, Y.X.; Dai, X.Y.; Yang, W.; Xu, X.W.; Liang, Y.X. Antifungal activity of chitooligosaccharides against the dermatophyte Trichophyton rubrum. Int. J. Biol. Macromol. 2015, 77, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Chokradjaroen, C.; Theeramunkong, S.; Yui, H.; Saito, N.; Rujiravanit, R. Cytotoxicity against cancer cells of chitosan oligosaccharides prepared from chitosan powder degraded by electrical discharge plasma. Carbohyd. Polym. 2018, 201, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, X.M.; Du, Y.G. Oligochitosan: A plant diseases vaccine—A review. Carbohyd. Polym. 2010, 82, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wu, Y.; Huang, W.C.; Yang, F.; Ren, X.E. Degradation of chitosan by hydrodynamic cavitation. Polym. Degrad. Stab. 2013, 98, 37–43. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wang, P.F.; Yuan, Y.; Ren, X.E.; Yang, F. Synergistic degradation of chitosan by impinging stream and jet cavitation. Ultrason. Sonochem. 2015, 27, 592–601. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Y.C.; Zhou, Y.; Ren, X.E.; Yang, F. Degradation of chitosan by swirling cavitation. Innov. Food Sci. Emerg. 2014, 23, 188–193. [Google Scholar] [CrossRef]
- Yan, J.C.; Xu, J.L.; Ai, S.; Zhang, K.M.; Yang, F.; Huang, Y.C. Degradation of chitosan with self-resonating cavitation. Arab. J. Chem. 2020, 13, 5776–5787. [Google Scholar] [CrossRef]
- Richard, W.J. Handbook of Fluid Dynamics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Yan, J.C.; Ai, S.; Yang, F.; Zhang, K.M.; Huang, Y.C. Study on mechanism of chitosan degradation with hydrodynamic cavitation. Ultrason. Sonochem. 2020, 64, 105046. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lu, Y.; Zhou, G.J.; Xia, X.H. A simple electrochemical method for the determination of hydroxyl free radicals without separation process. Talanta 2008, 74, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castro, P.; Vallejo, M.; San Román, M.F.; Ortiz, I. Insight on the fundamentals of advanced oxidation processes: Role and review of the determination methods of reactive oxygen species. J. Chem. Technol. Biotechnol. 2015, 90, 796–820. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Zhang, X.Y.; Liu, X.; Xu, L.L.; Liu, B.C.; Zhang, J.; Xu, H.; Han, Z.K.; Li, G. In-situ exfoliation and assembly of 2D/2D g-C3N4/TiO2(B) hierarchical microflower: Enhanced photo-oxidation of benzyl alcohol under visible light. Carbon 2022, 196, 401–409. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Raza, A.; Xu, L.L.; Li, G. Bismuth oxyhalide quantum dots modified sodium titanate necklaces with exceptional population of oxygen vacancies and photocatalytic activity. J. Colloid Interf. Sci. 2022, 625, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Wang, M.; Li, G. Recent advances in aerobic photo-oxidation over small-sized IB metal nanoparticles. Photochem 2022, 2, 528–538. [Google Scholar] [CrossRef]
- Zupanca, M.; Petkovšeka, M.; Zevnika, J.; Kozmusa, G.; Šmidb, A.; Dular, M. Anomalies detected during hydrodynamic cavitation when using salicylic acid dosimetry to measure radical production. Chem. Eng. J. 2020, 396, 125389. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, M.S.; Pandit, A.B. Experimental quantification of chemical effects of hydrodynamic cavitation. Chem. Eng. Sci. 2000, 55, 1633–1639. [Google Scholar] [CrossRef]
- Vichare, N.P.; Gogate, P.R.; Pandit, A.B. Optimization of hydrodynamic cavitation using a model reaction. Chem. Eng. Technol. 2000, 23, 683–690. [Google Scholar] [CrossRef]
- Zhang, X.D.; Fu, Y.; Li, Z.Y.; Zhao, Z.C. The collapse intensity of cavities and the concentration of free hydroxyl radical released in cavitation flow. Chin. J. Chem. Eng. 2008, 16, 547–551. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhu, X.F.; Cao, Y.; Zhang, K.M.; Huang, Y.C.; Yang, F.; Ren, X.E. Analysis of the influencing factors of the hydroxyl radical yield in a hydrodynamic cavitation bubble of a chitosan solution based on a numerical simulation. ACS Omega 2021, 6, 3736–3744. [Google Scholar] [CrossRef]
- Wang, K.; Yu, L.X.; Yin, S.; Li, H.N.; Li, H.M. Photocatalytic degradation of methylene blue on magnetically separable FePc/Fe3O4 nanocomposite under visible irradiation. Pure Appl. Chem. 2009, 81, 2327–2335. [Google Scholar] [CrossRef]
- Saharan, V.K.; Badve, M.P.; Pandit, A.B. Degradation of reactive red 120 dye using hydrodynamic cavitation. Chem. Eng. J. 2011, 178, 100–107. [Google Scholar] [CrossRef]
- Mancuso, G.; Langone, M.; Andreottola, G. A critical review of the current technologies in wastewater treatment plants by using hydrodynamic cavitation process: Principles and applications. J. Environ. Health Sci. 2020, 18, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Bagal, M.V.; Gogate, P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, I.; Jovanović, J.; Koturević, B.; Adnadjević, B. Transesterifcation of sunfower oil in the presence of the cosolvent assisted by hydrodynamic cavitation. BioEnerg. Res. 2022, 15, 1568–1578. [Google Scholar] [CrossRef]
- Amin, L.P.; Gogate, P.R.; Burgess, A.E.; Bremner, D.H. Optimization of a hydrodynamic cavitation reactor using salicylic acid dosimetry. Chem. Eng. J. 2010, 156, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Randhavane, S.B.; Khambete, A.K. Harnessing hydroxyl radicals generated by hydrodynamic cavitation reactor in simultaneous removal of chlorpyrifos pesticide and COD from aqueous solution. Desalin. Water Treat. 2017, 82, 346–354. [Google Scholar] [CrossRef]
- Randhavane, S.B.; Khambete, A.K. Hydrodynamic cavitation: An approach to degrade chlorpyrifos pesticide from real effluent. KSCE J. Civ. Eng. 2018, 22, 2219–2225. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, H.Z.; Li, Z.Y. Relationship between strength of hydrodynamic cavitation and amount of induced hydroxyl radical. J. Chem. Ind. Eng. 2007, 58, 27–32. (In Chinese) [Google Scholar]
- Gogate, P.R.; Pandit, A.B. Engineering design methods for cavitational reactors. II. Hydrodynamic cavitation. AIChE J. 2000, 46, 1641–1649. [Google Scholar] [CrossRef]
- Tao, Y.Q.; Cai, J.; Huai, X.L.; Liu, B. Global average hydroxyl radical yield throughout entire lifetime of cavitation bubbles. Chem. Eng. Technol. 2018, 41, 1035–1042. [Google Scholar] [CrossRef]
- Jyoti, K.K.; Pandit, A.B. Water disinfection by acoustic and hydrodynamic cavitation. Biochem. Eng. J. 2001, 7, 201–212. [Google Scholar] [CrossRef]
- Chakinala, A.G.; Gogate, P.R.; Burgess, A.E.; Bremner, D.H. Treatment of industrial wastewater effluents using hydrodynamic cavitation and the advanced Fenton process. Ultrason. Sonochem. 2008, 15, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.Q.; Cai, J.; Huai, X.L.; Liu, B.; Guo, Z.X. Application of hydrodynamic cavitation to wastewater treatment. Chem. Eng. Technol. 2016, 39, 1363–1376. [Google Scholar] [CrossRef]
- Franc, J.P.; Michel, J.M. Fundamentals of Cavitation; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Jomni, F.; Aitken, F.; Denat, A. Dynamics of microscopic bubbles generated by a corona discharge in insulating liquids: Influence of pressure. J. Electrostat. 1999, 47, 49–59. [Google Scholar] [CrossRef]
- Li, G.; Ye, S.C.; Zhang, Z.G.; Ma, Y.L. Research and analysis of mass transfer model for liquid-liquid phase dispersion extraction. J. Chem. Eng. Chin. U. 2015, 29, 1325–1332. (In Chinese) [Google Scholar]
- Cao, Y.; Li, J.; Jin, Y.; Luo, J.H.; Wang, Y.B. Liquid–liquid two-phase mass transfer characteristics in a rotating helical microchannel. Chin. J. Chem. Eng. 2019, 27, 2937–2947. [Google Scholar] [CrossRef]
- Su, Y.H.; Chen, G.W.; Zhao, Y.C.; Yuan, Q. Intensification of liquid–liquid two-phase mass transfer by gas agitation in a microchannel. AIChE J. 2009, 55, 1948–1958. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.M.; Zhang, X.; Xiao, L.X.; Kan, J.; Jin, C.H. Effects of ascorbate and hydroxyl radical degradations on the structural, physicochemical, antioxidant and film forming properties of chitosan. Int. J. Biol. Macromol. 2018, 114, 1086–1093. [Google Scholar] [CrossRef]
- Kang, B.; Dai, Y.D.; Zhang, H.Q.; Chen, D. Synergetic degradation of chitosan with gamma radiation and hydrogen peroxide. Polym. Degrad. Stabil. 2007, 92, 359–362. [Google Scholar] [CrossRef]




| Solution | Temperature (℃) | Density (kg/m3) | Viscosity (mPa·s) | Surface Tension (mN/m) |
|---|---|---|---|---|
| MB solution | 30 | 996.3 | 0.81 | 66.30 |
| 40 | 994.5 | 0.66 | 64.93 | |
| 50 | 989.5 | 0.56 | 61.49 | |
| 60 | 987.4 | 0.48 | 57.88 | |
| 70 | 983.4 | 0.44 | 57.24 | |
| CS solution | 30 | 1000.6 | 1.07 | 68.83 |
| 40 | 998.4 | 0.89 | 67.77 | |
| 50 | 995.7 | 0.73 | 67.30 | |
| 60 | 991.2 | 0.61 | 66.57 | |
| 70 | 987.1 | 0.52 | 65.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Xie, D.; Huang, Y.; Huang, C.; Zhang, K.; Zhang, X.; Wang, S. Investigation of Hydroxyl Radical Yield in an Impact-Jet Hydraulic Cavitator. Processes 2022, 10, 2194. https://doi.org/10.3390/pr10112194
Cao Y, Xie D, Huang Y, Huang C, Zhang K, Zhang X, Wang S. Investigation of Hydroxyl Radical Yield in an Impact-Jet Hydraulic Cavitator. Processes. 2022; 10(11):2194. https://doi.org/10.3390/pr10112194
Chicago/Turabian StyleCao, Yan, Dongdong Xie, Yongchun Huang, Chengdu Huang, Kunming Zhang, Xiangyu Zhang, and Shujun Wang. 2022. "Investigation of Hydroxyl Radical Yield in an Impact-Jet Hydraulic Cavitator" Processes 10, no. 11: 2194. https://doi.org/10.3390/pr10112194
APA StyleCao, Y., Xie, D., Huang, Y., Huang, C., Zhang, K., Zhang, X., & Wang, S. (2022). Investigation of Hydroxyl Radical Yield in an Impact-Jet Hydraulic Cavitator. Processes, 10(11), 2194. https://doi.org/10.3390/pr10112194







