Elements and Chemical Bonds Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures
Abstract
1. Introduction
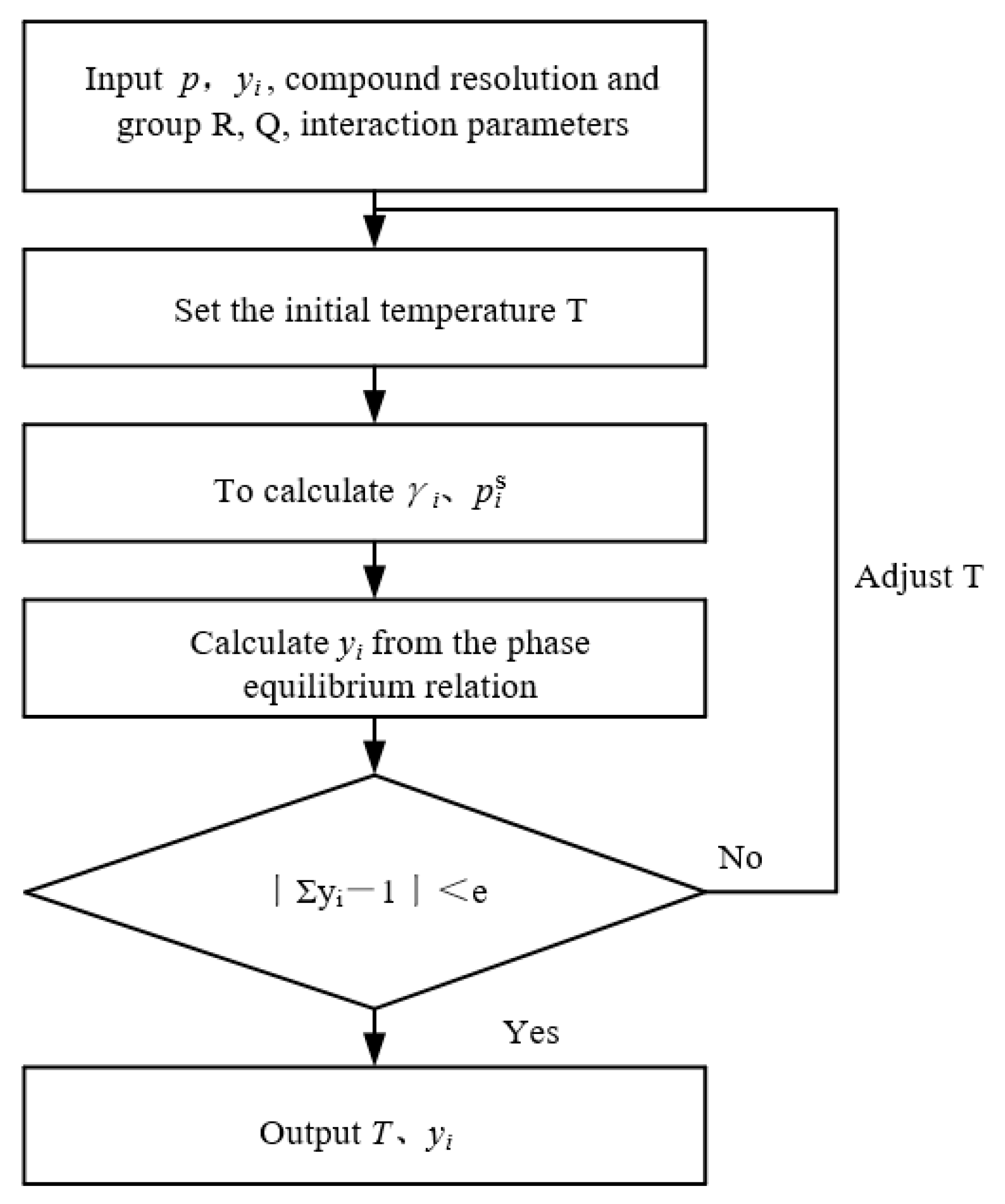
2. Method and Property Modeling
2.1. Data
2.2. Groups
2.3. Models
2.4. Group Interaction Parameters
3. Results and Discussion
- Binary systems included in the database;
- Binary systems not included in the database;
- Ternary systems.
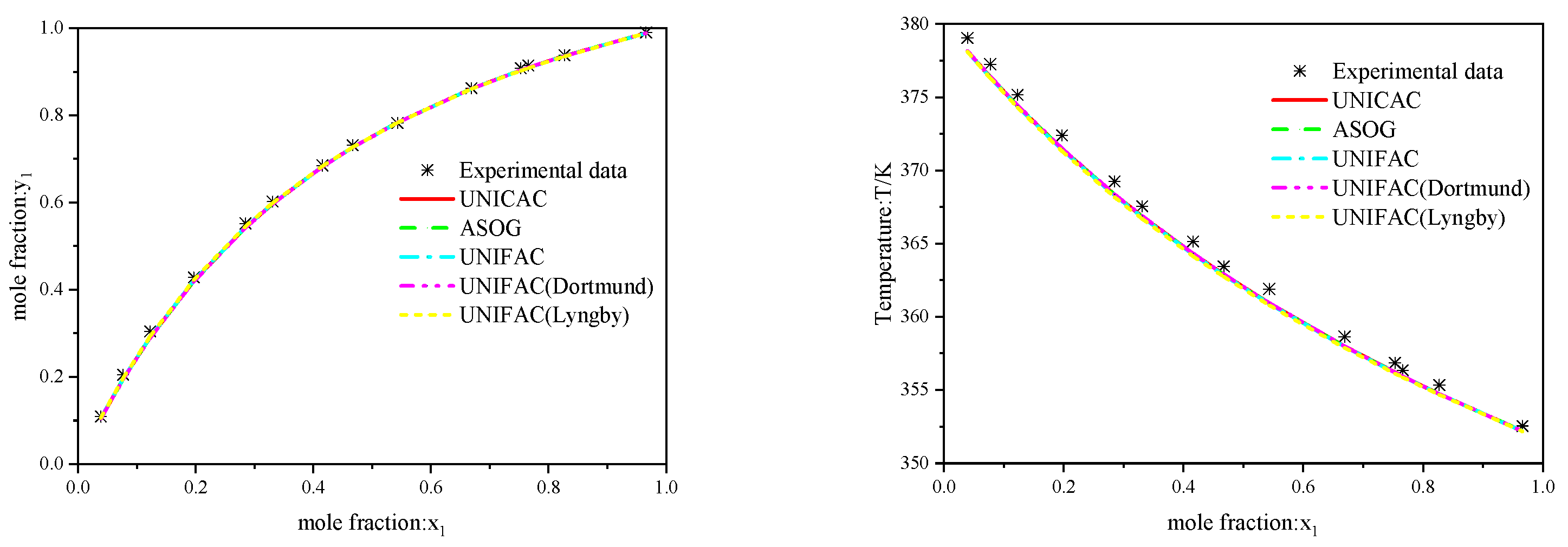
3.1. Binary Systems Included in the Database
3.2. Binary Systems Not Included in the Database
3.3. Ternary Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| n | The number of data sets for VLEs |
| N | The number of systems can be predicted using the functional groups of the selected method |
| P | Pressure |
| q | Pure component area parameter |
| Q | Group area parameter |
| r | Pure component volume parameter |
| R | Gas constant (without subscript) |
| Rk | Group volume parameter (with subscript) |
| T | Temperature |
| akl | UNICAC binary interaction parameter |
| x | Liquid-phase mole fraction |
| X | Liquid-phase group fraction |
| y | Vapor-phase mole fraction |
| z | Lattice coordination number, a constant here set, equal to ten |
| Activity coefficient of component i | |
| The group residual activity coefficient | |
| The residual activity coefficient of group k in a reference solution containing only molecules of type i | |
| The mole fraction of component i | |
| The area fraction | |
| The segment fraction | |
| C | Combinatorial |
| R | Residual |
| i, j, k | Components i, j, and k |
| ΔP | The mean absolute deviation for pressure to experimental values |
| ΔT | The mean absolute deviation for temperature to experimental values |
| Δy | The mean absolute deviation for vapor-phase composition to experimental values |
| ARDP | The mean relative deviation for pressure to experimental values |
| ARDT | The mean relative deviation for temperature to experimental values |
| ARDy | The mean relative deviation for vapor-phase composition to experimental values |
Appendix A
| k | H2O | C | H | O | N | S | F | Cl | Br | |
|---|---|---|---|---|---|---|---|---|---|---|
| j | ||||||||||
| H2O | 0.000 | 3050.144 | 2828.223 | 1216.658 | 1389.824 | −257.189 | 100.000 | 1206.203 | 100.000 | |
| C | −49.102 | 0.000 | 1700.283 | 571.297 | 205.332 | 757.536 | 138.321 | −201.219 | 536.176 | |
| H | −3.718 | 2873.440 | 0.000 | 98.958 | 123.882 | 342.010 | 39.479 | 461.176 | 407.430 | |
| O | −1062.727 | −305.461 | 1599.359 | 0.000 | 889.030 | −628.877 | 457.628 | −37.052 | −462.539 | |
| N | −1058.869 | 312.450 | 1277.751 | 1471.891 | 0.000 | 20.124 | −349.945 | −169.885 | −85.786 | |
| S | 846.310 | 229.472 | 780.556 | 537.378 | −143.997 | 0.000 | 100.000 | −25.555 | 100.000 | |
| F | 100.000 | 596.773 | 618.385 | −146.405 | 65.505 | 100.000 | 0.000 | 389.055 | −44.514 | |
| Cl | −464.586 | 101.578 | 274.945 | 211.270 | 1158.592 | 102.975 | 724.708 | 0.000 | −37.348 | |
| Br | 100.000 | 287.079 | 237.387 | 245.886 | 67.844 | 100.000 | −468.308 | −121.071 | 0.000 | |
| I | 100.000 | 441.250 | 211.749 | 661.227 | 100.000 | 100.000 | 100.000 | −170.467 | 100.000 | |
| Si | 100.000 | 265.550 | −33.247 | 292.652 | 100.000 | 100.000 | 100.000 | 321.030 | 100.000 | |
| C–C | −208.889 | 613.609 | 2327.050 | 1819.310 | 442.833 | 58.474 | −170.543 | 135.599 | 518.587 | |
| C=C | 106.845 | 2039.925 | 309.397 | 0.801 | 581.564 | −0.672 | 100.000 | 24.489 | 100.000 | |
| C≡C | 100.000 | −253.836 | 919.003 | 100.000 | 100.000 | 100.000 | 100.000 | 520.177 | 100.000 | |
| C–O | 21.533 | 544.100 | 2901.417 | 2596.894 | 657.528 | 638.406 | 374.031 | 707.922 | 853.301 | |
| C=O | 180.846 | 1656.696 | 1414.548 | 2663.021 | 355.356 | 597.766 | 413.476 | 413.183 | 100.000 | |
| O–H | −54.190 | 1668.997 | 2049.469 | 856.008 | 2044.495 | 468.492 | 1437.554 | 1256.615 | 2696.125 | |
| C–H | −460.178 | −830.150 | 1785.944 | −853.732 | 814.801 | −436.887 | 119.278 | 602.441 | −615.367 | |
| C–F | 100.000 | −774.669 | 1369.966 | −967.107 | 143.604 | 100.000 | 902.691 | −30.472 | −400.790 | |
| C–CL | −541.494 | 747.975 | 1172.662 | 732.129 | −26.174 | 708.055 | 424.076 | −481.656 | −188.007 | |
| C–Br | 100.000 | 261.758 | 376.489 | 170.260 | 47.283 | 100.000 | −184.911 | 275.332 | −59.922 | |
| C–I | 100.000 | 440.046 | 203.820 | 659.385 | 100.000 | 100.000 | 100.000 | −102.982 | 100.000 | |
| C–S | −818.786 | 474.728 | 1132.954 | 340.821 | 100.000 | −793.953 | 100.000 | −316.956 | 100.000 | |
| C–Si | 100.000 | 342.567 | 34.080 | 393.668 | 100.000 | 100.000 | 100.000 | −47.721 | 100.000 | |
| Si–Cl | 100.000 | −439.997 | 313.197 | 225.691 | 100.000 | 100.000 | 100.000 | 334.908 | 100.000 | |
| Si–H | 100.000 | 153.966 | 565.312 | 192.945 | 100.000 | 100.000 | 100.000 | −70.471 | 100.000 | |
| Si–O | 100.000 | 66.977 | 105.676 | 110.041 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C=S | 100.000 | −379.835 | 559.488 | −135.257 | 100.000 | 799.209 | 100.000 | −144.947 | 100.000 | |
| S–H | 100.000 | 156.071 | 102.836 | 100.000 | 100.000 | 37.410 | 100.000 | 100.000 | 100.000 | |
| S=O | 726.193 | 159.024 | 221.009 | 74.627 | 100.000 | −28.386 | 100.000 | −221.204 | 100.000 | |
| C–N | −1952.965 | −1652.983 | 1530.693 | −1625.916 | 820.715 | 93.345 | 358.580 | 413.852 | 32.076 | |
| C=N | 100.000 | 431.147 | −56.654 | 283.796 | 283.797 | 100.000 | 100.000 | 270.226 | 100.000 | |
| C≡N | 134.236 | 1241.637 | 1907.937 | 283.835 | −178.635 | 100.000 | 100.000 | 25.700 | 100.000 | |
| N–H | 2305.362 | 552.196 | 1130.348 | 1308.921 | 1356.805 | 100.000 | 100.000 | 1902.413 | 100.000 | |
| N–O | 100.000 | 295.673 | 477.315 | −147.975 | −147.974 | 100.000 | 100.000 | 100.000 | 100.000 | |
| NO2 | 100.000 | 749.446 | 511.939 | −97.284 | −363.979 | 144.011 | 100.000 | 5.617 | −259.222 | |
| benzene ring | −1449.963 | 1199.247 | 321.987 | 518.333 | 456.047 | 100.000 | −504.803 | −20.526 | 132.280 | |
| c–C–C | −371.653 | 303.435 | 166.746 | 1440.309 | 359.953 | 540.339 | 111.453 | 1701.947 | 100.000 | |
| c–C=C | −950.320 | 596.565 | 781.186 | −525.182 | 403.778 | −145.468 | 192.259 | −20.109 | 100.000 | |
| c–C–O | −1041.847 | 1463.739 | 1616.941 | −1002.002 | 313.169 | 100.000 | 100.000 | −820.396 | 100.000 | |
| c–C–S | 100.000 | 138.671 | 383.112 | −41.619 | 27.643 | −12.862 | 100.000 | 230.860 | 100.000 | |
| c–C–N | −906.042 | 1685.589 | 1555.754 | 1026.512 | −532.090 | 100.000 | 100.000 | −9.057 | 100.000 | |
| c–C=N | 69.075 | 353.717 | 420.888 | 507.191 | 162.534 | 100.000 | 100.000 | 101.612 | 100.000 | |
| k | I | Si | C–C | C=C | C≡C | C–O | C=O | O–H | C–H | |
| j | ||||||||||
| H2O | 100.000 | 100.000 | 968.856 | 1302.196 | 100.000 | 2351.668 | 270.868 | 221.262 | 2742.986 | |
| C | 18.127 | 127.745 | 209.497 | −150.495 | −16.663 | 119.445 | 1516.056 | 304.543 | 2404.675 | |
| H | 421.015 | 278.618 | 2328.983 | 240.528 | 244.831 | −126.927 | −93.161 | 73.297 | 2673.215 | |
| O | −742.230 | 542.032 | 895.263 | −433.118 | 100.000 | −810.412 | −1118.597 | −891.500 | −219.268 | |
| N | 100.000 | 100.000 | 1619.787 | 931.205 | 100.000 | 1696.658 | 368.314 | 135.428 | −98.845 | |
| S | 100.000 | 100.000 | 7.527 | 305.250 | 100.000 | −282.557 | 620.096 | 526.984 | 610.282 | |
| F | 100.000 | 100.000 | −98.852 | 100.000 | 100.000 | −289.695 | 283.065 | −274.047 | 533.812 | |
| Cl | −361.900 | −283.849 | −257.975 | 265.478 | 630.377 | −366.450 | 50.348 | −335.161 | 660.563 | |
| Br | 100.000 | 100.000 | 438.239 | 100.000 | 100.000 | 350.883 | 100.000 | 202.701 | −690.481 | |
| I | 0.000 | 100.000 | 435.601 | 100.000 | 100.000 | 299.981 | 566.429 | 509.232 | −316.375 | |
| Si | 100.000 | 0.000 | 177.628 | 100.000 | 100.000 | 171.594 | 233.123 | 171.593 | −188.973 | |
| C–C | 596.769 | 231.042 | 0.000 | −73.579 | −126.760 | 404.205 | 1591.987 | 1516.314 | −22.436 | |
| C=C | 100.000 | 100.000 | 1426.824 | 0.000 | 14.965 | 56.577 | −73.713 | −12.606 | 1688.310 | |
| C≡C | 100.000 | 100.000 | 474.952 | 514.193 | 0.000 | 100.000 | 100.000 | 100.000 | 919.003 | |
| C–O | −56.474 | 161.983 | −68.851 | 600.696 | 100.000 | 0.000 | 1035.930 | 2641.120 | 2520.279 | |
| C=O | 99.877 | 1001.694 | 2609.060 | 332.559 | 100.000 | 985.583 | 0.000 | 2401.832 | 862.502 | |
| O–H | 907.583 | 831.332 | 1610.931 | 1338.807 | 100.000 | 878.186 | −95.513 | 0.000 | 1972.909 | |
| C–H | −980.332 | −270.054 | −774.930 | −729.573 | 366.223 | 1240.032 | −693.036 | −941.209 | 0.000 | |
| C–F | 100.000 | 100.000 | −608.179 | 100.000 | 100.000 | 99.559 | 55.450 | −958.187 | 1358.684 | |
| C–CL | −526.103 | 125.445 | 529.495 | −244.739 | 698.727 | −313.712 | 167.680 | 116.231 | −88.721 | |
| C–Br | 100.000 | 100.000 | 763.867 | 100.000 | 100.000 | 188.669 | 100.000 | 12.756 | 450.176 | |
| C–I | −955.091 | 100.000 | 433.768 | 100.000 | 100.000 | 297.532 | 564.483 | 508.589 | −358.160 | |
| C–S | 100.000 | 100.000 | −40.450 | 100.000 | 100.000 | 257.259 | 100.000 | 257.259 | 1112.183 | |
| C–Si | 100.000 | −25.401 | 164.683 | 100.000 | 100.000 | 311.277 | 206.104 | 311.278 | −238.823 | |
| Si–Cl | 100.000 | −21.198 | −6.673 | 100.000 | 100.000 | 79.200 | 242.662 | 79.200 | 210.578 | |
| Si–H | 100.000 | −202.672 | −104.099 | 100.000 | 100.000 | 76.197 | 212.738 | 76.197 | 676.158 | |
| Si–O | 100.000 | 122.537 | −8.462 | 100.000 | 100.000 | 99.003 | 99.004 | 99.004 | 111.281 | |
| C=S | 100.000 | 100.000 | 106.553 | 297.841 | 100.000 | −517.212 | 270.810 | 209.479 | 563.369 | |
| S–H | 100.000 | 100.000 | 30.122 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 164.249 | |
| S=O | 100.000 | 100.000 | 306.549 | 100.000 | 100.000 | 180.701 | 100.000 | 180.700 | 156.449 | |
| C–N | 100.000 | 100.000 | 1140.892 | 206.548 | 100.000 | 483.348 | 366.950 | −1929.931 | 1602.708 | |
| C=N | 100.000 | 100.000 | −318.830 | 100.000 | 100.000 | 100.000 | 455.538 | −760.586 | 371.501 | |
| C≡N | 100.000 | 100.000 | 1180.078 | 446.813 | 100.000 | 617.379 | −380.671 | 27.610 | 1528.699 | |
| N–H | 100.000 | 100.000 | 199.241 | 100.000 | 100.000 | 1341.261 | 323.225 | −713.014 | 712.496 | |
| N–O | 100.000 | 100.000 | 324.138 | 100.000 | 100.000 | 100.000 | 100.000 | −147.975 | 547.948 | |
| NO2 | 100.000 | 100.000 | 168.900 | −37.728 | 100.000 | 31.076 | 100.000 | 31.073 | 462.391 | |
| benzene ring | 100.000 | 200.904 | −543.789 | 41.236 | 157.386 | −292.539 | 965.465 | −185.123 | −850.751 | |
| c–C–C | 100.000 | −92.927 | −90.262 | 438.407 | 100.000 | −111.877 | 1141.029 | 327.991 | 924.262 | |
| c–C=C | 100.000 | 100.000 | −734.337 | −527.144 | 100.000 | 724.998 | −253.632 | −994.849 | 640.254 | |
| c–C–O | 100.000 | 100.000 | −596.487 | −157.762 | 100.000 | 991.679 | 260.356 | 762.434 | −119.931 | |
| c–C–S | 100.000 | 100.000 | −205.340 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 383.112 | |
| c–C–N | 100.000 | 100.000 | −78.077 | 643.516 | 100.000 | 958.297 | 890.945 | −1315.329 | 1647.429 | |
| c–C=N | 100.000 | 100.000 | −377.239 | 735.308 | 100.000 | 732.853 | 116.042 | −1300.877 | 371.991 | |
| k | C–F | C–Cl | C–Br | C–I | C–S | C–Si | Si–Cl | Si–H | Si–O | |
| j | ||||||||||
| H2O | 100.000 | 1206.203 | 100.000 | 100.000 | −277.262 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C | 138.974 | 1580.026 | 536.175 | 18.169 | −62.264 | 149.354 | 332.342 | −207.322 | 76.969 | |
| H | 1482.545 | 294.968 | 407.443 | 421.023 | −403.976 | −435.751 | 454.069 | 142.780 | −93.798 | |
| O | 457.629 | −833.956 | −462.535 | −742.226 | −707.962 | 878.587 | 339.292 | 239.634 | 99.963 | |
| N | −349.947 | −175.735 | −85.799 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| S | 100.000 | −25.570 | 100.000 | 100.000 | −196.442 | 100.000 | 100.000 | 100.000 | 100.000 | |
| F | −101.796 | 389.056 | −44.512 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| Cl | 724.708 | 794.903 | −37.301 | −361.387 | −338.789 | 5.450 | 4.022 | −186.197 | 100.000 | |
| Br | −468.311 | −121.141 | 420.256 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| I | 100.000 | −170.478 | 100.000 | −884.219 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| Si | 100.000 | 388.771 | 100.000 | 100.000 | 100.000 | −44.635 | −117.154 | 138.844 | 117.939 | |
| C–C | −163.946 | 131.864 | 518.586 | 596.769 | 470.685 | 426.464 | 237.688 | 171.776 | 19.119 | |
| C=C | 100.000 | 24.511 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C≡C | 100.000 | 520.177 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–O | 374.031 | 892.752 | 853.301 | −56.508 | 644.208 | −170.901 | −6.566 | 41.375 | 169.707 | |
| C=O | 413.476 | 404.241 | 100.000 | 99.898 | 100.000 | 1386.286 | 558.328 | 413.466 | −63.068 | |
| O–H | 1437.554 | 1229.614 | 2696.125 | 907.583 | −81.309 | 1540.552 | 542.096 | 387.761 | −154.598 | |
| C–H | −643.936 | −812.503 | −615.293 | −980.336 | −465.344 | −959.113 | −762.710 | −54.605 | 159.073 | |
| C–F | 0.000 | −30.477 | −400.798 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–CL | 424.076 | 0.000 | −188.008 | −526.083 | 1138.813 | 100.000 | 208.033 | 100.000 | 100.000 | |
| C–Br | −184.917 | 275.312 | 0.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–I | 100.000 | −102.976 | 100.000 | 0.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–S | 100.000 | −316.892 | 100.000 | 100.000 | 0.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–Si | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 0.000 | −47.731 | 67.721 | 138.624 | |
| Si–Cl | 100.000 | 476.274 | 100.000 | 100.000 | 100.000 | −255.322 | 0.000 | 273.760 | 100.000 | |
| Si–H | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 70.159 | −70.331 | 0.000 | 100.000 | |
| Si–O | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 166.585 | 100.000 | 100.000 | 0.000 | |
| C=S | 100.000 | −144.945 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| S–H | 100.000 | 100.000 | 100.000 | 100.000 | 37.409 | 100.000 | 100.000 | 100.000 | 100.000 | |
| S=O | 100.000 | −221.206 | 100.000 | 100.000 | −189.311 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–N | 358.580 | 413.888 | 32.076 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C=N | 100.000 | 270.217 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C≡N | 100.000 | 25.695 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| N–H | 100.000 | 1902.413 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| N–O | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| NO2 | 100.000 | 5.666 | −259.227 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| benzene ring | −504.800 | −286.526 | 132.279 | 100.000 | 100.000 | 132.231 | 279.280 | 194.465 | 100.000 | |
| c–C–C | 111.454 | −398.397 | 100.000 | 100.000 | 281.620 | 100.000 | −178.159 | −92.909 | 100.000 | |
| c–C=C | 192.260 | −19.893 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| c–C–O | 100.000 | −819.599 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| c–C–S | 100.000 | 230.860 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| c–C–N | 100.000 | −9.061 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| c–C=N | 100.000 | 101.611 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| k | C=S | S–H | S=O | C–N | C=N | C≡N | N–H | N–O | NO2 | |
| j | ||||||||||
| H2O | 100.000 | 100.000 | −257.188 | 990.677 | 100.000 | 382.555 | 184.834 | 100.000 | 100.000 | |
| C | 94.306 | 161.761 | 45.536 | 111.353 | 572.409 | 648.163 | 40.330 | 638.395 | −17.545 | |
| H | −64.946 | 46.822 | −241.549 | −31.576 | −345.132 | −450.802 | 119.516 | 828.881 | 712.230 | |
| O | −241.929 | 100.000 | 25.715 | 1324.313 | 205.694 | −880.684 | 880.701 | −355.803 | −973.086 | |
| N | 100.000 | 100.000 | 100.000 | −914.472 | 291.981 | −956.003 | 1328.214 | −496.665 | −137.784 | |
| S | −351.720 | −94.221 | 33.313 | −143.998 | 100.000 | 100.000 | 100.000 | 100.000 | −143.997 | |
| F | 100.000 | 100.000 | 100.000 | 53.785 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| Cl | 99.021 | 100.000 | 62.633 | −381.884 | −442.091 | 721.657 | −373.448 | 100.000 | −29.934 | |
| Br | 100.000 | 100.000 | 100.000 | 67.924 | 100.000 | 100.000 | 100.000 | 100.000 | 67.807 | |
| I | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| Si | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–C | −434.381 | 69.707 | 335.332 | 1790.894 | −205.603 | 294.991 | 41.932 | 440.890 | −624.975 | |
| C=C | −0.439 | 100.000 | 100.000 | 364.448 | 100.000 | −55.299 | 100.000 | 100.000 | −317.034 | |
| C≡C | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–O | 568.968 | 100.000 | 456.565 | 235.841 | 100.000 | −261.918 | 323.491 | 100.000 | −73.121 | |
| C=O | 597.765 | 100.000 | 100.000 | −94.205 | 522.127 | −110.526 | 274.243 | 100.000 | 100.000 | |
| O–H | 476.542 | 100.000 | 4.210 | 922.708 | 996.941 | 49.129 | 1094.051 | 996.941 | 1169.604 | |
| C–H | −165.808 | 114.915 | −122.184 | 1398.731 | 226.569 | 652.083 | 116.868 | 393.119 | −704.437 | |
| C–F | 100.000 | 100.000 | 100.000 | 239.166 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–CL | −302.206 | 100.000 | 931.013 | 660.485 | −283.517 | 267.171 | −190.958 | 100.000 | −301.834 | |
| C–Br | 100.000 | 100.000 | 100.000 | 47.282 | 100.000 | 100.000 | 100.000 | 100.000 | 47.309 | |
| C–I | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–S | 100.000 | 186.230 | −6.368 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–Si | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| Si–Cl | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| Si–H | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| Si–O | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C=S | 0.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| S–H | 100.000 | 0.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| S=O | 100.000 | 100.000 | 0.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |
| C–N | 100.000 | 100.000 | 100.000 | 0.000 | 277.156 | 523.501 | −1012.995 | 100.000 | −799.139 | |
| C=N | 100.000 | 100.000 | 100.000 | 455.538 | 0.000 | 100.000 | 100.000 | −760.569 | 100.000 | |
| C≡N | 100.000 | 100.000 | 100.000 | 1278.710 | 100.000 | 0.000 | 1543.830 | 100.000 | 218.230 | |
| N–H | 100.000 | 100.000 | 100.000 | 146.828 | 100.000 | 376.480 | 0.000 | 100.000 | 100.000 | |
| N–O | 100.000 | 100.000 | 100.000 | 100.000 | −147.973 | 100.000 | 100.000 | 0.000 | 100.000 | |
| NO2 | 100.000 | 100.000 | 100.000 | −431.148 | 100.000 | 109.741 | 100.000 | 100.000 | 0.000 | |
| benzene ring | 100.000 | 100.000 | 100.000 | −130.231 | 100.000 | 490.002 | −836.341 | 100.000 | 214.480 | |
| c–C–C | −212.056 | 281.620 | 100.000 | 591.386 | 100.000 | 474.862 | −165.708 | 100.000 | 382.157 | |
| c–C=C | 100.000 | 100.000 | 100.000 | 149.690 | 100.000 | 332.719 | 100.000 | 100.000 | 149.689 | |
| c–C–O | 100.000 | 100.000 | 100.000 | −842.900 | 100.000 | 100.000 | −814.137 | 100.000 | 487.471 | |
| c–C–S | 100.000 | 100.000 | 100.000 | 27.668 | 100.000 | 100.000 | 100.000 | 100.000 | 27.668 | |
| c–C–N | 100.000 | 100.000 | 100.000 | 534.156 | 100.000 | 202.015 | 94.093 | 100.000 | 100.000 | |
| c–C=N | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 317.165 | 100.000 | 100.000 | 100.000 | |
| k | Benzene Ring | c–C–C | c–C=C | c–C–O | c–C–S | c–C–N | c–C=N | |||
| j | ||||||||||
| H2O | 2428.601 | 873.972 | 595.148 | 892.328 | 100.000 | 882.580 | 1299.519 | |||
| C | 2342.979 | −18.356 | −194.010 | −19.844 | 541.556 | −224.601 | −350.159 | |||
| H | −137.045 | 878.648 | −95.298 | −210.397 | 419.870 | 898.204 | 278.609 | |||
| O | −1125.752 | −364.521 | −1018.988 | −938.065 | −196.273 | 432.264 | 190.541 | |||
| N | 25.391 | −295.645 | −999.407 | 518.323 | −75.219 | −557.928 | −68.028 | |||
| S | 100.000 | −303.958 | 11.924 | 100.000 | 11.693 | 100.000 | 100.000 | |||
| F | −710.003 | −339.403 | 208.905 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| Cl | −493.659 | −21.130 | −327.417 | 167.558 | 232.735 | −199.888 | −199.938 | |||
| Br | 93.599 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| I | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| Si | 151.251 | −363.068 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C–C | −199.914 | 340.715 | 219.161 | 139.458 | −53.684 | 405.048 | −265.779 | |||
| C=C | 640.175 | 88.567 | −171.285 | −111.968 | 100.000 | 917.352 | 917.352 | |||
| C≡C | 184.563 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C–O | −259.606 | 90.208 | 280.205 | 270.645 | 100.000 | 591.009 | 591.005 | |||
| C=O | −101.292 | 1257.209 | −106.909 | 930.691 | 100.000 | −182.817 | 171.861 | |||
| O–H | 610.997 | 2118.223 | 2240.058 | 614.914 | 100.000 | 2023.057 | 2023.057 | |||
| C–H | −616.763 | −767.783 | 814.025 | 835.370 | 186.503 | 212.721 | −174.054 | |||
| C–F | 135.777 | −619.326 | 177.583 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C–Cl | −391.580 | 1024.101 | −427.959 | 386.027 | 133.660 | 887.304 | 887.304 | |||
| C–Br | 52.191 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C–I | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C–S | 100.000 | 10.317 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C–Si | 220.297 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| Si–Cl | −120.496 | 465.756 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| Si–H | −234.096 | 670.426 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| Si–O | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C=S | 100.000 | 88.545 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| S–H | 100.000 | 253.140 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| S=O | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C–N | −1837.934 | −1291.486 | 86.820 | 205.080 | 86.820 | 42.246 | 100.000 | |||
| C=N | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| C≡N | −284.559 | 685.778 | 685.778 | 100.000 | 100.000 | 479.422 | 479.422 | |||
| N–H | −51.561 | 1263.565 | 100.000 | 466.869 | 100.000 | 226.896 | 100.000 | |||
| N–O | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | |||
| NO2 | 179.307 | 561.474 | 194.215 | 731.096 | 194.215 | 100.000 | 100.000 | |||
| benzene ring | 0.000 | 625.329 | 583.208 | 642.880 | 100.000 | 71.630 | −19.934 | |||
| c–C–C | 410.757 | 0.000 | 529.782 | −197.735 | 184.022 | 916.388 | 293.016 | |||
| c–C=C | −1071.029 | −907.829 | 0.000 | −1074.457 | −702.276 | 123.804 | 123.695 | |||
| c–C–O | −1012.367 | 283.298 | −265.763 | 0.000 | 100.000 | 248.190 | 100.000 | |||
| c–C–S | 100.000 | 510.702 | −92.852 | 100.000 | 0.000 | 100.000 | 100.000 | |||
| c–C–N | 750.502 | −355.021 | −364.322 | −593.329 | 100.000 | 0.000 | −292.099 | |||
| c–C=N | −350.334 | 685.453 | −540.339 | 100.000 | 100.000 | −125.066 | 0.000 | |||
References
- Shang, Q.; Xiao, J.; Liu, X.; Ling, Y.; Liu, W.; Cui, G.; Shi, X.; Xia, S.; Tang, B. Isobaric vapor-liquid equilibrium and distillation process design for separating ketones in biomass pyrolysis oil. J. Chem. Thermodyn. 2022, 164, 106622. [Google Scholar] [CrossRef]
- Domańska, U. Experimental data of fluid phase equilibria-correlation and prediction models: A review. Processes 2019, 7, 277. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yeh, M.H.; Chen, Y.Y.; Chen, C.L. Design and control of a comprehensive ethylenediamine (EDA) process with external/internal heat integration. Sep. Purif. Technol. 2022, 293, 121137. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Folas, G.K. Thermodynamic Models for Industrial Applications: From Classical and Advanced Mixing Rules to Association Theories; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Prausnitz, J.M.; Lichtenthaler, R.N.; Azevedo, E.G. Molecular Thermodynamics of Fluid-Equilibria, 3rd ed.; Pearson Education: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Lohmann, J.; Joh, R.; Gmehling, J. From UNIFAC to modified UNIFAC (Dortmund). Ind. Eng. Chem. Res. 2001, 40, 957–964. [Google Scholar] [CrossRef]
- Tochigi, K.; Tiegs, D.; Gmehling, J. Determination of new ASOG parameters. J. Chem. Eng. Jpn. 1990, 23, 453–463. [Google Scholar] [CrossRef]
- Tochigi, K.; Gmehling, J. Determination of new ASOG parameters-extension and revision. J. Chem. Eng. Jpn. 2011, 44, 304–306. [Google Scholar] [CrossRef]
- Robles, P.A.; Morales, J.W.; Cisternas, L.A. Modeling of liquid-liquid equilibrium for binary and ternary systems containing ionic liquids with the hexafluorophosphate anion using the ASOG method. Fluid Phase Equilib. 2016, 429, 119–126. [Google Scholar] [CrossRef]
- Fredenslund, A.; Jones, R.L.; Prausnitz, J.M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Fredenslund, A.; Gmehling, J.; Rasmussen, P. Vapor-Liquid Equilibria Using UNIFAC: A Group-Contribution Method; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Jørgensen, S.S.; Kolbe, B.; Gmehling, J.; Rasmussen, P. Vapor-liquid equilibria by UNIFAC group contribution. revision and extension. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 714–722. [Google Scholar] [CrossRef]
- Gmehling, J.; Rasmussen, P.; Fredenslund, A. Vapor-liquid equilibria by UNIFAC group contribution. revision and extension 2. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 118–127. [Google Scholar] [CrossRef]
- Macedo, E.A.; Weidlich, U.; Gmehling, J.; Rasmussen, P. Vapor-liquid equilibria by UNIFAC group contribution. revision and extension 3. Ind. Eng. Chem. Process Des. Dev. 1983, 22, 676–678. [Google Scholar] [CrossRef]
- Tiegs, D.; Gmehling, J.; Rasmussen, P.; Fredenslund, A. Vapor-liquid equilibria by UNIFAC group contribution. Revision and extension 4. Ind. Eng. Chem. Res. 1987, 26, 159–161. [Google Scholar] [CrossRef]
- Hansen, H.K.; Rasmussen, P.; Fredenslund, A.; Schiller, M.; Gmehling, J. Vapor-liquid equilibria by UNIFAC group contribution. revision and extension 5. Ind. Eng. Chem. Res. 1991, 30, 2352–2355. [Google Scholar] [CrossRef]
- Wittig, R.; Lohmann, J.; Gmehling, J. Vapor-liquid equilibria by UNIFAC group contribution. 6 revision and extension. Ind. Eng. Chem. Res. 2003, 42, 183–188. [Google Scholar] [CrossRef]
- Larsen, B.L.; Rasmussen, P.; Fredenslund, A. A modified UNIFAC group-contribution model for prediction of phase equilibria and heats of mixing. Ind. Eng. Chem. Res. 1987, 26, 2274–2286. [Google Scholar] [CrossRef]
- Gmehling, J.; Li, J.; Schiller, M. A modified UNIFAC model. 2. present parameter matrix and results for different thermodynamic properties. Ind. Eng. Chem. Res. 1993, 32, 178–193. [Google Scholar] [CrossRef]
- Gmehling, J.; Lohmann, J.; Jakob, A.; Li, J.; Joh, R. A modified UNIFAC (Dortmund) model. 3. revision and extension. Ind. Eng. Chem. Res. 1998, 37, 4876–4882. [Google Scholar] [CrossRef]
- Gmehling, J.; Wittig, R.; Lohmann, J. A modified UNIFAC (Dortmund) model. 4. revision and extension. Ind. Eng. Chem. Res. 2002, 44, 1678–1688. [Google Scholar] [CrossRef]
- Jakob, A.; Grensemann, H.; Lohmann, J.; Gmehling, J. Further development of modified UNIFAC (Dortmund): Revision and extension 5. Ind. Eng. Chem. Res. 2006, 45, 7924–7933. [Google Scholar] [CrossRef]
- Constantinescu, D.; Gmehling, J. Further development of modified UNIFAC (Dortmund): Revision and extension 6. J. Chem. Eng. Data. 2016, 61, 2738–2748. [Google Scholar] [CrossRef]
- Gonzàlez, H.E.; Abildskov, J.; Gani, R. A method for prediction of UNIFAC group interaction parameters. AIChE J. 2007, 53, 1620–1632. [Google Scholar] [CrossRef]
- Klauck, M.; Richter, S.; Hähnel, T.; Schmelzer, J.; Kalies, G. Modified UNIFAC (Dortmund) parameters for the interaction between the amino group at cycloaliphatic hydrocarbon and the hydroxyl group. Ind. Eng. Chem. Res. 2019, 58, 21730–21738. [Google Scholar] [CrossRef]
- Qin, Y.B.; Zhang, H.; Liu, B.L.; Li, N.X. Experimental measurements and thermodynamic modeling of VLE for strong-zeotropic ternary system of 2,3,3,3-tetrafluoroprop-1-ene (R1234yf)+ ethane (R170)+ tetrafluoromethane (R14). Ind. Eng. Chem. Res. 2019, 58, 2333–2342. [Google Scholar] [CrossRef]
- Nayak, P.R.; Akhouri, B.P. Vapor-liquid equilibrium in methanol+ water system and modeling from 298.15 to 373.15 K. Mater. Today Proc. 2022, 59, 506–509. [Google Scholar] [CrossRef]
- Li, J.; Xia, L.; Xiang, S.G. A new method based on elements and chemical bonds for organic compounds critical properties estimation. Fluid Phase Equilib. 2016, 417, 1–6. [Google Scholar] [CrossRef]
- Gmehling, J.; Onken, U.; Arlt, W. Vapor-Liquid Equilibrium Data Collection; 34 parts DECHEMA chemistry data series; DECHEMA: Frankfurt, Germany, 1977; Volume I. [Google Scholar]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]




| Chemical Bonds | No. | /(cm3·mol−1) | /(109 cm2·mol−1) | Group Assignment Examples |
|---|---|---|---|---|
| H2O | 1 | 0.92 | 1.4 | water: 1(1) |
| C | 2 | 12.39 | 2.19 | butane: 4(2),10(3),3(12),10(18) |
| H | 3 | 4.36 | 1.09 | ethyl alcohol: 2(2),6(3),1(4),1(12),1(15),1(17), 5(18) |
| O | 4 | 8.51 | 1.70 | methyl alcohol: 1(2),4(3),1(4), 1(15),1(17), 3(18) |
| N | 5 | 9.39 | 1.82 | propylamine: 3(2),9(3),1(5),1(15),1(17) |
| S | 6 | 14.70 | 2.45 | n-butyl mercaptan: 4(2),10(3),1(6),3(12),10(18),1(23),1(29) |
| F | 7 | 8.01 | 1.63 | perfluorobenzene: 6(2),6(7),6(19),1(37) |
| Cl | 8 | 13.51 | 2.32 | chloroform: 1(2),1(3),3(8),1(18),3(20) |
| Br | 9 | 15.96 | 2.59 | bromoethane: 2(2),5(3),1(9),1(12),5(18),1(21) |
| I | 10 | 19.57 | 2.97 | ethyl iodide: 2(2),5(3),1(10),1(12),5(18),1(22) |
| Si | 11 | 23.35 | 3.34 | silicontetrachloride: 4(8),1(11),4(25) |
| C-C | 12 | −3.86 | −1.02 | isopentane: 5(2),12(3),4(12),12(18) |
| C=C | 13 | −5.46 | −1.24 | isoprene: 5(2),8(3),2(12),2(13),8(18) |
| C≡C | 14 | −4.84 | −1.34 | vinylacetylene: 4(2),4(3),1(12),1(13),1(14),4(18) |
| C-O | 15 | −4.54 | −1.05 | propanoicacid: 3(2),6(3),2(4),2(12),1(15),1(16),1(17),5(18) |
| C=O | 16 | −4.96 | −1.16 | allyl acetate: 5(2),8(3),2(4),2(12),1(13),2(15),1(16),8(18) |
| O-H | 17 | −2.92 | −0.80 | l-hexanol: 6(2),14(3),1(4),5(12),1(15),1(17),13(18) |
| C-H | 18 | −3.35 | −0.98 | benzene: 6(2),6(3),6(18),1(37) |
| C-F | 19 | −4.48 | −0.97 | dichlorodifluoromethane: 1(2),2(7),2(8),2(19),2(20) |
| C-Cl | 20 | −3.56 | −0.99 | 1,1-dichloroethane: 1(2),2(7),2(8),2(19),2(20) |
| C-Br | 21 | −3.72 | −0.90 | bromoform: 1(2),1(3),3(9),1(18),3(21) |
| C-I | 22 | −2.17 | −1.01 | methyl iodide: 1(2),3(3),1(10),3(18),1(22) |
| C-S | 23 | −4.45 | −1.02 | propyl mercaptan: 3(2),8(3),1(6),2(12),7(18),1(23),1(29) |
| C-Si | 24 | −5.47 | −1.11 | tetra-ethylsilane: 8(2),20(3),1(11),20(18),4(24) |
| Si-Cl | 25 | −4.07 | −1.19 | dichlorophenylsilane: 6(2),6(3),2(8),1(11),5(18),1(24),2(25),1(26),1(27) |
| Si-H | 26 | −3.70 | −0.99 | trichlorosilane: 1(3),3(8),1(11),3(25),1(26) |
| Si-O | 27 | −2.81 | −1.28 | hexamethyldisiloxane: 6(2),18(3),1(4),2(11),18(18),6(24),2(27), 1(26),1(27) |
| C=S | 28 | −0.342 | −0.464 | carbon disulfide: 1(2),2(6),2(28) |
| S-H | 29 | −2.05 | −0.69 | ethyl mercaptan: 2(2),6(3),1(6),1(12),6(18),1(23),1(29) |
| S=O | 30 | −3.19 | −1.04 | dimethylsulfoxide: 2(2),6(3),1(4),1(6),6(18),2(23),1(30) |
| C-N | 31 | −3.05 | −0.87 | diethylamine: 4(2),11(3),1(5),2(12),10(18),2(31),1(34) |
| C=N | 32 | −5.29 | −1.35 | methyl isocyanate: 2(2),3(3),1(4),1(5),1(16),3(18),1(31),1(32) |
| C≡N | 33 | −5.22 | −1.28 | methacrylonitrile: 4(2),5(3),1(5),2(12), 1(13),5(18),1(33) |
| N-H | 34 | −3.32 | −0.77 | aniline: 6(2),7(3),1(5),5(18),1(31),2(34),1(37) |
| N-O | 35 | −3.16 | −0.82 | methylethylketoxim: 4(2),9(3),1(4),1(5),3(12),1(17),8(18),1(32), 1(35) |
| NO2 | 36 | −9.07 | −2.46 | nitromethane: 1(2),3(3),2(4),1(5),3(18),1(31),1(36) |
| benzene | 37 | −29.93 | −7.58 | toluene: 7(2),8(3),1(12),8(18),1(37) |
| c-C-C | 38 | −4.47 | −1.12 | cyclohexane: 6(2),12(3),12(18),6(38) |
| c-C=C | 39 | −5.53 | −1.31 | furan: 4(2),4(3),1(4),4(18),1(38),2(39),2(40) |
| c-C-O | 40 | −4.54 | −1.03 | 1,4-dioxane: 4(2),8(3),2(4),8(18),2(38),4(40) |
| c-C-S | 41 | −4.02 | −0.99 | thiophene: 4(2),4(3),1(6),4(18),1(38),2(39),2(41) |
| c-C-N | 42 | −2.87 | −0.95 | morpholine: 4(2),9(3),1(4),1(5),8(18),1(34),2(38),2(40), 2(42) |
| c-C=N | 43 | −5.89 | −0.79 | pyridine: 5(2),5(3),1(5),5(18),2(38),2(39),1(42),1(43) |
| Methods | N | ARDy | ARDP/ARDT |
|---|---|---|---|
| UNICAC | 1085 | 5.18 | 2.69 |
| UNIFAC | 1050 | 5.26 | 4.18 |
| UNIFAC (Dortmund) | 1048 | 4.94 | 3.75 |
| UNIFAC (Lyngby) | 830 | 5.71 | 4.22 |
| ASOG | 1056 | 6.70 | 4.47 |
| System Family | N | UNICAC | UNIFAC | UNIFAC (Dortmund) | UNIFAC (Lyngby) | ASOG | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ARDT/ ARDT | ARDy | ARDT/ ARDT | ARDy | ARDT/ ARDT | ARDy | ARDT/ ARDT | ARDy | ARDT/ ARDT | ARDy | ||
| watery system | 74 | 4.35 | 10.05 | 1.75 | 6.71 | 5.20 | 6.14 | 1.94 | 9.29 | 8.42 | 12.51 |
| aromatic system | 291 | 1.83 | 3.96 | 2.90 | 4.65 | 2.74 | 4.67 | 2.69 | 7.38 | 4.09 | 5.30 |
| silicon containing system | 12 | 2.82 | 2.39 | 1.66 | 0.68 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| sulfur system | 18 | 1.04 | 1.93 | 5.48 | 3.26 | 5.08 | 2.22 | n.a. | n.a. | 1.37 | 2.57 |
| acid system | 78 | 3.22 | 8.98 | 13.94 | 16.33 | 11.77 | 14.43 | 23.47 | 11.38 | 10.79 | 28.68 |
| ester containing system | 126 | 2.61 | 4.99 | 3.31 | 4.82 | 2.85 | 4.20 | 3.85 | 3.82 | 2.92 | 5.36 |
| aldehyde containing system | 21 | 2.94 | 5.65 | 0.46 | 4.68 | 1.42 | 4.33 | 0.11 | 12.08 | 4.06 | 8.45 |
| ketone containing system | 144 | 2.38 | 4.79 | 1.63 | 3.25 | 1.44 | 4.03 | 4.95 | 3.30 | 1.91 | 3.53 |
| fluorine containing system | 27 | 2.62 | 4.05 | 2.57 | 6.20 | 0.46 | 8.53 | n.a. | n.a. | 4.68 | 6.28 |
| chloride containing system | 221 | 3.02 | 5.84 | 3.91 | 5.22 | 2.06 | 4.16 | 1.91 | 4.14 | 4.06 | 6.46 |
| bromine containing system | 9 | 2.31 | 1.65 | 13.21 | 2.72 | 3.13 | 1.87 | n.a. | n.a. | 22.30 | 2.92 |
| iodine containing system | 8 | 2.09 | 2.71 | 11.32 | 1.43 | 11.23 | 1.30 | n.a. | n.a. | 1.02 | 2.06 |
| nitrogen containing system | 119 | 4.42 | 4.70 | 7.44 | 6.47 | 4.96 | 5.04 | 3.04 | 5.43 | 10.98 | 15.27 |
| alcohol containing system | 399 | 2.07 | 4.19 | 2.86 | 4.62 | 3.06 | 4.74 | 2.64 | 4.65 | 2.77 | 4.42 |
| System | System Number | P or T | n | Δy | ΔT or ΔP |
|---|---|---|---|---|---|
| Component 1 + Component 2 | |||||
| ethanol + 2-methyl-1-propanol | 1 | 101.33 kPa | 14 | 0.0052 | 0.72 K |
| toluene + 3-methyl-1-butanol | 2 | 101.33 kPa | 15 | 0.0196 | 0.58 K |
| acetone + methanol | 3 | 328.15 K | 11 | 0.0132 | 1.36 kPa |
| 1-hexene + 2-butanol | 4 | 333.15 K | 12 | 0.0160 | 3.59 kPa |
| hexane + 2-butanol | 5 | 333.15 K | 11 | 0.0130 | 2.25 kPa |
| hexane + 1-pentanol | 6 | 323.15 K | 13 | 0.0079 | 0.42 kPa |
| benzene + 2-methyl-1-propanol | 7 | 101.33 kPa | 31 | 0.0149 | 0.19 K |
| toluene + 1-pentanol | 8 | 383.15 K | 23 | 0.0126 | 1.92 kPa |
| toluene + 4-methyl-2-pentanone | 9 | 323.15 K | 26 | 0.0065 | 0.32 kPa |
| acetone + benzene | 10 | 318.15 K | 11 | 0.0091 | 0.72 kPa |
| methyl formate + dimethyl carbonate | 11 | 101.33 kPa | 14 | 0.0036 | 0.32 K |
| methanol + epoxy chloropropane | 12 | 101.33 kPa | 10 | 0.0103 | 0.87 K |
| trichlorosilane + silicon tetrachloride | 13 | 98.70 kPa | 11 | 0.0086 | 0.50 K |
| Diethyl disulfide + 2,2,4-trimethylpentane | 14 | 368.15 K | 8 | 0.0389 | 7.59 kPa |
| 1,1-difluoroethane+ methanol | 15 | 101.33 kPa | 4 | 0.3815 | 25.16 K |
| water + propanoic acid | 16 | 343.2 K | 9 | 0.3090 | 23.98 kPa |
| System Number | UNICAC | UNIFAC | UNIFAC (Dortmund) | UNIFAC (Lyngby) | ASOG | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ARDy | ARDP/T | ARDy | ARDP/T | ARDy | ARDP/T | ARDy | ARDP/T | ARDy | ARDP/T | |
| 1 | 1.43 | 0.20 | 1.33 | 0.20 | 1.57 | 0.19 | 1.13 | 0.23 | 1.42 | 0.20 |
| 2 | 3.60 | 1.21 | 3.67 | 0.99 | 2.92 | 1.76 | 3.33 | 1.10 | 3.96 | 1.17 |
| 3 | 2.88 | 1.46 | 1.08 | 0.17 | 0.86 | 0.31 | 1.68 | 0.59 | 1.12 | 0.61 |
| 4 | 2.27 | 5.33 | 1.07 | 2.03 | 0.79 | 1.05 | 0.95 | 1.98 | 0.94 | 1.27 |
| 5 | 1.67 | 3.54 | 1.19 | 1.95 | 0.79 | 1.29 | 1.08 | 1.62 | 1.19 | 2.79 |
| 6 | 1.15 | 1.78 | 2.72 | 7.79 | 0.91 | 1.25 | 2.52 | 7.23 | 3.12 | 8.50 |
| 7 | 2.33 | 0.05 | 2.12 | 0.07 | 1.69 | 0.09 | 1.79 | 0.08 | 2.47 | 0.07 |
| 8 | 2.80 | 2.32 | 2.52 | 2.47 | 2.03 | 0.65 | 2.14 | 0.73 | 2.84 | 1.42 |
| 9 | 1.99 | 2.85 | 4.91 | 4.98 | 0.56 | 0.42 | 0.42 | 0.88 | 2.72 | 2.18 |
| 10 | 2.52 | 1.61 | 1.52 | 0.93 | 1.47 | 1.81 | 1.74 | 0.72 | 1.24 | 0.44 |
| 11 | 0.00 | 0.00 | 0.73 | 0.11 | n.a. | n.a. | n.a. | n.a. | 2.22 | 0.30 |
| 12 | 0.03 | 0.00 | 7.82 | 0.95 | 15.87 | 1.93 | 0.27 | 0.02 | 9.33 | 0.99 |
| 13 | 0.04 | 0.00 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| 14 | 0.23 | 0.15 | 6.34 | 4.74 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| 15 | 1.05 | 0.09 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 26.26 | 5.05 |
| 16 | 0.52 | 1.04 | 7.46 | 9.43 | 5.13 | 6.62 | n.a. | n.a. | 5.96 | 5.59 |
| Average | 1.53 | 1.35 | 3.18 | 2.63 | 2.88 | 1.45 | 1.55 | 1.38 | 4.63 | 2.18 |
| System Number | Component | UNICAC | ASOG | UNIFAC | UNIFAC (Dortmund) | UNIFAC (Lyngby) |
|---|---|---|---|---|---|---|
| 11 | methyl formate | √ | √ | √ | × | × |
| 11 | diethyl carbonate | √ | √ | √ | × | × |
| 13 | trichlorosilane | √ | × | × | × | × |
| 13 | silicon tetrachloride | √ | × | × | × | × |
| 14 | ethyl disulfide | √ | × | √ | × | × |
| 15 | 1,1-difluoroethane | √ | √ | × | × | × |
| 16 | propanoic acid | √ | √ | √ | √ | × |
| System | P or T | n | Δy1 | Δy2 | ΔT or Δp |
|---|---|---|---|---|---|
| Component 1 + Component 2 + Component 3 | |||||
| acetone + methanol + ethanol | 101.33 kPa | 83 | 0.0228 | 0.0123 | 1.09 K |
| methanol + ethanol + 1-propanol | 101.33 kPa | 45 | 0.0224 | 0.0122 | 0.75 K |
| acetone + methanol + 2-propanol | 328.15 K | 27 | 0.0206 | 0.0163 | 2.02 kPa |
| 2,3-dimethyl-butane + methanol + acetone | 101.33 kPa | 27 | 0.0247 | 0.0236 | 2.36 K |
| methanol + 2-methylbutane+ isoprene | 101.33 kPa | 13 | 0.0489 | 0.0534 | 0.69 K |
| methanol + heptane + toluene | 101.33 kPa | 8 | 0.0065 | 0.0049 | 0.21 K |
| acetone + ethanol + hexane | 328.15 K | 21 | 0.0271 | 0.0141 | 12.20 kPa |
| hexane + ethanol + benzene | 328.15 K | 43 | 0.0206 | 0.0253 | 4.81 kPa |
| ethanol + benzene + heptane | 53.33 kPa | 50 | 0.0232 | 0.0176 | 1.08 K |
| ethanol + benzene + heptane | 101.33 kPa | 47 | 0.0264 | 0.0199 | 0.99 K |
| benzene + heptane + 1-propanol | 348.15 K | 77 | 0.0273 | 0.0153 | 1.83 kPa |
| acetone + 2-methyl-butane + isoprene | 101.33 kPa | 15 | 0.0251 | 0.0205 | 2.22 K |
| 2-butanone + 3-pentanone + 4-methyl-2-pentanone | 101.33 kPa | 64 | 0.0214 | 0.0085 | 0.54.K |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, X.; Wang, Y.; Sun, X.; Zhao, W.; Xia, L.; Xiang, S. Elements and Chemical Bonds Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures. Processes 2022, 10, 2141. https://doi.org/10.3390/pr10102141
Liu H, Li X, Wang Y, Sun X, Zhao W, Xia L, Xiang S. Elements and Chemical Bonds Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures. Processes. 2022; 10(10):2141. https://doi.org/10.3390/pr10102141
Chicago/Turabian StyleLiu, Haodong, Xinyu Li, Yuxin Wang, Xiaoyan Sun, Wenying Zhao, Li Xia, and Shuguang Xiang. 2022. "Elements and Chemical Bonds Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures" Processes 10, no. 10: 2141. https://doi.org/10.3390/pr10102141
APA StyleLiu, H., Li, X., Wang, Y., Sun, X., Zhao, W., Xia, L., & Xiang, S. (2022). Elements and Chemical Bonds Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures. Processes, 10(10), 2141. https://doi.org/10.3390/pr10102141





