Abstract
Liquid ammonia as a non-aqueous medium has many physical properties close to water, such as small molecular weight and strong permeability. It has been widely used for the ecological processing of cellulosic fibers to improve their luster, softness and dyeing properties. However, there are few reports on the dyeing of wool treated with liquid ammonia, especially at a lower temperature. Herein, a continuous liquid ammonia finishing machine was used to batch process wool followed by dyeing in a commonly-used wool dyeing machine. The results showed that many scale flakes and some cuticle cracking were seen on the fiber surface, and the disulfide bonds of cystine were broken down after liquid ammonia treatment, which promoted the diffusion of dyestuff into the fiber. Moreover, the uptakes and K/S value of wool dyed with Lanaset and Lanasol CE dyes were higher than the untreated wool, and the dyeing temperature could decrease to 85 °C, while the degree of fiber strength reduction merely decreased by 3–5%. Furthermore, for the reactive dyes, the dyeing temperature can reduce to 70 °C with the chemical auxiliaries Miralan LTD, while the degree of strength reduction decrease by 8–10%. Liquid ammonia treatment can be used for dyeing at a lower temperature than boiling temperature (100 °C), reduce energy consumption and reduce the degree of fiber strength reduction of wool. The method shows considerable to great value and is significant in providing a feasible approach for the industrial application of low-temperature dyeing technology.
1. Introduction
Wool is a complex protein compound composed of the cuticle, cortex, medulla, and cell membrane complex. The cuticle is neatly arranged with a hydrophobic lipid layer composed of eicosanoic acid and 18-methyleicosanoic acid, which can form ester and thioester bonds with proteins. The cross-linking can increase the density of the wool surface and form a diffusion barrier to dyes and chemical agents [1]. Therefore, the dyeing of wool fibers is usually at boiling temperature for a long time, promoting the diffusion of the dyes through the cuticles along the membrane complex into the fiber’s interior and then into the cortex under the condition of high-temperature swelling. However, the degree of fiber strength reduction is serious after boiling it for a long time, which deteriorates the luster and brightness of wool fiber, and the cost of energy consumption is high. Therefore, low-temperature dyeing technologies of wool have been a research hotspot for many years. These intended methods can change the cuticle or the cell membrane complex structure of wool to improve the dyestuffs adsorption and diffusion capacity, decrease the diffusion activation energy of the dye and then eventually achieve low-temperature dyeing. Low-temperature dyeing is of great significance in reducing the degree of fiber strength reduction of wool and the processing difficulty of spinning and weaving.
In recent years, low-temperature dyeing of wool fiber mainly focused on the research of ultrasonic waves, plasma, enzymes and chemical additives. It has been reported that ultrasonic waves can break the cuticle, improve the dye uptake and reduce the dyeing temperature to 70–80 °C [2,3,4,5,6,7]. Wakida et al. studied the role of plasma technology [8,9,10,11,12] in wool fiber dyeing. Surface etching and hydrophilic groups increased when the fiber was treated with an inert gas plasma, and the dyeing and anti-felting properties were synchronously improved. Generally, wool fibers treated with plasma can be dyed at 60–70 °C. Enzymes and chemical auxiliaries have also been used for the low-temperature dyeing of wool. The main effect of enzymes on wool is to hydrolyze the CMC layer and improve the intercellular diffusion rate of dye molecules [13,14]. Synergistic effects of enzymes combined with other physical or chemical pretreatments have also been reported [15,16,17]. It not only hydrolyzes the membrane complex, but also makes some parts of disulfide bonds break down. The adsorption and diffusion rate of dyestuffs increase, causing a decrease in the dyeing temperature. Chemical additives break the cuticle, break the disulfide bonds, or increase the content of salt and hydrogen bonds to increase the adsorption and dissolution of dyestuffs [18,19,20,21]. However, ultrasonic waves and plasma are difficult to industrialize due to the limitation of equipment, while enzymes are difficult to store, and it is difficult to control the quality due to the strict requirements on temperature and pH value. Chemical additive methods are the most widely used for low-temperature dyeing in the industry due to the advantages such as easy storage, facile operation, and pronounced effect. However, the chemical easily breaks the unique style of the wool itself. Therefore, developing a mild and industrially feasible low-temperature dyeing technology for wool is of great practical significance and value.
Liquid ammonia finishing is a modern international integration of mechanical, chemical, automation control and other high-tech integrated green environmental protection technology. The application of liquid ammonia in wool is feasible, the liquid ammonia finishing machine is widely used in cotton textile processing. The surface characteristics, thermodynamic qualities, and dyeing properties of wool have been studied in various laboratories [22,23,24,25,26]. Wakida [25] et al. considered that wool’s physical or chemical structure was not altered after liquid ammonia treatment. Therefore, it seems likely that the treatment induced a slight relaxation in the surface of the endocuticle or the cell membrane complex, which resulted in the improvement in dyeing properties.
Unlike previous research, this paper focuses on the changes in wool surface properties and low-temperature dyeing properties after pretreatment using an industrial continuous liquid ammonia finishing machine. The surface characteristics and chemical structure of wool were evaluated by scanning electron microscopy (SEM), x-ray photoelectron spectroscopy (XPS), and Raman spectrum. Then the wool was dyed with the metal-complex dye Lanaset and the reactive dye Lanasol CE. The dyeing temperature, dyeing rate, K/S values and fiber strength were investigated and compared before and after the liquid ammonia treatment.
2. Materials and Methods
2.1. Materials
Australian wool tops with diameters of 19.5 µm were provided by Ruyi Limited, China.
Metal-complex dyestuff Lanaset Blue 2R (C.I. Acid Blue 225), Lanaset Red G, reactive dyestuff Lanasol Red CE, Lanasol Blue CE and Miralan LTD were all provided by Huntsman Company.
2.2. Liquid Ammonia Treatment
The liquid ammonia finishing of wool was impregnated in the liquid ammonia at −33.4 °C for one minute by a continuous liquid ammonia finishing machine (Kyoto Machinery, Kyoto, Japan), and the excess ammonia was removed by steaming at 110 °C. Then the treated wool was washed at 50–70 °C and dried at 110 °C. The schematic diagram of the working principle is shown in Figure 1.
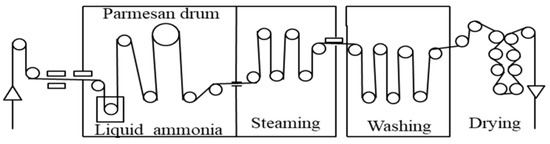
Figure 1.
Schematic diagram of a continuous liquid ammonia finishing machine.
2.3. Dyeing with Metal-Complex Dyes
Lanaset Blue 2R and Lanaset Red G were used to dye untreated wool (W) and liquid ammonia-treated wool (LAT). The concentration of dyestuffs was 3% on weight of fabric (o.w.f), and the liquor ratio was 1:10. The pH value was adjusted to 5.0 by an acetic acid–sodium acetate buffer solution. The temperature was subsequently increased from 40 °C to 85 °C at a rate of 1 °C/min, while the dyeing time was from 0 to 120 min. The dyeing curves of metal-complex dyestuff with different temperatures are shown in Figure 2.
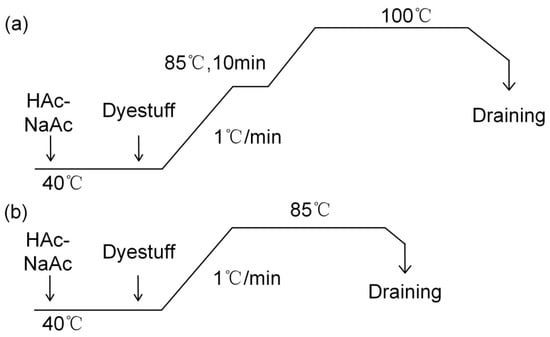
Figure 2.
Dyeing curves of Lanaset Red G and Blue 2R with different temperatures: (a) 100 °C; and (b) 85 °C.
2.4. Reactive Dyeing
Lanasol Red CE and Lanasol Blue CE were used for reactive dyeing of untreated wool (W) and liquid ammonia treated wool (LAT). The concentration of dyestuffs and levelling agent Miralan LTD was 3% (o.w.f) and 1% (o.w.f), respectively. The liquor ratio was 1:10, and the pH value was adjusted to 5.0 by acetic acid-sodium acetate buffer solution. The temperature was subsequently increased at a rate of 1 °C/min from 40 °C to 70 °C, while dyeing time was from 0 to 120 min. After dyeing, the solid-color processing of reactive dyes was at 65 °C for 20 min. The pH value was adjusted to 9 by sodium carbonate. Then the dyed wool was neutralized by acetic acid for 10 min and finally washed for 5 min. The dyeing curves of reactive dyestuff with different temperatures are shown in Figure 3.
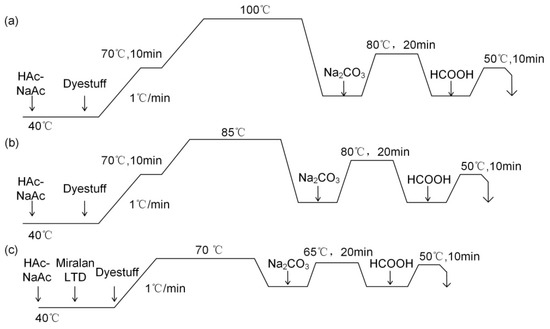
Figure 3.
Dyeing curves of Lanasol Red CE and Blue CE with different temperatures: (a) 100 °C; (b) 85 °C; (c) 70 °C.
2.5. Surface Morphology Analysis
Regulus8100 scanning electron microscope (Hitachi, Japan) was used to test the surface morphology of wool fibers. The voltage and magnification were 5 kV and 2000 times, respectively.
2.6. Chemical Structure Analysis
An In Via Laser Micro-Raman Spectrometer (Renishaw, Gloucestershire) was used to measure the surface structure of the wool fiber. The scanning range was from 4000 cm−1 to 650 cm−1.
2.7. Dye Uptake
The absorbance of the dye liquor before and after dyeing at its maximum absorption wavelength (Lanaset Red G, Lanaset Blue 2R, Lanasol Red CE, and Lanasol Blue CE was 497 nm, 602 nm, 510 nm, and 386 nm, respectively) and was measured by UV1800 (Shimadzu, Kyoto, Japan) ultraviolet spectrophotometer. The formula of dye uptake E(%) is expressed by:
where A0 is the absorbance of the initial dye solution, and C0 is its dilution multiple; A1 is the absorbance of the residual solution after dyeing, and C1 is its dilution multiple.
2.8. K/S Value
K/S value was measured by Datacolor800 spectrophotometer (D65/10°). Each group was tested 10 times at 20 ± 2 °C temperature and 65 ± 2% relative humidity, and the average value was taken. The K/S value was calculated by the Kubelka–Munk Equation [27] in Formula (2):
where K is the adsorption coefficient, S is the scattering coefficient, and R is the reflectivity of the maximum wave strength.
2.9. Color Difference (ΔE)
CIE L*a*b* color space values were evaluated by Datacolor800 spectrophotometer, the measurement conditions were D65 daylight and 10° standard observer. All measurements were carried out at 20 ± 2 °C temperature and 65 ± 2% relative humidity, and the average value was taken. CIE ΔE [28] was measured by Equation (3):
where is the difference in CIE L* values between the treated sample and the reference sample, is the difference in CIE a* values, and is the difference in CIE b* values.
2.10. Rubbing Fastness
The sample was cut into 5 cm × 5 cm size and then mounted on the bed of the crock meter. Then, a cotton standard rubbing cloth (5 cm × 5 cm) was mounted on the finger of the crock meter and rubbed against the sample fabric for 10 s and 10 cycles. The rubbing fastness of the sample was evaluated by using grayscale according to the standard of ISO 105-X12-2016 (E).
2.11. Potting Fastness
According to the ISO 105-E09-2010 standard, the samples were boiled in boiling water for 30 min. Standard grayscale was used to evaluate the discoloration of the sample and the staining of adjacent fabric such as cotton and wool adjacent fabrics.
2.12. Single Fiber Break Strength
The single fiber break strength of wool was measured by XQ-2 fiber strength depth tester (Xinyi, Shanghai, China), and it was tested at 20 ± 2 °C temperature and 65 ± 2% relative humidity by the standard method of IWTO-30. According to sampling regulations, 100–200 mg representative samples were drawn from the batch as specified. The number of fibers tested was not less than 300 roots.
3. Results and Discussion
3.1. Surface Morphology
The surface morphology of wool fibers before and after liquid ammonia treatment was observed by electron microscopy, just as shown in Figure 4.
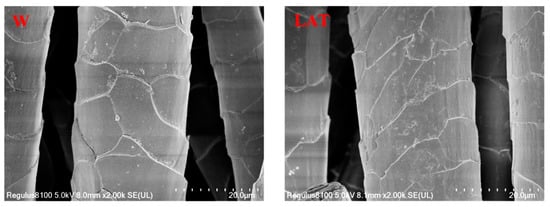
Figure 4.
SEM (×2000) photos of wool fiber.
The SEM photos show the unique structure of wool with irregular scales. After liquid ammonia treatment, some parts of the scales were broken, and some fragments were found on the surface. Furthermore, the warping angle of the scales became smaller, and the scaly edge became thinner compared to untreated wool. This may be because liquid ammonia could break the surface scale of wool to some extent. On the other hand, the surface scales may be further broken by the action of rollers in the process of liquid ammonia treatment in a continuous liquid ammonia finishing machine.
3.2. Chemical Structure Analysis
The exocuticle is the part of the cuticle with the highest sulfur content in which the cystine exists in the form of a large number of disulfide bonds. It has been reported that [29,30,31,32,33], the −S−S−bonds, amide bands, aromatic amino acids and cysteine −S−O, −S−H bands are the more apparent bands in Raman spectra of wool.
Figure 5 shows the characteristic vibration band of wool, and in the range of 500−700 cm−1, it is an S−S stretching vibration band, while 2565 cm−1 is the S−H stretching vibration of cystine. The S−S stretching vibration band has three different conformation forms, which are at 505 cm−1 (gauche to gauche), 515 cm−1 (gauche to trans) and 536 cm−1 (trans to trans), respectively. After liquid ammonia treatment, the intensity of characteristic bands decreased, and there were two new peaks at 2420 cm−1 and 572 cm−1, respectively. The peak at 2420 cm−1 may be the stretching vibration of the S-H band that was affected by liquid ammonia. In contrast, the peak at 572 cm−1 indicated that the S−S bond conformation changed, and a new conformation was generated.
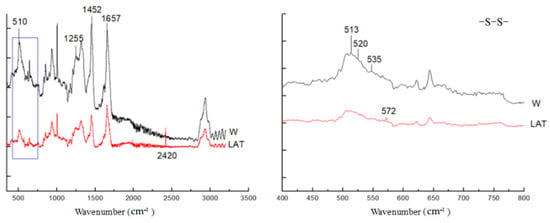
Figure 5.
Raman spectra of wool fiber.
The peak at 1452 cm−1 is the bending vibration of −CH2 and −CH3 on the side chain of amino acids, which is not affected by conformational changes in the backbone of the peptide chain. In this paper, the Raman spectra of wool are normalized by the band intensity of the peak. Furthermore, I510 represents the band intensity of frequency 510 cm−1, while I1452 represents the intensity of frequency of 1452 cm−1. According to the research of Jurdana et al. [29], I510/1452 is content of the disulfide bond in cystine of wool fiber. As seen in Figure 5, after liquid ammonia treatment, the I510/1452 ratio of wool was 0.5, lower than the untreated wool of 0.845. This shows that liquid ammonia can break the cysteine disulfide bond, reducing the diffusion barrier of dye molecules and improving dye uptake in wool.
3.3. Dyeing Properties
3.3.1. Dyeing with Metal-Complex Dyes
Lanaset series dyes were produced by Huntsman, which are modified 1:2 metal-complex dyes with good compatibility, dyeing rate, dyeing reproducibility and high fastness.
The dyeing rate of W and LAT dyed at 100 °C and 85 °C are shown in Figure 6.
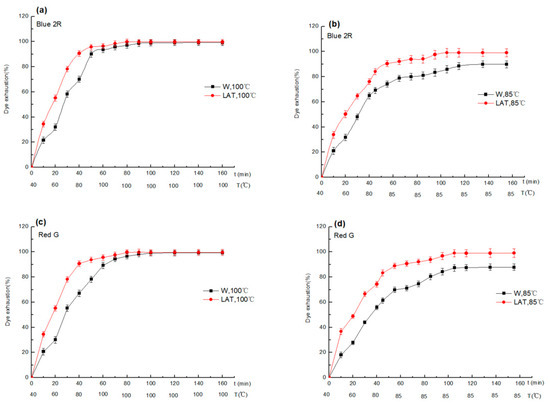
Figure 6.
Dyeing rate of W and LAT dyed with Lanaset dyes at different temperatures: (a) Blue 2R-100 °C; (b) Blue 2R-85 °C; (c) Red G-100 °C; and (d) Red G-85 °C.
Figure 6 shows that the dyeing rate of LAT was in line with the Langmuir adsorption isotherm curve. The dyeing rate increased faster at the initial stage and then gradually increased until dyeing equilibrium. The dyeing rate of LAT was significantly higher than that of W, especially in the initial dyeing stage. The main reasons for that may be as follows: (a) the barrier of surface scale to wool dyeing can be reduced due to the breakage of scales by liquid ammonia treatment so that more dye molecules penetrate the fiber; (b) more hydrophilic groups were exposed due to breakage of the lipid layer by liquid ammonia, increasing the affinity of dye and wool fiber; (c) the dyeing site of wool was increased due to the disulfide bond that can break down to form a sulfoalanine by liquid ammonia.
The influence of liquid ammonia on dyeing decreased with temperature rise, especially when wool was dyed at 100 °C. The surface scales open entirely under hot and humid conditions, the dye molecules can quickly penetrate fiber, and the dyeing exhaustion reached the maximum. Therefore, hot and humid conditions are the main reason for dyeing exhaustion at high-temperature dyeing.
The dyeing equilibrium time of W dyed at 100 °C can be reached for holding 40 min, while LAT for 30 min. However, when wool was dyed at 85 °C, the dyeing equilibrium time of W and LAT was 80 min and 60 min, respectively. The equilibrium dyeing rate could reach 99% when LAT was dyed at 85 °C, which has an excellent dyeing effect. The dyeing rate of Blue 2R was slightly higher than that of Red G, but the difference was slight. Blue 2R and Red G have excellent compatibility and form the three primary colors of Lanaset series dyes with Grey G.
The dyeing properties of W and LAT at 100 °C and 85 °C for 60 min are shown in Table 1.

Table 1.
Dyeing properties of W and LAT dyed at 100 °C and 85 °C for 60 min.
As shown in Table 1, the dyeing properties of LAT dyed at 85 °C, such as K/S value, color difference and fastness were similar to that of W dyed at 100 °C. However, the breaking strength of a single fiber changed significantly. The single fiber strength of LAT was 5.35 cN, which decreased by 2.6% compared to W. When wool was dyed at 100 °C with Lanaset Blue 2R, the single fiber strength of W and LAT decreased by 12.4% and 13.3%, respectively, while that dyed at 85 °C decreased by 5.6% and 6.0%, respectively. For the Lanaset Red G, the single fiber strength of W and LAT dyed at 100 °C was decreased by 11.6% and 12.7%, respectively, while that dyed at 85 °C decreased by 4.9% and 5.4%, respectively. This indicated that the wool treated with liquid ammonia could be dyed at a lower temperature of 85 °C, which can weaken the fiber’s strength reduction of high-temperature dyeing and be profitable for the spinning and weaving process.
3.3.2. Reactive Dyeing
The reactive dyes used in this paper were Lanasol CE series produced by Huntsman Company. Lanasol CE is α-bromoacrylamide type dyestuff, which has a double active group. The Br atom can undergo nucleophilic substitution reaction with the amino group. In contrast, the vinyl can undergo nucleophilic addition reaction with the amino group, forming a cross-linking covalent bond and enhancing the bond with the dyes. After liquid ammonia treatment, the content of -NH2,-NH- increased, which enhanced the interaction with dye molecules and made the dye more easily adsorbed to wool. The reaction mechanism of Lanasol CE dye and wool is shown in Figure 7.
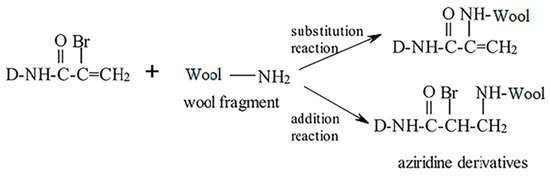
Figure 7.
Dying mechanism of Lanasol CE dye and wool [34].
Miralan LTD is a polyethoxy surfactant of an ethoxy fatty acid amine derivative. The ethylene oxidation unit in the molecular structure can produce a liquid layer with a high concentration of dyes and additives on the outer layer of the fiber, which reduces the aggregation of dyes, promotes the dissolution of dyes and makes the dye molecule easier to enter the fiber [35,36,37].
Lanasol CE dyestuff has a bifunctional group, which is susceptible to the influence of different types of additives during dyeing. In this paper, the levelling agent Miralan LTD was added in the dye bath when wool was dyed at 70 °C, and the influences of liquid ammonia and Miralan LTD on reactive dyeing were investigated.
The dyeing rate of W and LAT dyed at 100 °C, 85 °C and 70 °C are shown in Figure 8.
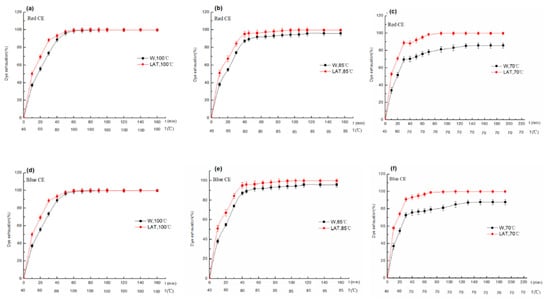
Figure 8.
Dyeing rate of W and LAT dyed at different temperatures: (a) Red CE-100 °C; (b) Red CE-85 °C; (c) Red CE-70 °C; (d) Blue CE-100 °C; (e) Blue CE-85 °C; and (f) Blue CE-70 °C.
Figure 8 shows that the dyeing rate of LAT is higher than W, especially at a low temperature. The dyeing rate of LAT can reach about 50% at 50 °C, while it is nearly about 95% when temperature rises to 85 °C. This is because after liquid ammonia treatment, the surface scales were broken, reducing the barrier of dye molecules penetrating the fiber. On the other hand, more hydrophilic groups were exposed due to the breakage of the lipid layer by liquid ammonia, increasing the affinity of dye and wool fiber. Furthermore, the nucleophilic substitution reaction between dyes and wool fiber may increase because the disulfide bond was broken down to form a sulfoalanine by liquid ammonia. When wool is dyed at 100 °C, dye molecules can quickly enter the fiber to make the dyeing rate maximum because surface scales are completely open under hot and humid conditions. The dyeing rate of LAT is consistent to W at this moment.
Miralan LTD can reduce aggregation and promote the dissolution and dispersion of dyes. Under the co-effect of liquid ammonia and Miralan LTD, dye molecules are easier to uniformly and rapidly attach to fiber, and the dyeing rate is higher than W under the same conditions. When wool was dyed with Lanasol Red CE, the dyeing rate of LAT was 88.8% when the temperature was raised to 70 °C, which was 26.7% higher than that of W. Furthermore, it can go above 95% after holding for 30 min at 70 °C.
Liquid ammonia can shorten dyeing equilibrium time, which can be reached for holding 30 min at 100 °C, while LAT for 20 min. The dyeing equilibrium times of W and LAT dyed at 85 °C were 70 min and 50 min, respectively, while there were 120 and 80 min when W and LAT dyed at 70 °C. Compared to Lanasol Red CE and Lanasol Blue CE, it was found that the dyeing rate of Blue CE was higher than that of Red CE.
The dyeing properties of W and LAT dyed at 100 °C, 85 °C and 70 °C, respectively, for 60 min are shown in Table 2.

Table 2.
Dyeing properties of W and LAT dyed at 100 °C, 85 °C and 70 °C for 60 min.
As shown in Table 2, the K/S value, color difference and fastness of LAT dyed at 85 °C or 70 °C were similar to that of W dyed at 100 °C, but the breaking strength of single fiber changed significantly. When wool was dyed with Lanasol Red CE, the single fiber strength of W dyed at 100 °C, 85 °C and 70 °C were decreased by 12.9%, 6.6% and 4.2%, respectively, while those of LAT decreased by 13.3%, 8.8% and 3.7%, respectively. Compared to Red CE, the single fiber strength of wool was reduced more when wool was dyed with Blue CE. Single fiber strength of W dyed at 100 °C, 85 °C and 70 °C were decreased by 15.3%, 7.8% and 4.6%, respectively, while those of LAT decreased by 15.7%, 10.1% and 4.5%, respectively. This indicated that LAT can dye at a lower temperature of 70 °C under the co-effect of liquid ammonia and Miralan LTD with a little degree of strength reduction. The fiber strength was mainly influenced by temperature.
4. Conclusions
This work provided a green, low-carbon and environmentally friendly dyeing method, which can reduce the dosage of chemical additives and the difficulty of dyeing wastewater treatment, and then achieve energy conservation and emission reduction of wool dyeing. After liquid ammonia treatment, the wool’s surface scale and disulfide bond were partly broken, reducing the dye molecules’ diffusion barrier and promoting their adsorption and diffusion to wool. The dyeing rate of LAT is higher than W, especially at a low temperature. Compared to W dyed at 100 °C, the dyeing temperature of LAT can reduce to 85 °C. Moreover, with the addition of Miralan LTD, the temperature of LAT dyed with Lanasol CE can be lowered to 70 °C. The K/S value, color difference and fastness of LAT fiber dyed at 85 °C or 70 °C were similar to that of W dyed at 100 °C. However, the single fiber-breaking strength of wool was significantly influenced by temperature. The single fiber strength decreased most when wool dyed at 100 °C, while it decreased only about 5% when dyed at 70 °C.
Author Contributions
Writing—original draft preparation, writing—review and editing, X.S.; Methodology, Z.J. and H.M.; Resources, project administration, funding acquisition, Q.W.; Conceptualization, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key R&D Program of China (2017YFB0309200) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX18_1832).
Data Availability Statement
Not applicable.
Acknowledgments
Thanks for the support from International Joint Research Laboratory for Eco-Textile Technology at Jiangnan University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zahn, H.; Messinger, H.; Hocker, H. Covalently linked fatty acids at the surface of wool: Part of the cuticle cell envelop. Text. Res. J. 1994, 64, 554–555. [Google Scholar] [CrossRef]
- Islam, G.M.N.; Ke, G.Z.; Haque, A.N.M.A.; Islam, M.A. Effect of ultrasound on dyeing of wool fabric with acid dye. J. Ind. Eng. Chem. 2017, 8, 425–431. [Google Scholar] [CrossRef][Green Version]
- McNeil, S.J.; McCall, R.A. Ultrasound for wool dyeing and finishing. Ultrason. Sonochem. 2011, 18, 401–406. [Google Scholar] [CrossRef]
- Yükseloşlu, S.M.; Bolat, N. The use of conventional and ultrasonic energy in dyeing of 100% wool woven fabrics. Tekst. Konfeksiyon. 2010, 20, 162–167. [Google Scholar]
- Kamel, M.M.; EI-Shishtawy, R.M.; Yussef, B.M.; Mashaly, H. Ultrasonic assisted dyeing. Dye. Pigment. 2005, 65, 103–110. [Google Scholar] [CrossRef]
- Nazmul, G.M.N.; Ke, G.Z. Ultrasonic effects on the kinetics and thermodynamics of dyeing wool fiber with reactive dye. Fiber. Polym. 2020, 21, 1071–1077. [Google Scholar] [CrossRef]
- Khatri, M.; Ahmed, F.; Jatoi, A.W.; Mahar, R.B.; Khatri, Z.; Kim, I.S. Ultrasonic dyeing of cellulose nanofibers. Ultrason. Sonochem. 2016, 31, 350–354. [Google Scholar] [CrossRef]
- Panda, P.K.; Rastogi, D.; Jassal, M.; Agrawal, A.K. Effect of atmospheric pressure helium plasma on felting and low temperature dyeing of wool. J. Appl. Polym. Sci. 2012, 124, 4289–4297. [Google Scholar] [CrossRef]
- Mendhe, P.; Arolkar, G.; Shukla, S.; Deshmukh, R. Low-temperature plasma processing for the enhancement of surface properties and dyeability of wool fabric. J. Appl. Polym. Sci. 2016, 133, 43097. [Google Scholar] [CrossRef]
- Cai, Z.S.; Qin, Y.P. Dyeing properties of wool fabrics treated with atmospheric pressure plasmas. J. Appl. Polym. Sci. 2008, 109, 1257–1261. [Google Scholar] [CrossRef]
- Wakida, T.; Cho, S.; Choi, S.; Tokino, S.; Lee, M. Effect of low temperature plasma treatment on color of wool and nylon 6 fabrics dyed with natural dyes. Text. Res. J. 1998, 68, 848–853. [Google Scholar] [CrossRef]
- Haji, A. Natural dyeing of wool with henna and yarrow enhanced by plasma treatment and optimized with response surface methodology. J. Text. I. 2020, 111, 467–475. [Google Scholar] [CrossRef]
- Riva, A.; Algaba, I.; Prieto, R. Dyeing kinetics of wool fabrics pretreated with a protease. Color. Technol. 2002, 118, 59–63. [Google Scholar] [CrossRef]
- Cui, L.; Yu, Y.Y.; Fan, X.R.; Wang, P.; Wang, Q. Effect of protease treatment on dyeing properties of wool fabrics for single bath. Eng. Life Sci. 2009, 9, 135–139. [Google Scholar] [CrossRef]
- Periolatto, M.; Ferrero, F.; Giansetti, M.; Mossotti, R.; Innocenti, R. Enzyme-aided wool dyeing with a neutral protease at reduced temperatures. Eng. Life Sci. 2010, 10, 474–479. [Google Scholar] [CrossRef]
- El-Gabry, L.; El-Nouby, G.; Allam, O.G.; El-Sayed, H. Effect of mechanical and enzymatic treatments on some properties of coarse wool. J. Nat. Fibers. 2008, 5, 461–475. [Google Scholar] [CrossRef]
- Alhariri, D.K.; Rattee, I.D.; Seltzer, I. Improvements in the dyeing of wool at low temperatures by the use of amine or ammonia pretreatments. J. Soc. Dye. Colours 1978, 94, 149–155. [Google Scholar] [CrossRef]
- El-Sayed, H.; El-Hawary, N. The use of modified fenton chemistry for reducing energy consumption during dyeing of wool and nylon 6 fabrics with acid dyes. J. Nat. Fibers 2021, 1–13. [Google Scholar] [CrossRef]
- Akoh, F.; Mehdi, E.B.; Cherkaoui, O.; Tahiri, M. The esterification and dyeing properties of raw wool fibers. IOP Conf. Ser. Mater. Sci. Eng. 2020, 827, 012053. [Google Scholar] [CrossRef]
- El-Hawary, N.S.; Elshemy, N.S.; El-Sayed, H. New thiol-disulfide exchangers as anti-setting agents for wool fabric during dyeing. Fiber Polym. 2016, 17, 1391–1396. [Google Scholar] [CrossRef]
- Cao, W.Z.; Pei, L.J.; Zhang, H.J.; Wang, J.P. Sustainable wool fabric pad dyeing using reactive dyes in silicone non-aqueous medium. Environ. Chem. Lett. 2021, 19, 737–741. [Google Scholar] [CrossRef]
- Włochowicz, A.; Stelmasiak, E. Influence of ammonia on the fine structure of wool fibre. Acta Polym. 1983, 34, 428–433. [Google Scholar] [CrossRef]
- Hermiston, B.N.; Pitts, J.M.D. The surface characteristics of wool fibres treated in liquid ammonia. J. Text. I. 1976, 67, 72–76. [Google Scholar] [CrossRef]
- Wakida, T.; Lee, M.; Sato, Y.; Yanai, Y. Dyeing properties of wool treated with liquid ammonia. J. Soc. Dyers. Colour. 1993, 109, 393–397. [Google Scholar] [CrossRef]
- Navik, R.; Shafiq, F.; Khan, A.; Datta, M.; Peng, X.Y.; Kamruzzaman, M.; Cai, Y.J. Preparation and characterizations of polypyrrole on liquid ammonia pre-treated wool fabric. Fiber Polym. 2017, 18, 1115–1123. [Google Scholar] [CrossRef]
- Włochowicz, A.; Stelmasiak, E. Change in thermal properties of wool after treatment with liquid ammonia. J. Therm. Anal. Calorim. 1983, 26, 17–22. [Google Scholar] [CrossRef]
- Osa, R.A.D.L.; Iparragirre, I.; Ortiz, D.; Saiz, J.M. The extended Kubelka-Munk theory and its application to spectroscopy. Chem. Text. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Tse, S.T.; Kan, C.W. Effect of laser treatment on pigment printing on denim fabric-A study of colour properties. Fiber Polym. 2022, 23, 728–735. [Google Scholar] [CrossRef]
- Jurdana, L.E.; Ghiggino, K.P.; Nugent, K.W.; Leaver, I.H. Confocal Laser Raman Microprobe Studies of Keratin Fibers. Text. Res. J. 1995, 65, 593–600. [Google Scholar] [CrossRef]
- Jones, D.C.; Carr, C.M.; Cooke, W.D.; Lewis, D.M. Investigating the photo-oxidation of wool using FT-Raman and FT-IR spectroscopies. Text. Res. J. 1998, 68, 739–748. [Google Scholar] [CrossRef]
- Liu, H.L.; Yu, W.D. Study of the structure transformation of wool fibers with Raman spectroscopy. J. Appl. Polym. Sci. 2007, 103, 1–7. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, N.; Wang, Q.; Wang, P.; Yu, Y.Y.; Yuan, J.G. A controlled, highly effective and sustainable approach to the surface performance improvement of wool fibers. J. Mol. Liq. 2021, 322, 114952. [Google Scholar] [CrossRef]
- Wojciechowska, E.; Rom, M.; Włochowicz, A.; Wysocki, M.; Wesełucha-Birczyńska, A. The use of Fourier transform-infrared (FTIR) and Raman spectroscopy (FTR) for the investigation of structural changes in wool fiber keratin after enzymic treatment. J. Mol. Struct. 2004, 704, 315–321. [Google Scholar] [CrossRef]
- Lewis, D.M. The dyeing of wool with reactive dyes. Color. Technol. 1982, 98, 165–175. [Google Scholar] [CrossRef]
- Ding, C.; Yu, J.Y.; Zhang, R.Y.; Li, H.; Cheng, L.D.; Qin, G. Research on low damage and temperature dyeing performance of superfine wool. J. Text. Res. 2016, 37, 72–75. [Google Scholar] [CrossRef]
- Abdel-Fattah, S.H.; El-Khatib, E.M. Low Temperature Chrome Dyeing of Wool. J. Nat. Fiber. 2007, 2, 53–61. [Google Scholar] [CrossRef]
- Hassan, M.M.; Bhagvandas, M. Sustainable low liquor ratio dyeing of wool with acid dyes: Effect of auxiliaries on agglomeration of dye molecules in a dyebath and dyeing uniformity. J. Clean. Prod. 2017, 152, 464–473. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).