Optimization of Extraction of Natural Antimicrobial Pigments Using Supercritical Fluids: A Review
Abstract
:1. Introduction
2. Green Extraction
- (a)
- Innovation through using sustainable plant resources
- (b)
- Use of alternative solvents, principally water and safe solvents
- (c)
- Reduced energy consumption by energy recovery using innovative technologies
- (d)
- Production of co-products instead of waste to include bio- and agro-refining industries
- (e)
- Reduced unit operations and safe-controlled processes
- (f)
- Nondenatured and biodegradable extracts without contaminants
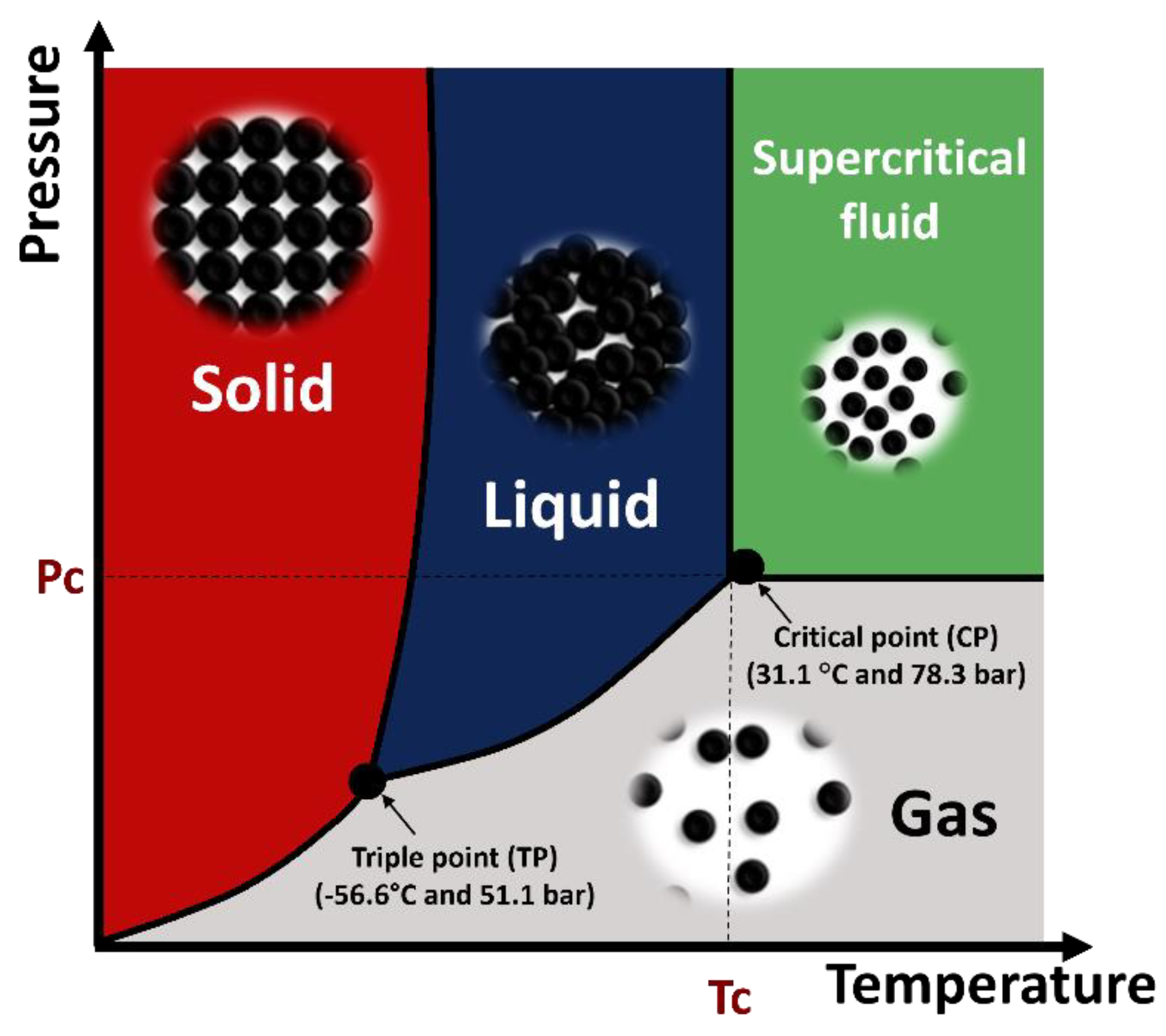
3. Supercritical Fluids (SCFs)
3.1. History of Supercritical Fluids
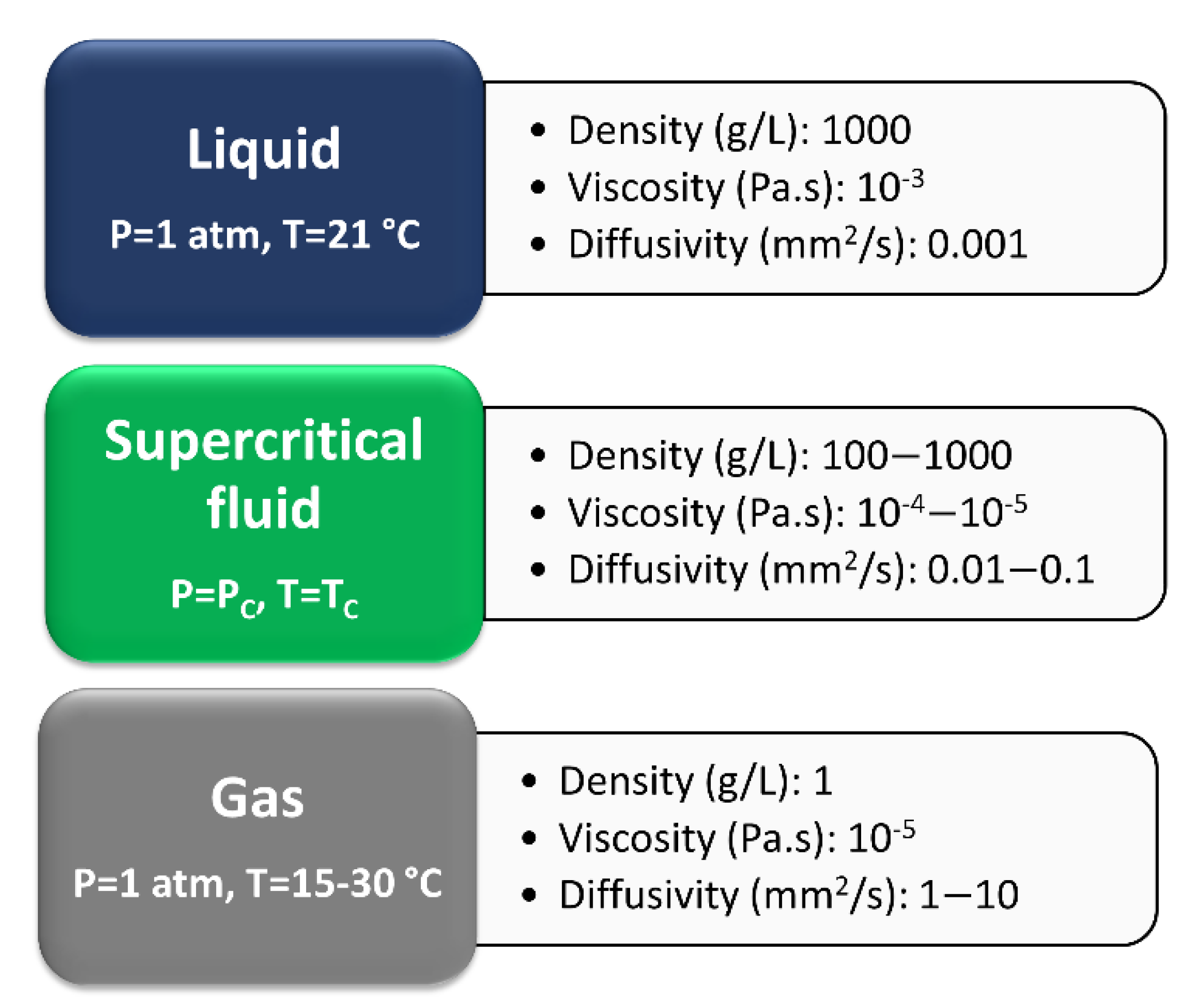
3.2. Properties of Supercritical Fluids
- Treatment of polluted soils;
- Manufacture of micron- and submicron-sized powders, as well as processes in or with SFCs;
4. Supercritical Fluid Extraction (SFE)
4.1. Supercritical Fluid Extraction Principles
4.2. Supercritical Fluid Extraction Instrument
4.3. Supercritical Fluid Extraction Mechanism
5. Critical Parameters in the SFE
5.1. Temperature
5.2. Pressure
5.3. Co-Solvent
5.4. Extraction Time
5.5. Flow Rate
5.6. Raw Matrix
6. Major Advantages and Disadvantages of SFE
- SCFs are highly diffusible and have relatively low viscosities. Therefore, they have a greater ability than liquid solvents to penetrate porous solid materials, leading to faster extraction;
- Compared with traditional procedures, SFE significantly reduces the amount of time required for extraction, from hours or days to a few minutes (less than 2 h);
- Continuous reflux of supercritical fluid into a sample can provide quantitative or complete extraction.
- SCFs have a better selectivity than liquid solvents because their solvation power can be tuned by changing the temperature and/or pressure.
- This adjustable solvation power of SCFs is helpful for extracting complicated substances, such as plant materials.
- The solute can be easily separated from the solvent using depressurization, which saves time.
- SFE is often performed at low temperatures, making it an excellent approach for studying thermally labile chemicals. This may lead to the identification of novel natural components.
- SFE uses no or significantly less toxic organic solvents and is considered to be environmentally friendly.
- SCFs can be recycled and reused to reduce waste generation.
- SFE may enable direct coupling with chromatographic techniques, which can be an efficient way to extract and immediately quantify extremely volatile compounds.
- For specialized purposes, SFE scales can be set up for small-scale analytical, preparative, pilot plant-scale, and large-scale industrial [70].
- However, there are some drawbacks of SCFs, which are listed as follows [71]:
- The phases of equilibrium between a solvent and a solute can be challenging.
- When co-solvents are used to change the polarity of a fluid, they remain in the extract and require further purification.
- It is challenging to continue adding solids to the raw material owing to the high pressure involved in this process.
- Compared with solvent extraction techniques, less material can be extracted.
- High operational costs.
- Low equipment availability.
7. Comparison between SFE and Traditional Methods
8. Extraction of Functional and Antimicrobial Pigments Using Supercritical Fluids
8.1. Carotenoids Extraction
| Raw Material | SFE conditions (Solvent System, Temp, P, T, FR, PS, and SW) | Yield | Biological Activities | Remarks/Results | Ref. | |
|---|---|---|---|---|---|---|
| General structure | ||||||
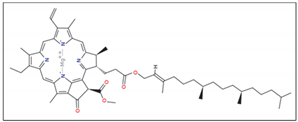 | 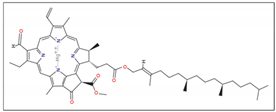 | |||||
| Chlorophyll (a) | Chlorophyll (b) | |||||
Olive | Solvent system | CO2/Ethanol | - | Antioxidant Antimicrobial | Main pigments: Chlorophyll (a) Chlorophylls (b) Carotenoids | [94] |
| Temp | 60 °C | |||||
| P | 35 MPa | |||||
| T | 13 min | |||||
| FR | 10.00 mL/min | |||||
| Elaeagnus angustifolia  | Solvent system | Pure CO2 for leaves | Carotenoids: 0.0183 g/Kg Chlorophyll a: 0.0438 g/Kg chlorophyll b 0.001 g/Kg | Antimicrobial | Main pigments: Chlorophylls Carotenoids | [95] |
| Temp | 55 °C | |||||
| P | 15 MPa | |||||
| T | 60 min | |||||
| SW | 7 g | |||||
Hop cone | Solvent system | Pure CO2 | - | Antimicrobial | Main pigments: Chlorophylls (a, b) | [96] |
| Temp | 50 °C | |||||
| P | 30 MPa | |||||
Rosemary | Solvent system | Pure CO2 + 3%, 10% (EtOH: Water 50/50 v/v), or 30% (EtOH) | The yields of Carotenoids: 53 g/Kg Chlorophyll a: 100 g/Kg Chlorophyll b: 100 g/Kg | Antioxidant Antibacterial Antifungal | Main pigments: Chlorophylls Carotenoids Optimal conditions for carotenoids: Pure CO2, 25 °C, and 20 MPa Optimal conditions for chlorophylls: 30% of ethanol as co-solvent. | [97] |
| Temp | 25 °C | |||||
| P | 20/10 MPa | |||||
| T | 20/40/50/30 min | |||||
| FR | 3.0 mL/min | |||||
| SW | 1 g | |||||
| Solvent system | Pure CO2 | 3.52% | Optimal conditions: 30 MPa and 50 °C | [98] | ||
| Temp | 30, 40, and 50 °C | |||||
| P | 10, 20 and 30 MPa | |||||
| T | 240 min | |||||
| FR | 0.2 kg/h | |||||
| Dunaliella salina  | Solvent system | Pure CO2 | Carotenoids: 0.115 g/Kg Chlorophylls: 0.033 g/Kg | Antioxidant | Main pigments: Chlorophylls Carotenoids Optimal conditions: 40 MPa and 55 °C | [99] |
| Temp | 40–60 °C | |||||
| P | 10–50 MPa | |||||
| T | 180 min | |||||
| FR | 3 L/min | |||||
| SW | 5.0 g | |||||
| Solvent system | CO2 + 5% (Ethanol/Hexane/Acetone/Methanol) | β-carotene: 25 g (for CO2 + ethanol), 6 g (for pure CO2) | Ethanol was the best co-solvent | [100] | ||
| Temp | 35, 45, and 55 °C | |||||
| P | 20 and 30 MPa | |||||
| T | 105 min | |||||
| PS | <0.355 mm | |||||
| SW | 10 g | |||||
Spinach | Solvent system | CO2 + 0, 5, and 10% Ethanol | 72% lutein 50% chlorophylls | Anti-inflammatory Antioxidant | Main pigments: Chlorophylls Lutein Optimal conditions: 56 °C, 3.6 h, and 39 MPa, with 10% ethanol | [101] |
| Temp | 40, 50, 60 °C | |||||
| P | 10, 30, 50 MPa | |||||
| T | 60,120,180 min | |||||
| FR | 10 g/min | |||||
| SW | 25 g | |||||
Chlorella sorokiniana | Solvent system | CO2 + Ethanol (0–10%) | - | Anti-obesity Antioxidant | Main pigments: Chlorophylls (chlorophyll a and chlorophyll b) Carotenoids Optimal conditions: 50.1 °C, 20.29 MPa, and 4.5% ethanol | [102] |
| Temp | 40–60 °C | |||||
| P | 10–30 MPa | |||||
| T | 180 min | |||||
| FR | 1 kg/h | |||||
| SW | 55 g | |||||
| Raw Material | SFE Conditions (Solvent System, Temp, P, T, FR, PS, and SW) | Yield | Biological Activities | Remarks/Results | Ref. | |
|---|---|---|---|---|---|---|
General structure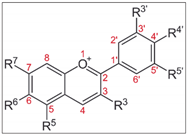 | ||||||
Roselle | Solvent system | CO2 + (5, 7.5, and 10)% Ethanol | Anthocyanins: 26.73%. | Antioxidant Wound healing Anticholesterol Antihypertensive | Optimal conditions: 8.90 MPa, 70 °C, and 9.49% ethanol | [103] |
| Temp | 50, 60, and 70 | |||||
| P | 8, 10, and 12 MPa | |||||
| T | 70 min | |||||
| FR | 6 mL/min | |||||
| PS | 355 µm | |||||
| SW | 1.5 g | |||||
| Solvent system | CO2 + 5–10% Ethanol or Water | Anthocyanin: 11.97 g/Kg | Main pigments: Cyanidin 3-sambubioside 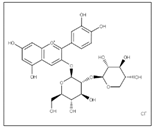 Optimal conditions: 27 MPa, 58 °C, and co-solvent ratio of 8.86% | [104] | ||
| Temp | 40–70 °C | |||||
| P | 10–30 MPa | |||||
| T | 120 min | |||||
| FR | 4 mL/min | |||||
| PS | <0.355 mm | |||||
| SW | 1.5 g | |||||
Juçara | Solvent system | CO2 + 10% acidified mixture of ethanol and water | Anthocyanins: 22 g/Kg | Antioxidant | SFE was more selective for anthocyanins. | [105] |
| Temp | 60 °C | |||||
| P | 20 MPa | |||||
| T | 46 min | |||||
| FR | 2.08 × 10−4 kg/s | |||||
| SW | 2.5 g | |||||
Chokeberry | Solvent system | CO2 + Ethanol (20, 50, and 80%) | Phenolic compounds: 15.2 g/Kg (Anthocyanins accounted for 50–67% of the total phenolics) | Antioxidant | Optimal conditions: 35 °C, 10 MPa, and 80% m/m ethanol addition Anthocyanins are impacted by the use of an acidified co-solvent. | [106] |
| Temp | 35, 50, and 65 °C | |||||
| P | 7.5,10, and 12.5 MPa | |||||
| T | 75 min | |||||
| FR | 1.8 g/min | |||||
| SW | 10 g | |||||
Haskap berry | Solvent system | CO2 + Water | Anthocyanins: 52.7% | Antioxidant Anti-inflammatory Antitumor | Optimal conditions: 65 °C, 45 MPa, 15 min of static time, and 20 min of dynamic time | [107] |
| Temp | 35, 55 and 65 °C | |||||
| P | 10, 27.5, and 45 MPa | |||||
| T | Static time:15, 60, and 120 min Dynamic time:0, 20, and 60 min | |||||
| FR | 10 mL/min | |||||
| Raw Material | SFE Conditions (Solvent System, Temp, P, T, FR, PS, and SW) | Yield | Biological Activities | Remarks/Results | Ref. | |
|---|---|---|---|---|---|---|
| Quinones | ||||||
| Walnut green husk  | Solvent system | CO2 + Ethanol | Juglone: 0.03726 g | Antibacterial Antifungal Antioxidant Antitumor | Main pigments: Juglone 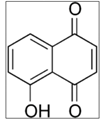 Optimal conditions: 35 °C, 15 MPa, and 375 μm | [108] |
| Temp | 35, 40, 45 and 50 °C | |||||
| P | 9, 11, 13 and 15 MPa | |||||
| T | Static time: 20 min | |||||
| PS | 375, 605, 855 and 1500 μm | |||||
| SW | 12 g | |||||
| Solvent system | CO2 + Ethanol | Juglone: 11.92 g/Kg | Antifungal Antioxidant | - | [109] | |
| Temp | 50 °C | |||||
| P | 30 MPa | |||||
| T | 195 min | |||||
| FR | 10 mL/min | |||||
| PS | ≤1 mm | |||||
| Curcuminoids | ||||||
Turmeric | Solvent system | CO2 + Water or Ethanol | Curcumin: 23.4% | Antimalarial | Main pigments: Curcumin 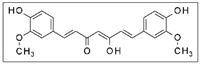 The supercritical extract had a low curcumin content but significant antimalarial activity. | [110] |
| Temp | 40 °C | |||||
| P | 40 MPa | |||||
| T | 360 min | |||||
| FR | 4 × 10−2 g/s | |||||
| PS | 0.823 mm | |||||
| Solvent system | Pure CO2 | 31 g/Kg | Antimicrobial Antifungal | Main pigments: Turmerones Optimal conditions: 30 MPa and 40 °C | [111] | |
| Temp | 40 °C | |||||
| P | 9–66 MPa | |||||
| FR | 1.8 g/min | |||||
| SW | 60 g | |||||
| Iridoids | ||||||
Momordica charantia Vine | Solvent system | CO2 + ethyl acetate or ethanol | - | Antibacterial | Main pigments: Plumericin 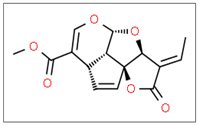 Optimal conditions: 50 °C, 25 MPa, and 5 L/min in the presence of ethyl acetate and ethanol as co-solvents. Plumericin exhibited antibacterial activity against 8 harmful bacterial strains, especially Enterococcus faecalis and B. subtilis. | [112] |
| Temp | 50 °C | |||||
| P | 25 MPa | |||||
| T | 180 min | |||||
| FR | 5 L/min for CO2 0.003 L/min for co-solvent | |||||
| SW | 3000 g | |||||
| Phycocyanins | ||||||
| Spirulina maxima  | Solvent system | Pure CO2 | - | Antioxidant Anti- inflammatory Antiviral, Anticancer Cholesterol-lowering | Main pigments: C-phycocyanin 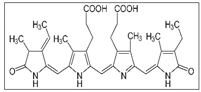 Chlorophylls Carotenoids Optimal conditions: 60 °C, 24.13 MPa | [113] |
| Temp | 40, 50, and 60 °C | |||||
| P | 24.13, 31.03, and 37.92 MPa | |||||
| T | 60,90, and 120 min Static time: 30,45, and 60 min Dynamic time: 30,45, and 60 min | |||||
| Black sesame pigment | ||||||
Sesame dregs | Solvent system | Pure CO2 | Black sesame pigment: 3.58% | Antioxidant | Optimal conditions: 30 °C, 4 MPa, and 10.80 L/min | [114] |
| Temp | 20, 30, and 40 °C | |||||
| P | 10,14, and 18 MPa | |||||
| FR | 0.48, 0.80, and 1.12 L/min | |||||
8.2. Chlorophylls Extraction Using SCFs
8.3. SFE of Anthocyanins
8.4. Quinones Extraction Using the SFE Technique
8.5. Curcuminoids Extraction Using SCFs
8.6. Iridoids Extraction via SCFs
8.7. SFE of Phycocyanins
8.8. SFE of Black Sesame Pigment
9. Antimicrobial Effect of Natural Pigments
- Coagulation of cytoplasmic contents
- Prevention of enzyme generation
- Inactivation of the function of the outer membrane
- Fluctuation of the proton engine force of the cells
- Interaction with extracellular proteins
- Alteration of cytoplasmic membrane
- Blockage of metabolic pathway
10. Future Trends
11. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPR | Back pressure regulator |
| PC | Critical pressure |
| TC | Critical temperature |
| FR | Flow rate |
| PS | Particle size |
| SW | Sample weight |
| scCO2 | Supercritical carbon dioxide |
| SCF | Supercritical fluid |
| SCFs | Supercritical fluids |
| SFE | Supercritical fluid extraction |
References
- Kamboj, A.; Mahajan, S. Consumer Awareness for Natural Dyes. Indian J. Health Wellbeing 2017, 8, 1012–1014. [Google Scholar]
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.U.; Khan, A.L.; Shinwari, Z.K.; Al-Harrasi, A. Therapeutic Applications of Bacterial Pigments: A Review of Current Status and Future Opportunities. 3 Biotech 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Mohammad, F. Recent Advancements in Natural Dye Applications: A Review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Rehman, F.; Sanbhal, N.; Naveed, T.; Farooq, A.; Wang, Y.; Wei, W. Antibacterial Performance of Tencel Fabric Dyed with Pomegranate Peel Extracted via Ultrasonic Method. Cellulose 2018, 25, 4251–4260. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, A.; Panwar, S. Anti-UV and Anti-Microbial Properties of Some Natural Dyes on Cotton. Indian J. Fibre Text Res. 2005, 30, 190. [Google Scholar]
- Yusuf, M.; Shahid, M.; Khan, M.I.; Khan, S.A.; Khan, M.A.; Mohammad, F. Dyeing Studies with Henna and Madder: A Research on Effect of Tin (II) Chloride Mordant. J. Saudi Chem. Soc. 2015, 19, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Hwang, E.-K.; Kim, H.-D. Colorimetric Assay and Antibacterial Activity of Cotton, Silk, and Wool Fabrics Dyed with Peony, Pomegranate, Clove, Coptis chinenis and Gallnut Extracts. Materials 2009, 2, 10–21. [Google Scholar] [CrossRef]
- Butola, B.S.; Roy, A. Chitosan Polysaccharide as a Renewable Functional Agent to Develop Antibacterial, Antioxidant Activity and Colourful Shades on Wool Dyed with Tea Extract Polyphenols. Int. J. Biol. Macromol. 2018, 120, 1999–2006. [Google Scholar]
- Inprasit, T.; Motina, K.; Pisitsak, P.; Chitichotpanya, P. Dyeability and Antibacterial Finishing of Hemp Fabric Using Natural Bioactive Neem Extract. Fibers Polym. 2018, 19, 2121–2126. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of Fruits and Vegetable Wastes and By-Products to Produce Natural Pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Fernandes, F.; Pereira, E.; Círić, A.; Soković, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Ocimum basilicum Var. Purpurascens Leaves (Red Rubin Basil): A Source of Bioactive Compounds and Natural Pigments for the Food Industry. Food Funct. 2019, 10, 3161–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, M.D.; Melo, M.M.; Coimbra, J.M.; dos Reis, K.C.; Schwan, R.F.; Silva, C.F. Solid Coffee Waste as Alternative to Produce Carotenoids with Antioxidant and Antimicrobial Activities. Waste Manag. 2018, 82, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Sitohy, M.; Mosa, B.; Ismaiel, A.; Enan, G.; Osman, A. Antimicrobial Activity and Chemical Constitution of the Crude, Phenolic-Rich Extracts of Hibiscus sabdariffa, Brassica oleracea and Beta vulgaris. Molecules 2019, 24, 4280. [Google Scholar] [CrossRef] [PubMed]
- Pargai, D.; Jahan, S.; Gahlot, M. Functional properties of natural dyed textiles. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; IntechOpen: London, UK, 2020; pp. 1–19. [Google Scholar]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural Colorants: Pigment Stability and Extraction Yield Enhancement via Utilization of Appropriate Pretreatment and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Danilović, B.; Đorđević, N.; Milićević, B.; Šojić, B.; Pavlić, B.; Tomović, V.; Savić, D. Application of Sage Herbal Dust Essential Oils and Supercritical Fluid Extract for the Growth Control of Escherichia coli in Minced Pork during Storage. LWT 2021, 141, 110935. [Google Scholar] [CrossRef]
- Ahmad, T.; Masoodi, F.A.; Rather, A.S.; Wani, S.M.; Gull, A. Supercritical Fluid Extraction: A Review. J. Biol. Chem. Chron. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Fomo, G.; Madzimbamuto, T.N.; Ojumu, T.V. Applications of Nonconventional Green Extraction Technologies in Process Industries: Challenges, Limitations and Perspectives. Sustainability 2020, 12, 5244. [Google Scholar] [CrossRef]
- Miranda, P.H.S.; dos Santos, A.C.; de Freitas, B.C.B.; de Souza Martins, G.A.; Boas, E.V.; de, B.V.; Damiani, C. A Scientific Approach to Extraction Methods and Stability of Pigments from Amazonian Fruits. Trends Food Sci. Technol. 2021, 113, 335–345. [Google Scholar] [CrossRef]
- Patil, P.D.; Patil, S.P.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Nadar, S.S. Enzyme-Assisted Supercritical Fluid Extraction: An Integral Approach to Extract Bioactive Compounds. Trends Food Sci. Technol. 2021, 116, 357–369. [Google Scholar] [CrossRef]
- Shah, N.A.; Prasad, R.V.; Patel, B.B. Optimization of Supercritical Fluid Extraction of Paprika (Cv. Reshampatti) Oil, Capsaicin and Pigments. Flavour Fragr. J. 2020, 35, 469–477. [Google Scholar] [CrossRef]
- Wani, F.A.; Rashid, R.; Jabeen, A.; Brochier, B.; Yadav, S.; Aijaz, T.; Makroo, H.A.; Dar, B.N. Valorisation of Food Wastes to Produce Natural Pigments Using Non-thermal Novel Extraction Methods: A Review. Int. J. Food Sci. Technol. 2021, 56, 4823–4833. [Google Scholar] [CrossRef]
- Bell, A.T. Chemistry and Chemical Engineering; Springer: Berlin, Germany, 1997; Volume 52, ISBN 9781493990597. [Google Scholar]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Budisa, N.; Schulze-Makuch, D. Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zosel, K. Process for the Decaffeination of Coffee. U.S. Patent 1977, 27 January 1981. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branch, J.A.; Bartlett, P.N. Electrochemistry in Supercritical Fluids. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20150007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AE, K.; Singh, A.; NC, S.; JP, P. Novel Eco-Friendly Techniques for Extraction of Food Based Lipophilic Compounds from Biological Materials. Nat. Prod. Chem. Res. 2016, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Kulazynski, M.; Stolarski, M.; Faltynowicz, H.; Narowska, B.; Swiatek, L.; Lukaszewicz, M. Supercritical Fluid Extraction of Vegetable Materials. Chem. Chem. Technol. 2016, 10, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Abou Elmaaty, T.; Abd El-Aziz, E. Supercritical Carbon Dioxide as a Green Media in Textile Dyeing: A Review. Text. Res. J. 2018, 88, 1184–1212. [Google Scholar] [CrossRef]
- Gopaliya, P.; Kamble, P.R.; Kamble, R.; Chauhan, C.S. A Review Article on Supercritical Fluid Chromatography. Int. J. Pharma Res. Rev. 2014, 3, 59–66. [Google Scholar]
- Ibáñez, E.; Mendiola, J.A.; Castro-Puyana, M. Supercritical Fluid Extraction. Encycl. Food Health 2015, 8, 227–233. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green Solvents for Green Technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Karğılı, U.; Aytaç, E. Supercritical Fluid Extraction of Cannabinoids (THC and CBD) from Four Different Strains of Cannabis Grown in Different Regions. J. Supercrit. Fluids 2022, 179, 105410. [Google Scholar] [CrossRef]
- Bader, C.D.; Neuber, M.; Panter, F.; Krug, D.; Muller, R. Supercritical Fluid Extraction Enhances Discovery of Secondary Metabolites from Myxobacteria. Anal. Chem. 2020, 92, 15403–15411. [Google Scholar] [CrossRef] [PubMed]
- Penthala, R.; Kumar, R.S.; Heo, G.; Kim, H.; Lee, I.Y.; Ko, E.H.; Son, Y.-A. Synthesis and Efficient Dyeing of Anthraquinone Derivatives on Polyester Fabric with Supercritical Carbon Dioxide. Dyes Pigments 2019, 166, 330–339. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; El-Taweel, F.; Elsisi, H.; Okubayashi, S. Water Free Dyeing of Polypropylene Fabric under Supercritical Carbon Dioxide and Comparison with Its Aqueous Analogue. J. Supercrit. Fluids 2018, 139, 114–121. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Sofan, M.; Elsisi, H.; Kosbar, T.; Negm, E.; Hirogaki, K.; Tabata, I.; Hori, T. Optimization of an Eco-Friendly Dyeing Process in Both Laboratory Scale and Pilot Scale Supercritical Carbon Dioxide Unit for Polypropylene Fabrics with Special New Disperse Dyes. J. CO2 Util. 2019, 33, 365–371. [Google Scholar] [CrossRef]
- Penthala, R.; Heo, G.; Kim, H.; Lee, I.Y.; Ko, E.H.; Son, Y.A. Synthesis of Azo and Anthraquinone Dyes and Dyeing of Nylon-6, 6 in Supercritical Carbon Dioxide. J. CO2 Util. 2020, 38, 49–58. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Elsisi, H.; Negm, I. Dyeing Characteristics of Polypropylene Fabric Dyed with Special Disperse Dyes Using Supercritical Carbon Dioxide. Fibers Polym. 2021, 22, 1314–1319. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.M.; El-Taweel, F.M.; Elsisi, H.G. Water-Free Dyeing of Polyester and Nylon 6 Fabrics with Novel 2-Oxoacetohydrazonoyl Cyanide Derivatives under a Supercritical Carbon Dioxide Medium. Fibers Polym. 2018, 19, 887–893. [Google Scholar] [CrossRef]
- Elmaaty, T.A.; Sofan, M.; Kosbar, T.; Elsisi, H.; Negm, I. Green Approach to Dye PET and Nylon 6 Fabrics with Novel Pyrazole Disperse Dyes under Supercritical Carbon Dioxide and Its Aqueous Analogue. Fibers Polym. 2019, 20, 2510–2521. [Google Scholar] [CrossRef]
- Hirogaki, K.; Koizumi, K.; Hirata, T.; Tabata, I.; Teruo, H.; El-Taweel, F.; Elmaaty, A.T. Investigation of Dyeing Condition for Cotton Fabric with Reactive Disperse Dye under Supercritical Carbon Dioxide. J. Text. Eng. 2018, 64, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zheng, H.; Zheng, L. Optimization of Eco-Friendly Reactive Dyeing of Cellulose Fabrics Using Supercritical Carbon Dioxide Fluid with Different Humidity. J. Nat. Fibers 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Elsisi, H.; Negm, E.; Ayad, S.; Sofan, M. Novel Nano Silica Assisted Synthesis of Azo Pyrazole for the Sustainable Dyeing and Antimicrobial Finishing of Cotton Fabrics in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2022, 179, 105354. [Google Scholar] [CrossRef]
- Zaghloul, D.N.; Abou Elmaaty, T.; Nakamura, K.; Tabata, I.; Hori, T.; Hirogaki, K. Influence of Additive Organic Base on Dyeing of Cotton Fabric under Supercritical Carbon Dioxide Using Fluorotriazine Reactive Disperse Dye and Investigation of Optimal Dyeing Conditions. J. Supercrit. Fluids 2021, 174, 105243. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Kazumasa, H.; Elsisi, H.; Mousa, A.; Sorour, H.; Gaffer, H.; Hori, T.; Hebeish, A.; Tabata, I.; Farouk, R. Pilot Scale Water Free Dyeing of Pure Cotton under Supercritical Carbon Dioxide. Carbohydr. Polym. Technol. Appl. 2020, 1, 100010. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Sofan, M.; Ayad, S.; Negm, E.; Elsisi, H. Novel Synthesis of Reactive Disperse Dyes for Dyeing and Antibacterial Finishing of Cotton Fabric under ScCO2. J. CO2 Util. 2022, 61, 102053. [Google Scholar] [CrossRef]
- Raventós, M.; Duarte, S.; Alarcón, R. Application and Possibilities of Supercritical CO2 Extraction in Food Processing Industry: An Overview. Food Sci. Technol. Int. 2002, 8, 269–284. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental Design of Supercritical Fluid Extraction—A Review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Bhusnure, O.G.; Gholve, S.B.; Giram, P.S.; Borsure, V.S.; Jadhav, P.P.; Satpute, V.V.; Sangshetti, J.N. Importance of Supercritical Fluid Extraction Techniques in Pharmaceutical Industry: A Review. Indo Am. J. Pharm. Res. 2015, 5, 6876. [Google Scholar]
- Lam, C.T.; Sin, L.T.; Bee, S.T.; Siwayanan, P. A Review of Natural Products from Plants Using Carbon Dioxide Supercritical Fluid Extraction. IEM J. 2020, 81, 1–16. [Google Scholar]
- Chandrakant, K.K.; Pravin, D.J.; Bharat, H.S.; Sachin, K.; Amol, K.P. A overview o supercritical fluid extractio for herbal drugs. Pharmacologyonline 2011, 2, 575–596. [Google Scholar]
- Reverchon, E.; de Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent Developments in Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: Role of Key Parameters, Technological Achievements and Challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical Fluid Extraction in Plant Essential and Volatile Oil Analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Challenges in the Production of Pharmaceutical and Food Related Compounds by SC-CO2 Processing of Vegetable Matter. J. Supercrit. Fluids 2018, 134, 269–273. [Google Scholar] [CrossRef]
- Rad, H.B.; Sabet, J.K.; Varaminian, F. Study of Solubility in Supercritical Fluids: Thermodynamic Concepts and Measurement Methods-a Review. Braz. J. Chem. Eng. 2020, 36, 1367–1392. [Google Scholar] [CrossRef] [Green Version]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P.; Machmudah, S.; Goto, M. Selective Extraction of Lutein from Alcohol Treated Chlorella Vulgaris by Supercritical CO2. Chem. Eng. Technol. 2012, 35, 255–260. [Google Scholar] [CrossRef]
- Xie, L.; Cahoon, E.; Zhang, Y.; Ciftci, O.N. Extraction of Astaxanthin from Engineered Camelina sativa Seed Using Ethanol-Modified Supercritical Carbon Dioxide. J. Supercrit. Fluids 2019, 143, 171–178. [Google Scholar] [CrossRef]
- Brondz, I.; Sedunov, B.; Sivaraman, N. Influence of Modifiers on Supercritical Fluid Chromatography (SFC) and Supercritical Fluid Extraction (SFE), Part I. Int. J. Anal. Mass Spectrom. Cromatogr. 2017, 5, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Macías-Sánchez, M.D.; Serrano, C.M.; Rodríguez, M.R.; de la Ossa, E.M. Kinetics of the Supercritical Fluid Extraction of Carotenoids from Microalgae with CO2 and Ethanol as Cosolvent. Chem. Eng. J. 2009, 150, 104–113. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils—A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of Supercritical CO2 in Lipid Extraction—A Review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
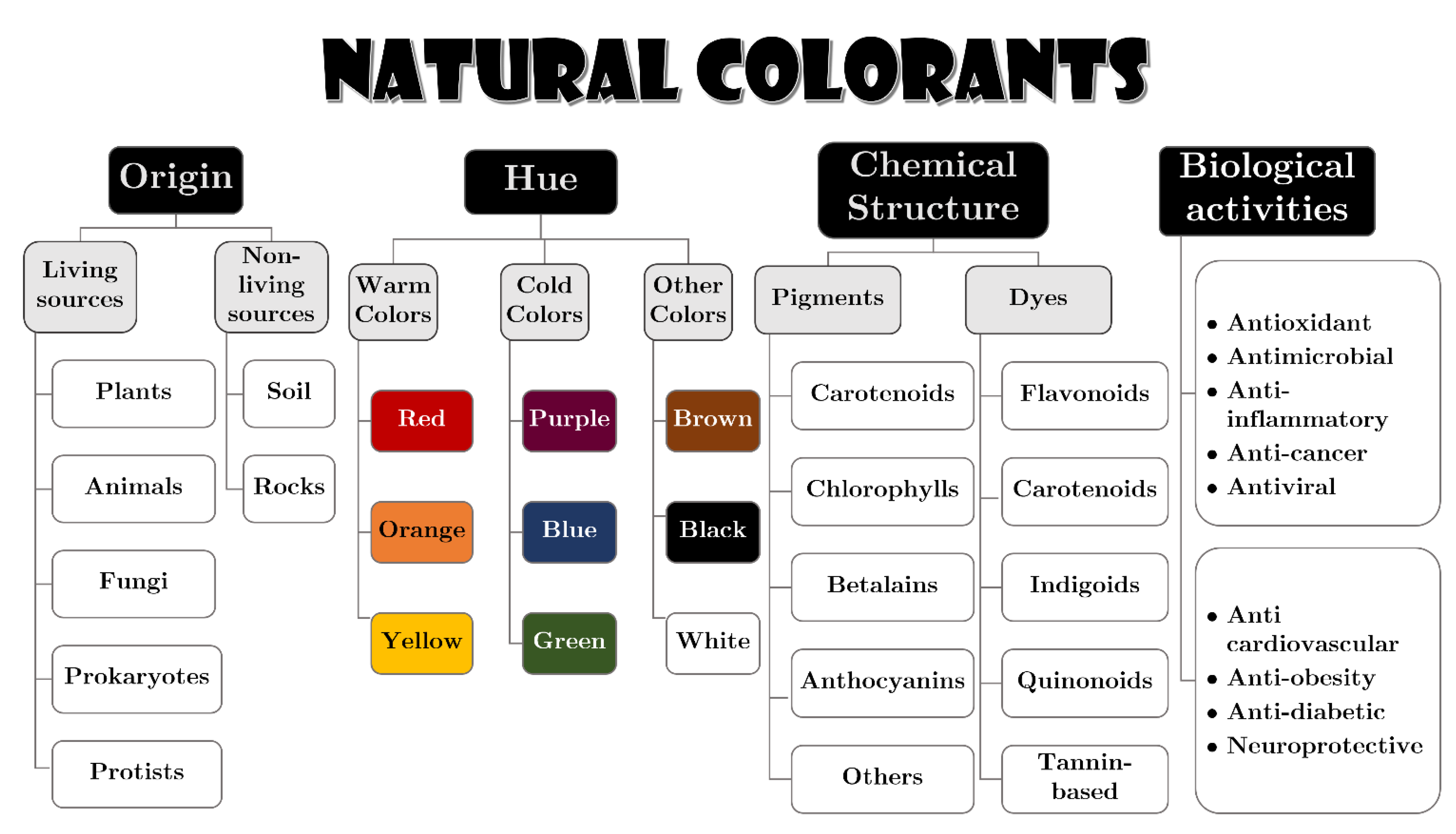
- Rodríguez-mena, A.; Ochoa-martínez, L.A.; Marina, S.; Herrera, G.-; Rutiaga-quiñones, O.M.; Francisco, R. Natural Pigments of Plant Origin: Classification, Extraction and Application in Foods. Food Chem. 2022, 398, 133908. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural Colorants from Plant Pigments and Their Encapsulation: An Emerging Window for the Food Industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Nambela, L.; Haule, L.V.; Mgani, Q. A Review on Source, Chemistry, Green Synthesis and Application of Textile Colorants. J. Clean. Prod. 2020, 246, 119036. [Google Scholar] [CrossRef]
- Uddin, M.A.; Rahman, M.M.; Haque, A.N.M.A.; Smriti, S.A.; Datta, E.; Farzana, N.; Chowdhury, S.; Haider, J.; Muhammad Sayem, A.S. Textile Colouration with Natural Colourants: A Review. J. Clean. Prod. 2022, 349, 131489. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Sanchez-Camargo, A.d.P.; Benvenutti, L.; Ferro, D.M.; Dias, J.L.; Ferreira, S.R.S. High-Pressure Fluid Technologies: Recent Approaches to the Production of Natural Pigments for Food and Pharmaceutical Applications. Trends Food Sci. Technol. 2021, 118, 850–869. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Q.; Zhou, J.; Li, S.; Liu, J.; Chen, H. Insight into the Progress on Natural Dyes: Sources, Structural Features, Health Effects, Challenges, and Potential. Molecules 2022, 27, 3291. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of Mango Peel: Proximate Composition, Supercritical Fluid Extraction of Carotenoids, and Application as an Antioxidant Additive for an Edible Oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Impregnation of Mango Leaf Extract into a Polyester Textile Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; García-Pérez, J.S.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; Ontiveros-Valencia, A.; Rostro-Alanis, M.; García-García, R.M.; Torres, J.A.; et al. Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira platensis. Mar. Drugs 2017, 15, 174. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Lim, D.J.; Chun, B.S. Antioxidant and Antimicrobial Activity of Oils Obtained from a Mixture of Citrus By-Products Using a Modified Supercritical Carbon Dioxide. J. Ind. Eng. Chem. 2018, 57, 339–348. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Chun, B.S. Optimization of Carotenoids and Antioxidant Activity of Oils Obtained from a Co-Extraction of Citrus (Yuzu ichandrin) by-Products Using Supercritical Carbon Dioxide. Biomass Bioenergy 2017, 106, 1–7. [Google Scholar] [CrossRef]
- Urbonaviciene, D.; Viskelis, P. The Cis-Lycopene Isomers Composition in Supercritical CO2 Extracted Tomato by-Products. LWT—Food Sci. Technol. 2017, 85, 517–523. [Google Scholar] [CrossRef]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 Extraction and Antioxidant Activity of Lycopene and β-Carotene-Enriched Oleoresin from Tomato (Lycopersicum esculentum L.) Peels by-Product of a Tunisian Industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Bruno, A.; Durante, M.; Marrese, P.P.; Migoni, D.; Laus, M.N.; Pace, E.; Pastore, D.; Mita, G.; Piro, G.; Lenucci, M.S. Shades of Red: Comparative Study on Supercritical CO2 Extraction of Lycopene-Rich Oleoresins from Gac, Tomato and Watermelon Fruits and Effect of the α-Cyclodextrin Clathrated Extracts on Cultured Lung Adenocarcinoma Cells’ Viability. J. Food Compos. Anal. 2018, 65, 23–32. [Google Scholar] [CrossRef]
- De Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and Modelling of Supercritical CO2 Extraction Process of Carotenoids from Carrot Peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Kraujalis, P.; Tamkutė, L.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Recovery of Bioactive Substances from Rowanberry Pomace by Consecutive Extraction with Supercritical Carbon Dioxide and Pressurized Solvents. J. Ind. Eng. Chem. 2020, 85, 152–160. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zha, X.; Mei, Y.; Xia, J.; Jiao, Z. Supercritical Carbon Dioxide Extraction of β-Carotene and α-Tocopherol from Pumpkin: A Box-Behnken Design for Extraction Variables. Anal. Methods 2017, 9, 294–303. [Google Scholar] [CrossRef]
- Cobb, B.F.; Kallenbach, J.; Hall, C.A.; Pryor, S.W. Optimizing the Supercritical Fluid Extraction of Lutein from Corn Gluten Meal. Food Bioprocess Technol. 2018, 11, 757–764. [Google Scholar] [CrossRef]
- Saravana, P.S.; Getachew, A.T.; Cho, Y.J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of Co-Solvents on Fucoxanthin and Phlorotannin Recovery from Brown Seaweed Using Supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Achparaki, M.; Thessalonikeos, E.; Tsoukali, H.; Mastrogianni, O.; Zaggelidou, E.; Chatzinikolaou, F.; Vasilliades, N.; Raikos, N.; Isabirye, M.; Raju, D.V.N.; et al. We Are IntechOpen, the World’s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%; Intech: London, UK, 2012; p. 13. [Google Scholar]
- Tepić, A.; Zeković, Z.; Kravić, S.; Mandić, A. Pigment Content and Fatty Acid Composition of Paprika Oleoresins Obtained by Conventional and Supercritical Carbon Dioxide Extraction. CyTA—J. Food 2009, 7, 95–102. [Google Scholar] [CrossRef]
- Careri, M.; Furlattini, L.; Mangia, A.; Musci, M.; Anklam, E.; Theobald, A.; von Holst, C. Supercritical Fluid Extraction for Liquid Chromatographic Determination of Carotenoids in Spirulina pacifica Algae: A Chemometric Approach. J. Chromatogr. A 2001, 912, 61–71. [Google Scholar] [CrossRef]
- Difonzo, G.; Aresta, A.; Cotugno, P.; Ragni, R.; Squeo, G.; Su mmo, C.; Massari, F.; Pasqualone, A.; Faccia, M.; Zambonin, C.; et al. Supercritical CO2 Extraction of Phytocompounds from Olive Pomace Subjected to Different Drying Methods. Molecules 2021, 26, 598. [Google Scholar] [CrossRef]
- Carradori, S.; Cairone, F.; Garzoli, S.; Fabrizi, G.; Iazzetti, A.; Giusti, A.M.; Menghini, L.; Uysal, S.; Ak, G.; Zengin, G.; et al. Phytocomplex Characterization and Biological Evaluation of Powdered Fruits and Leaves from Elaeagnus angustifolia. Molecules 2020, 25, 2021. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Czerwonka, D.; Piotrowska, U.; Seweryn, A.; Nizioł-łukaszewska, Z.; Sobczak, M. Use of Hop Cone Extract Obtained under Supercritical CO2 Conditions for Producing Antibacterial All-Purpose Cleaners. Green Chem. Lett. Rev. 2018, 11, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Sequential Extraction of Carnosic Acid, Rosmarinic Acid and Pigments (Carotenoids and Chlorophylls) from Rosemary by Online Supercritical Fluid Extraction-Supercritical Fluid Chromatography. J. Chromatogr. A 2021, 1639, 461709. [Google Scholar] [CrossRef] [PubMed]
- Genena, A.K.; Hense, H.; Smânia, A.; de Souza, S.M. Rosemary (Rosmarinus officinalis)—A Study of the Composition, Antioxidant and Antimicrobial Activities of Extracts Obtained with Supercritical Carbon Dioxide. Cienc. Tecnol. Aliment. 2008, 28, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Pour Hosseini, S.R.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental Optimization of SC-CO2 Extraction of Carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Tirado, D.F.; Calvo, L. The Hansen Theory to Choose the Best Cosolvent for Supercritical CO2 Extraction of Β-Carotene from Dunaliella salina. J. Supercrit. Fluids 2019, 145, 211–218. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of Supercritical Carbon Dioxide Extraction of Lutein and Chlorophyll from Spinach By-Products Using Response Surface Methodology. LWT 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Morcelli, A.; Cassel, E.; Vargas, R.; Rech, R.; Marcílio, N. Supercritical Fluid (CO2+ethanol) Extraction of Chlorophylls and Carotenoids from Chlorella sorokiniana: COSMO-SAC Assisted Prediction of Properties and Experimental Approach. J. CO2 Util. 2021, 51, 101649. [Google Scholar] [CrossRef]
- Idham, Z.; Nasir, H.M.; Yunus, M.A.C.; Yian, L.N.; Peng, W.L.; Hassan, H.; Setapar, S.H.M. Optimisation of Supercritical CO2 Extraction of Red Colour from Roselle (Hibiscus sabdariffa Linn.) Calyces. Chem. Eng. Trans. 2017, 56, 871–876. [Google Scholar] [CrossRef]
- Idham, Z.; Putra, N.R.; Aziz, A.H.A.; Zaini, A.S.; Rasidek, N.A.M.; Mili, N.; Yunus, M.A.C. Improvement of Extraction and Stability of Anthocyanins, the Natural Red Pigment from Roselle Calyces Using Supercritical Carbon Dioxide Extraction. J. CO2 Util. 2021, 56, 101839. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.; del, P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of Phenolic Compounds and Anthocyanins from Juçara (Euterpe edulis Mart.) Residues Using Pressurized Liquids and Supercritical Fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Woźniak, L.; Marszalek, K.; Skapska, S.; Jedrzejczak, R. The Application of Supercritical Carbon Dioxide and Ethanol for the Extraction of Phenolic Compounds from Chokeberry Pomace. Appl. Sci. 2017, 7, 322. [Google Scholar] [CrossRef] [Green Version]
- Jiao, G.; Kermanshahi pour, A. Extraction of Anthocyanins from Haskap Berry Pulp Using Supercritical Carbon Dioxide: Influence of Co-Solvent Composition and Pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
- Ramezani, N.; Raji, F.; Rezakazemi, M.; Younas, M. Juglone Extraction from Walnut (Juglans regia L.) Green Husk by Supercritical CO2: Process Optimization Using Taguchi Method. J. Environ. Chem. Eng. 2020, 8, 103776. [Google Scholar] [CrossRef]
- Romano, R.; Aiello, A.; Meca, G.; de Luca, L.; Pizzolongo, F.; Masi, P. Recovery of Bioactive Compounds from Walnut (Juglans regia L.) Green Husk by Supercritical Carbon Dioxide Extraction. Int. J. Food Sci. Technol. 2021, 56, 4658–4668. [Google Scholar] [CrossRef]
- Martinez-Correa, H.A.; Paula, J.T.; Kayano, A.C.A.V.; Queiroga, C.L.; Magalhães, P.M.; Costa, F.T.M.; Cabral, F.A. Composition and Antimalarial Activity of Extracts of Curcuma longa L. Obtained by a Combination of Extraction Processes Using Supercritical CO2, Ethanol and Water as Solvents. J. Supercrit. Fluids 2017, 119, 122–129. [Google Scholar] [CrossRef]
- Topiar, M.; Sajfrtova, M.; Karban, J.; Sovova, H. Fractionation of Turmerones from Turmeric SFE Isolate Using Semi-Preparative Supercritical Chromatography Technique. J. Ind. Eng. Chem. 2019, 77, 223–229. [Google Scholar] [CrossRef]
- Saengsai, J.; Kongtunjanphuk, S.; Yoswatthana, N.; Kummalue, T.; Jiratchariyakul, W. Antibacterial and Antiproliferative Activities of Plumericin, an Iridoid Isolated from Momordica charantia Vine. Evid. Based Complement. Altern. Med. 2015, 2015, 823178. [Google Scholar] [CrossRef] [Green Version]
- Dejsungkranont, M.; Chen, H.H.; Sirisansaneeyakul, S. Enhancement of Antioxidant Activity of C-Phycocyanin of Spirulina Powder Treated with Supercritical Fluid Carbon Dioxide. Agric. Nat. Resour. 2017, 51, 347–354. [Google Scholar] [CrossRef]
- Bai, L.; Cheng, X.; Xu, J.; Wang, X.; Zhao, H.; Tao, Y.; Huang, H. Black Sesame Pigment Extract from Sesame Dregs by Subcritical CO2: Extraction Optimization, Composition Analysis, Binding Copper and Antioxidant Protection. LWT 2019, 100, 28–34. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Raes, K.; van Speybroeck, V.; Roosen, M.; de Clerck, K.; de Meester, S. Non-Food Applications of Natural Dyes Extracted from Agro-Food Residues: A Critical Review. J. Clean. Prod. 2021, 301, 126920. [Google Scholar] [CrossRef]
- Kamboj, A.; Jose, S.; Singh, A. Antimicrobial Activity of Natural Dyes—A Comprehensive Review. J. Nat. Fibers 2021, 1–15. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]

| Solvent | Molecular Weight (g/mol) | Critical Temperature (k) | Critical Pressure (MPa) | Critical Density (g/cm3) |
|---|---|---|---|---|
| Carbon dioxide | 44.01 | 304.1 | 7.38 | 0.469 |
| Water | 18.015 | 647.3 | 22.064 | 0.348 |
| Methane | 16.04 | 190.4 | 4.60 | 0.162 |
| Ethane | 30.07 | 305.3 | 4.87 | 0.203 |
| Propane | 44.09 | 369.8 | 4.25 | 0.217 |
| Ethylene | 28.05 | 282.4 | 5.04 | 0.215 |
| Propylene | 42.08 | 364.9 | 4.60 | 0.232 |
| Methanol | 32.04 | 512.6 | 8.09 | 0.272 |
| Ethanol | 46.07 | 513.9 | 6.14 | 0.276 |
| Benzene | 78.11 | 562 | 4.89 | 0.876 |
| Acetone | 58.08 | 508.1 | 4.70 | 0.278 |
| Pentane | 72.15 | 469.6 | 3.369 | 0.273 |
| Butane | 58.12 | 425.16 | 3.796 | 0.225 |
| Hexane | 86.178 | 507.44 | 3.031 | 0.233 |
| Parameter | The Optimal Range | |
|---|---|---|
| Temperature | 35–60 °C | |
| Pressure | Around 40 MPa (in the case of scCO2) | |
| Co- solvent | Concentration | Below 1–10% (in the case of scCO2) |
| Type | Ethanol (in the case of food industry)Methanol (in the case of analytical operations) | |
| Time | Less than 2 h | |
| Flow rate | 1–10 L/min (in the case of scCO2) | |
| Raw material | Particle size | 0.25 to 2.0 mm |
| Moisture content | 4–14% | |
| Pre-treatment | Freeze-dried samples | |
| Parameter | SFE | Traditional Methods |
|---|---|---|
| Solvent | SCF, few amount of harmful organic solvents | Large volumes of harmful organic solvents |
| Speed | Rapid | Many steps and long processing time |
| Purity | Highly pure extracts (No solvent residue) | Less pure (Solvent residue) |
| Recovery | Simple | Need additional operations for solvent removal |
| Selectivity | Selective | Less selective |
| Dissolving power | Pressure-tunable dissolving power | Constant dissolving power |
| Cost | Expensive | Inexpensive |
| Raw Material | SFE Conditions (Solvent System, Temp, P, T, FR, PS, and SW) | Yield | Biological Activities | Remarks/Results | Ref. | |
|---|---|---|---|---|---|---|
General structure | ||||||
Mango | Solvent system | CO2 + Ethanol (5–15)% | Carotenoids: 1.9 g/kg | Antioxidant Antibacterial | Optimal conditions: 60 °C, 25 MPa, and 15% w/w ethanol | [78] |
| Temp | 40–60 °C | |||||
| P | 25–35 MPa | |||||
| T | 180 min | |||||
| FR | 6.7 g/min | |||||
| SW | 5 g | |||||
| Solvent system | CO2 + 50% Methanol | - | Mango leaf extract was used to impregnate polyester textiles using supercritical CO2 | [79] | ||
| Temp | 100 °C | |||||
| P | 12 MPa | |||||
| T | 180 min | |||||
| FR | 10 g/min | |||||
| SW | 30 g | |||||
Paprika | Solvent system | Pure CO2 | - | Antimicrobial | Main pigments: Capsanthin 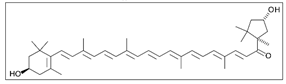 Capsorubin 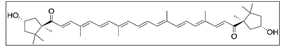 Optimal conditions: 65 °C, 40 MPa, 1 mm, and 90 min | [23] |
| Temp | 35–75 °C | |||||
| P | 10–50 MPa | |||||
| T | 60–180 min | |||||
| FR | 3 L/min | |||||
| PS | 0.25–1.25 mm | |||||
| SW | 25 g | |||||
| Arthrospira platensis  | Solvent system | CO2 + 96% Ethanol | β-carotene: 0.52446 g/kg Lutein: 0.00144 g/Kg | Antimicrobial Antioxidant | Main pigments: β-carotene 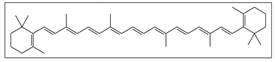 Lutein  Optimal conditions for β-carotene: 60 °C, 45 MPa, 15 min of static time, and 25 min of dynamic time Optimal conditions for lutein: 60 °C, 45 MPa, 5 min of static time, and 55 min of dynamic time | [80] |
| Temp | 40, 60 °C | |||||
| P | 15, 45 MPa | |||||
| T | Static time: 5, 15 min Dynamic time: 25, 55 min | |||||
| FR | 25 g/min | |||||
| PS | 1 mm | |||||
| SW | 35 g | |||||
Citrus | Solvent system | Pure CO2 | Carotenoids: 1.952 g/Kg | Antimicrobial Antioxidant [81] | Optimal conditions: 25.196 MPa, 44.88 °C, and 1.91 mixing ratio. | [82] |
| Temp | 40–50 °C | |||||
| P | 20–30 MPa | |||||
| T | 120 min | |||||
| FR | 27 g/min | |||||
Tomato | Solvent system | Pure CO2 | Oleoresin yield: 251.15 g/kg (~62% lycopene) | Antioxidant Anti-inflammatory Anticancer | Main pigments: Lycopene  Optimal conditions: 52 ℃, 55 MPa, and 180 min | [83] |
| Temp | 40–80 °C | |||||
| P | 20–55 MPa | |||||
| T | 120–240 min | |||||
| FR | 0.0018 g/mL | |||||
| PS | < 0.20 mm | |||||
| SW | 15 g | |||||
| Solvent system | Pure CO2 | Lycopene: 0.729 g/kg β-carotene: 0.016 g/kg | Optimal conditions: 40 MPa, 80 °C, and 4g CO2/min | [84] | ||
| Temp | 50–80 °C | |||||
| P | 30–50 MPa | |||||
| T | 105 min | |||||
| FR | 3–4 g/min | |||||
| PS | 0.3–1 mm | |||||
| SW | 10 g | |||||
| Tomato Watermelon Gac    | Solvent system | Pure CO2 | Lycopene: 63, 52, and 60% from gac, tomato, and watermelon, respectively. | Antioxidant | Main pigments: Lycopene 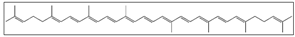 Dehydrated matrices are suitable for SFE. | [85] |
| Temp | 60 °C | |||||
| P | 35 MPa | |||||
| T | 30–180 min | |||||
| FR | 4 mL/min | |||||
| SW | 25 g | |||||
Carrot | Solvent system | CO2 + 5, 10, and 15% Ethanol | Carotenoids: 86.1%. | Antioxidant | Optimal conditions: 59.0 °C, 34.9 MPa, and 15.5% ethanol | [86] |
| Temp | 50, 60 and 70 °C | |||||
| P | 15, 25 and 35 MPa | |||||
| T | 80 min | |||||
| FR | 15 g/min | |||||
| PS | 205 μm | |||||
| SW | 5.0 g | |||||
Rowanberry | Solvent system | Pure CO2 | Carotenoid: 6.630 ± 0.403 g/ Kg β-carotene: 3.295 ± 0.200 g/Kg | Antioxidant | Main pigments: β-carotene 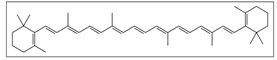 Optimal conditions: 45 MPa, 60 °C and 180 min | [87] |
| Temp | 40–60 °C | |||||
| P | 25–45 MPa | |||||
| T | 360 min | |||||
| FR | 3.0 mL/min | |||||
| SW | 20 g | |||||
Pumpkin | Solvent system | CO2 + Ethanol | β-carotene: 0.205 g/Kg | Antioxidant | Optimal conditions: 47.75 °C, 30 MPa and 67% mass of seeds | [88] |
| Temp | 40–50 °C | |||||
| P | 20–30 MPa | |||||
| T | 60 min | |||||
| FR | 15 L/ h | |||||
| SW | 100 g | |||||
| Corn gluten meal  | Solvent system | CO2 + 5–15% ethanol | Lutein: 85.4 × 10−6 g | - | Main pigments: Lutein 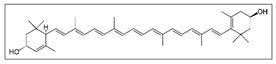 Optimal conditions: 40 °C, 47.02 MPa, and 15% ethanol | [89] |
| Temp | 40–80 °C | |||||
| P | 37.92–51.71MPa | |||||
| T | 60–480 min | |||||
| FR | 2 mL/min | |||||
| SW | 2.5 g | |||||
| Brown Seaweed  | Solvent system | CO2 + (Ethanol/soybean oil /canola oil/sunflower oil) | Fucoxanthin: 1.421 g/Kg. Phlorotannin: 0.927 g/Kg. | Fucoxanthin: Anti-inflammatory Antioxidant Anticancer Phlorotannins: Antioxidant Antibacterial Anti-inflammatory Anti-allergic. | Main pigments: Fucoxanthin 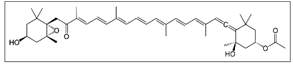 Phlorotannin  Optimal conditions for fucoxanthin: 50.62 °C, 30 MPa, and 2.00% sunflower oil as a co-solvent Optimal conditions for phlorotannins: 48.98 °C, 30 MPa, and 2.00% with water | [90] |
| Temp | 45–55 °C | |||||
| P | 20–30 MPa | |||||
| T | 120 min | |||||
| FR | 27 g/min | |||||
| SW | 100 g | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Elmaaty, T.; Sayed-Ahmed, K.; Elsisi, H.; Magdi, M. Optimization of Extraction of Natural Antimicrobial Pigments Using Supercritical Fluids: A Review. Processes 2022, 10, 2111. https://doi.org/10.3390/pr10102111
Abou Elmaaty T, Sayed-Ahmed K, Elsisi H, Magdi M. Optimization of Extraction of Natural Antimicrobial Pigments Using Supercritical Fluids: A Review. Processes. 2022; 10(10):2111. https://doi.org/10.3390/pr10102111
Chicago/Turabian StyleAbou Elmaaty, Tarek, Khaled Sayed-Ahmed, Hanan Elsisi, and Mai Magdi. 2022. "Optimization of Extraction of Natural Antimicrobial Pigments Using Supercritical Fluids: A Review" Processes 10, no. 10: 2111. https://doi.org/10.3390/pr10102111
APA StyleAbou Elmaaty, T., Sayed-Ahmed, K., Elsisi, H., & Magdi, M. (2022). Optimization of Extraction of Natural Antimicrobial Pigments Using Supercritical Fluids: A Review. Processes, 10(10), 2111. https://doi.org/10.3390/pr10102111








