Lithium-Ion Cell Characterization, Using Hybrid Current Pulses, for Subsequent Battery Simulation in Mobility Applications
Abstract
1. Introduction
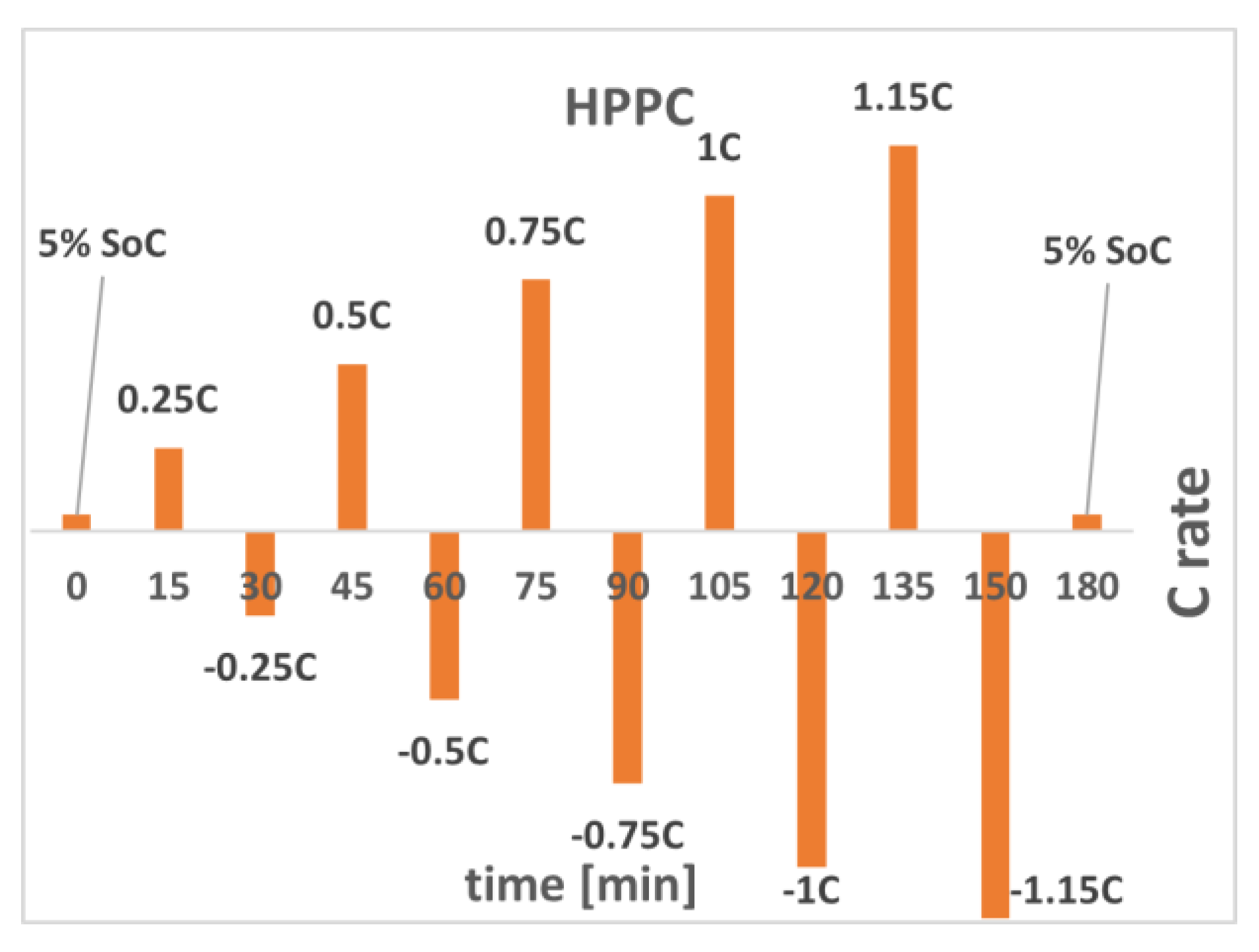
2. The HPPC Method and the Developed EEC
3. Experimental Setup
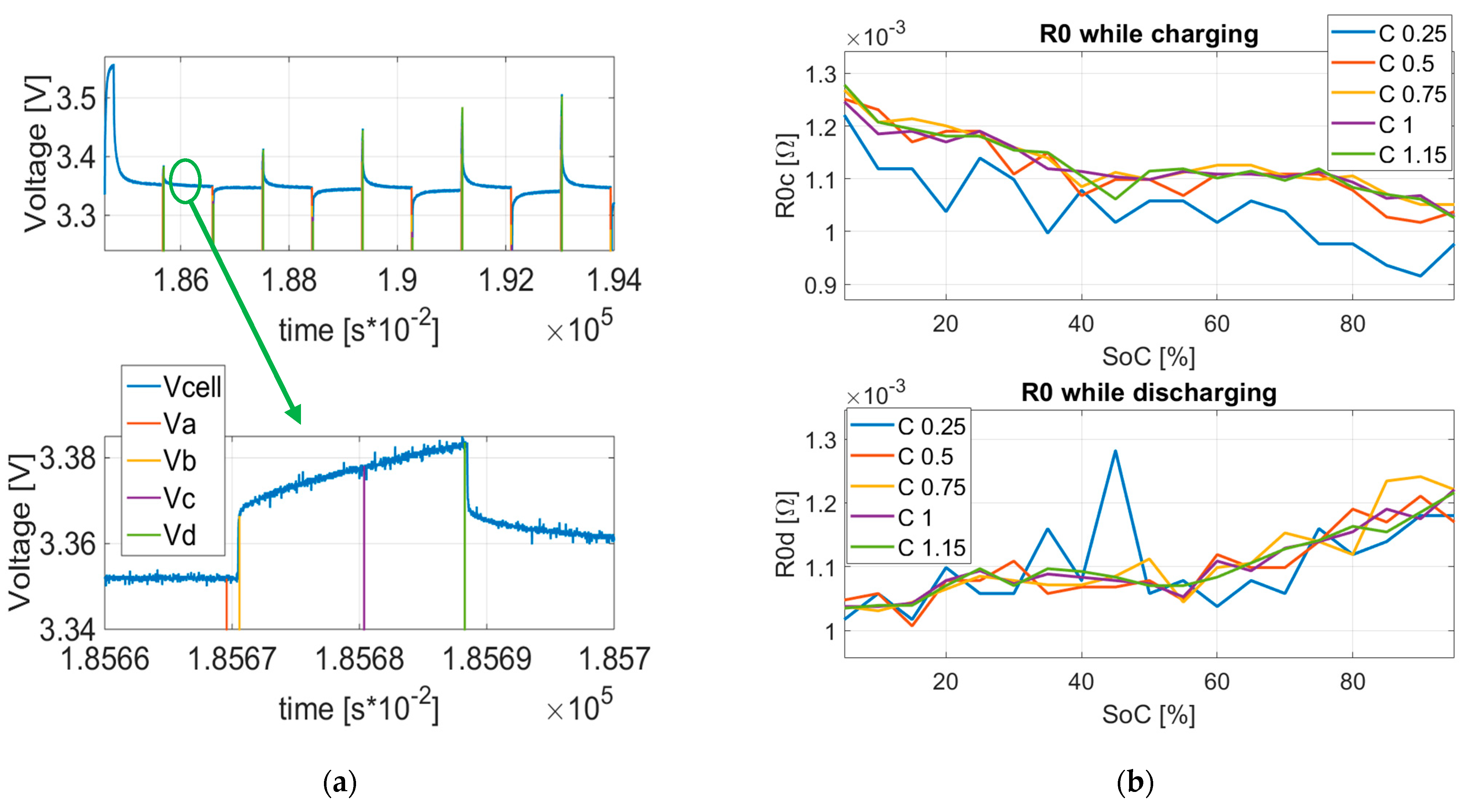
4. Characterization Results
5. The Model Implementation
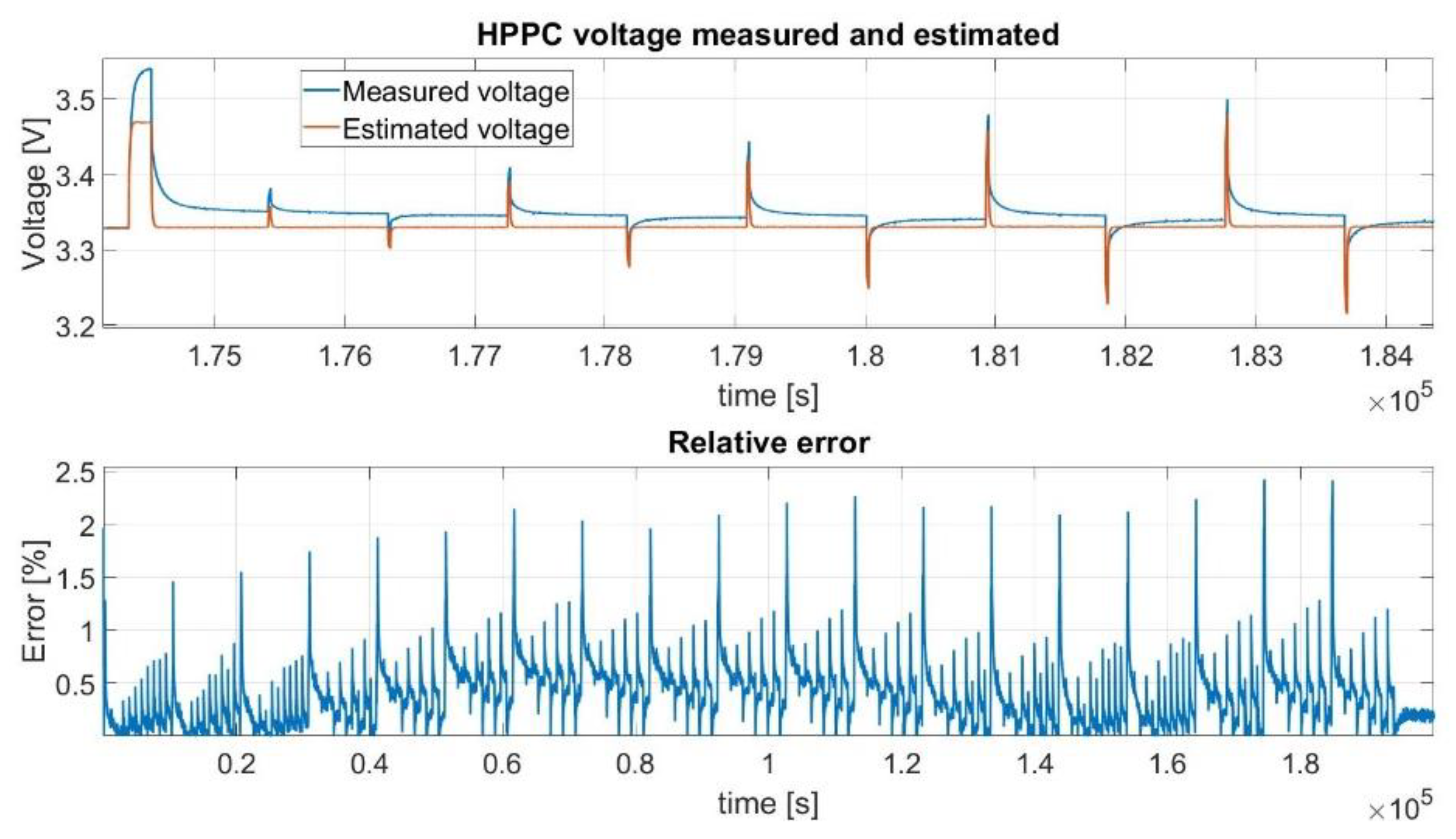
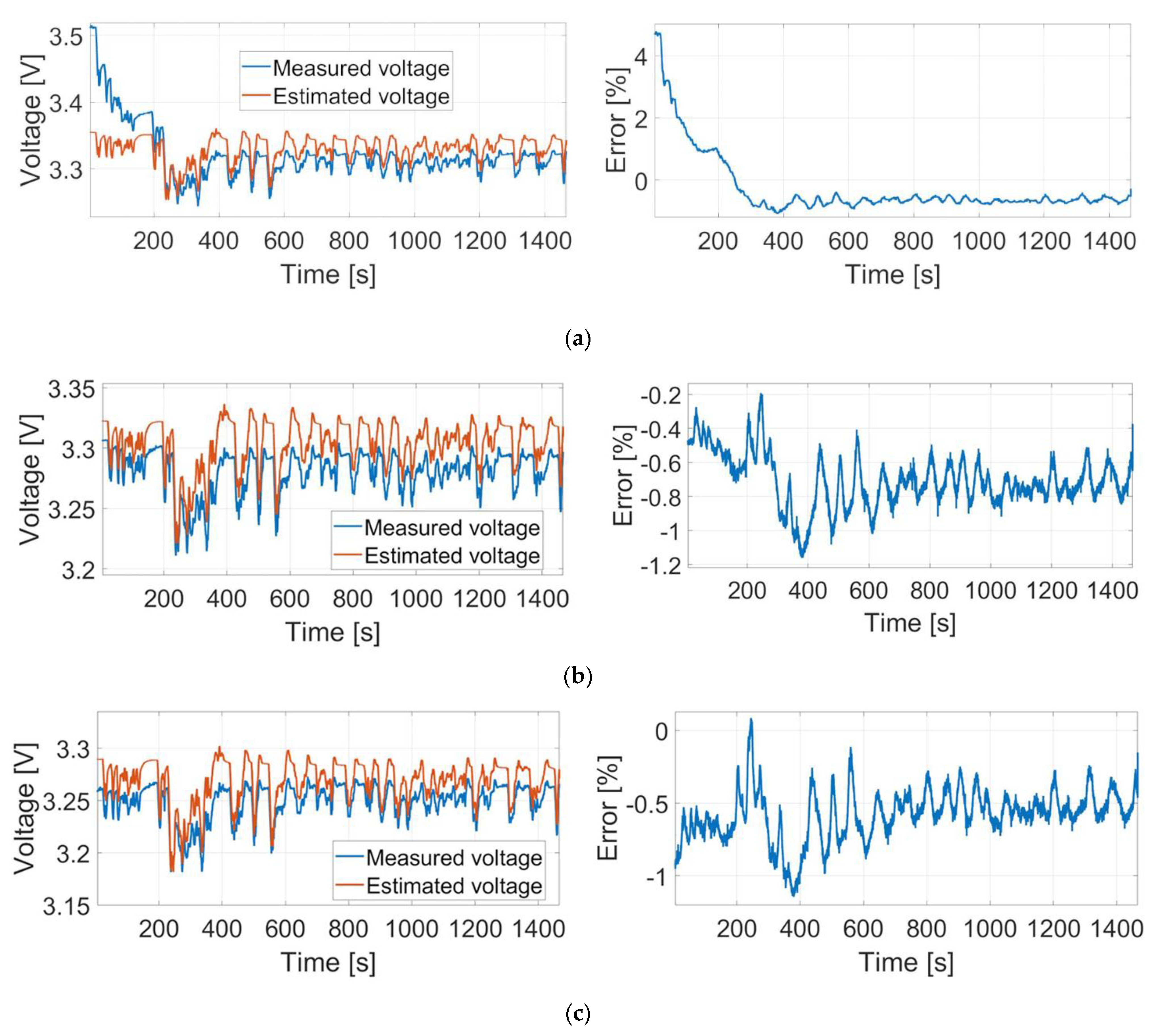
6. The Obtained Results
6.1. HPPC
6.2. FTP-72
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| LFP | lithium iron phosphate |
| HPPC | hybrid pulse power characterization |
| EV | electric vehicle |
| EVSE | electric vehicle supply equipment |
| FTP-72 | federal test procedure driving cycle |
| EU | European union |
| EIS | electrochemical impedance spectroscopy |
| RC | resistor–capacitor |
| OCV | open circuit voltage |
| A | amperes |
| C | is a measure of the rate at which a battery is discharged relative to its capacity |
| EEC | electrical equivalent circuit |
| RT | real-time |
| CAN | controller area network |
| 1D, 2D | one dimension, two dimensions |
| RMSE | root mean square error |
| the capacitances for the parallel branches, 1 and 2, of the battery’s equivalent circuit | |
| the current passing one cell | |
| the Ah capacity for one cell | |
| ohmic resistor for one cell | |
| the resistances for the parallel branches, 1 and 2, of the battery’s equivalent circuit | |
| the state-of-charge | |
| the drop off voltage for the ohmic resistor | |
| the drop off voltage for the parallel branches, 1 and 2, of the battery’s equivalent circuit | |
| voltage measured at different moments of time during each pulse of HPPC | |
| the open-circuit average voltage | |
| the voltage for one cell | |
| the hysteresis voltage | |
| the open-circuit voltage | |
| the charging open-circuit voltage | |
| the discharging open-circuit voltage | |
| hysteresis coefficient | |
| time constants for the parallel branches, 1 and 2, of the battery’s equivalent circuit |
References
- European Automobile Manufacturers Association. Fuel Types of New Cars: Electric 10.5%, Hybrid 11.9%, Petrol 47.5% Market Share Full-Year 2020; European Automobile Manufacturers Association: Brussels, Belgium, 2021. [Google Scholar]
- European Automobile Manufacturers Association. Charging Points: Growth Not Keeping Pace with Rising Demand for Electric Vehicles, New Data Show; European Automobile Manufacturers Association: Brussels, Belgium, 2020. [Google Scholar]
- Zhang, L.; Ning, Z.; Peng, H.; Mu, Z.; Sun, C. Effects of Vibration on the Electrical Performance of Lithium-Ion Cells Based on Mathematical Statistics. Appl. Sci. 2017, 7, 802. [Google Scholar] [CrossRef]
- Grietus, M.; Noshin, O.; Stijn, P.; Filip, L.; Bavo, V.; Wouter De, N.; Peter, V.B.; Daan, S.; Joeri, V.M. Enhanced test methods to characterise automotive battery cells. J. Power Source 2011, 196, 10079–10087. [Google Scholar]
- Andre, D.; Meiler, M.; Steiner, K.; Wimmer, C.; Soczka-Guth, T.; Sauer, D.U. Characterization of high-power lithium-ion batteries by electrochemical impedance spectroscopy. I. Experimental investigation. J. Power Source 2011, 196, 5334–5341. [Google Scholar] [CrossRef]
- Stroe, D.-I.; Swierczynski, M.; Stroe, A.-I.; Knudsen Kær, S. Generalized Characterization Methodology for Performance Modelling of Lithium-Ion Batteries. Batteries 2016, 2, 37. [Google Scholar] [CrossRef]
- Nacu, R.C.; Fodorean, D. Battery Cells Characterization for Subsequent Operation in Battery Models used in Mobile Charging Station Designing. In Proceedings of the Electric Vehicles International Conference (EV), Bucharest, Romania, 3–4 September 2019. [Google Scholar]
- Rizzello, A.; Scavuzzo, S.; Ferraris, A.; Airale, A.G.; Carello, M. Temperature-Dependent Thévenin Model of a Li-Ion Battery for Automotive Management and Control. In Proceedings of the IEEE International Conference on Environment and Electrical Engineering and IEEE Industrial and Commercial Power Systems Europe (EEEIC/I&CPS Europe), Madrid, Spain, 9–12 June 2020. [Google Scholar]
- Wang, W.; Malysz, P.; Khan, K.; Gauchia, L.; Emadi, A. Modeling, parameterization, and benchmarking of a lithium ion electric bicycle battery. In Proceedings of the IEEE Energy Conversion Congress and Exposition (ECCE), Milwaukee, WI, USA, 18–22 September 2016. [Google Scholar]
- Rahman, M.A.; Anwar, S.; Izadian, A. Electrochemical Model-Based Condition Monitoring via Experimentally Identified Li-Ion Battery Model and HPPC. Energies 2017, 10, 1266. [Google Scholar] [CrossRef]
- Tran, M.-K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A Review of Lithium-Ion Battery Thermal Runaway Modeling and Diagnosis Approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- FreedomCAR Battery Test Manual for Power-Assist Hybrid Electric Vehicles; Technical Report DOE/ID-11069; Idaho National Engineering & Environmental Laboratory: Idaho Falls, ID, USA, 2003.
- Stroe, D.I. Lifetime Models for Lithium-Ion Batteries Used in Virtual Power Plant Applications. Ph.D. Thesis, Aalborg University, Aalborg, Denmark, 2014. [Google Scholar]
- Zhang, L.; Peng, H.; Ning, Z.; Mu, Z.; Sun, C. Comparative Research on RC Equivalent Circuit Models for Lithium-Ion Batteries of Electric Vehicles. Appl. Sci. 2017, 7, 1002. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.; Zhang, W.; Shi, W.; Liu, Q. Modeling charge polarization voltage for large lithium-ion batteries in electric vehicles. J. Ind. Eng. Manag. 2013, 6, 686–697. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Ji, W.; Zhao, X. SOC Estimation of E-Cell Combining BP Neural Network and EKF Algorithm. Processes 2022, 10, 1721. [Google Scholar] [CrossRef]
- Barletta, G.; DiPrima, P.; Papurello, D. Thévenin’s Battery Model Parameter Estimation Based on Simulink. Energies 2022, 15, 6207. [Google Scholar] [CrossRef]
- Poopanya, P.; Sivalertporn, K.; Phophongviwat, T. A Comparative Study on the Parameter Identification of an Equivalent Circuit Model for an Li-ion Battery Based on Different Discharge Tests. World Electr. Veh. J. 2022, 13, 50. [Google Scholar] [CrossRef]
- Thanagasundram, S.; Arunachala, R.; Makinejad, K.; Teutsch, T.; Jossen, A. A Cell Level Model for Battery Simulation. In Proceedings of the European Electric Vehicle Congress, Brussels, Belgium, 20–22 September 2012. [Google Scholar]
- Wu, C.; Fu, R.; Xu, Z.; Chen, Y. Improved state of charge estimation for high power lithium ion batteries considering current dependence of internal resistance. Energies 2017, 10, 1486. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Wei, X. Performance and characteristic research in LiFePO4 battery for electric vehicle applications. In Proceedings of the IEEE Vehicle Power and Propulsion Conference, Dearborn, MI, USA, 7–11 September 2009. [Google Scholar]
- Huang, Y.; Li, Y.; Jiang, L.; Qiao, X.; Cao, Y.; Yu, J. Research on Fitting Strategy in HPPC Test for Li-ion battery. In Proceedings of the IEEE Sustainable Power and Energy Conference (iSPEC), Beijing, China, 20–24 October 2019. [Google Scholar]
- López, V.; Jove, E.; Zayas Gato, F.; Pinto-Santos, F.; Piñón-Pazos, A.J.; Casteleiro-Roca, J.-L.; Quintian, H.; Calvo-Rolle, J.L. Intelligent Model for Power Cells State of Charge Forecasting in EV. Processes 2022, 10, 1406. [Google Scholar] [CrossRef]



| Parameter | Unit | Value |
|---|---|---|
| Nominal Voltage | V | 3.2 |
| Maximal charge voltage | V | 4 |
| Deep discharge voltage | V | 2.5 |
| Operating Voltage | V | 2.8–4 |
| Capacity | Ah | 67 |
| Max discharging current | A | 600 |
| Optimal discharging current | A | 30 |
| Max charging current | A | 180 |
| Optimal charging current | A | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacu, R.C.; Fodorean, D. Lithium-Ion Cell Characterization, Using Hybrid Current Pulses, for Subsequent Battery Simulation in Mobility Applications. Processes 2022, 10, 2108. https://doi.org/10.3390/pr10102108
Nacu RC, Fodorean D. Lithium-Ion Cell Characterization, Using Hybrid Current Pulses, for Subsequent Battery Simulation in Mobility Applications. Processes. 2022; 10(10):2108. https://doi.org/10.3390/pr10102108
Chicago/Turabian StyleNacu, Rares Catalin, and Daniel Fodorean. 2022. "Lithium-Ion Cell Characterization, Using Hybrid Current Pulses, for Subsequent Battery Simulation in Mobility Applications" Processes 10, no. 10: 2108. https://doi.org/10.3390/pr10102108
APA StyleNacu, R. C., & Fodorean, D. (2022). Lithium-Ion Cell Characterization, Using Hybrid Current Pulses, for Subsequent Battery Simulation in Mobility Applications. Processes, 10(10), 2108. https://doi.org/10.3390/pr10102108







