Photocatalytic and Electrochemical Activity of Magnesium Oxide Nanoballs Synthesized via a Hydrothermal Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MgO Nanoballs
2.3. Photocatalytic Activity Study
2.4. Electrochemical Measurement
2.5. Characterisation
3. Results and Discussion
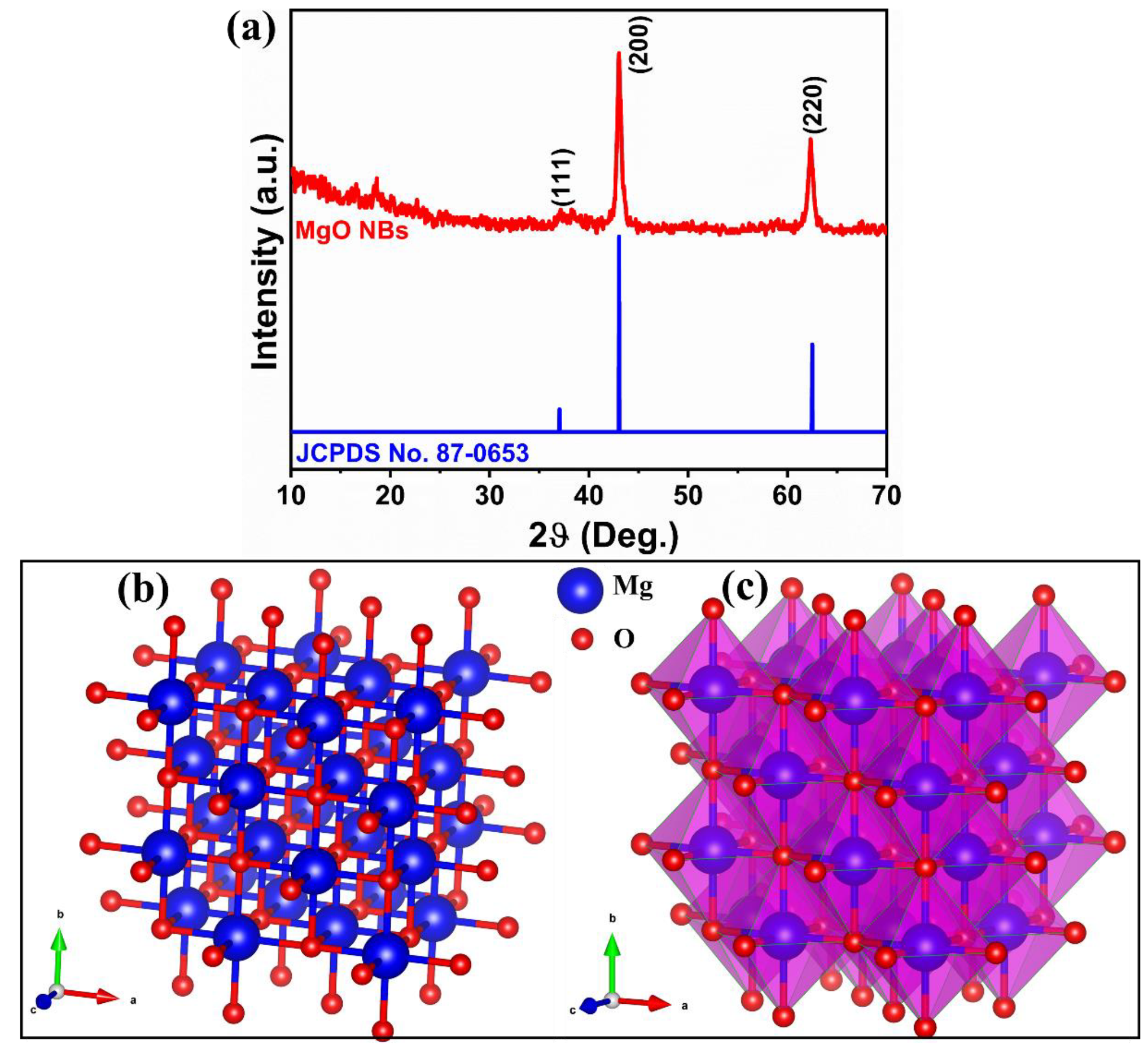
3.1. X-ray Diffraction Analysis
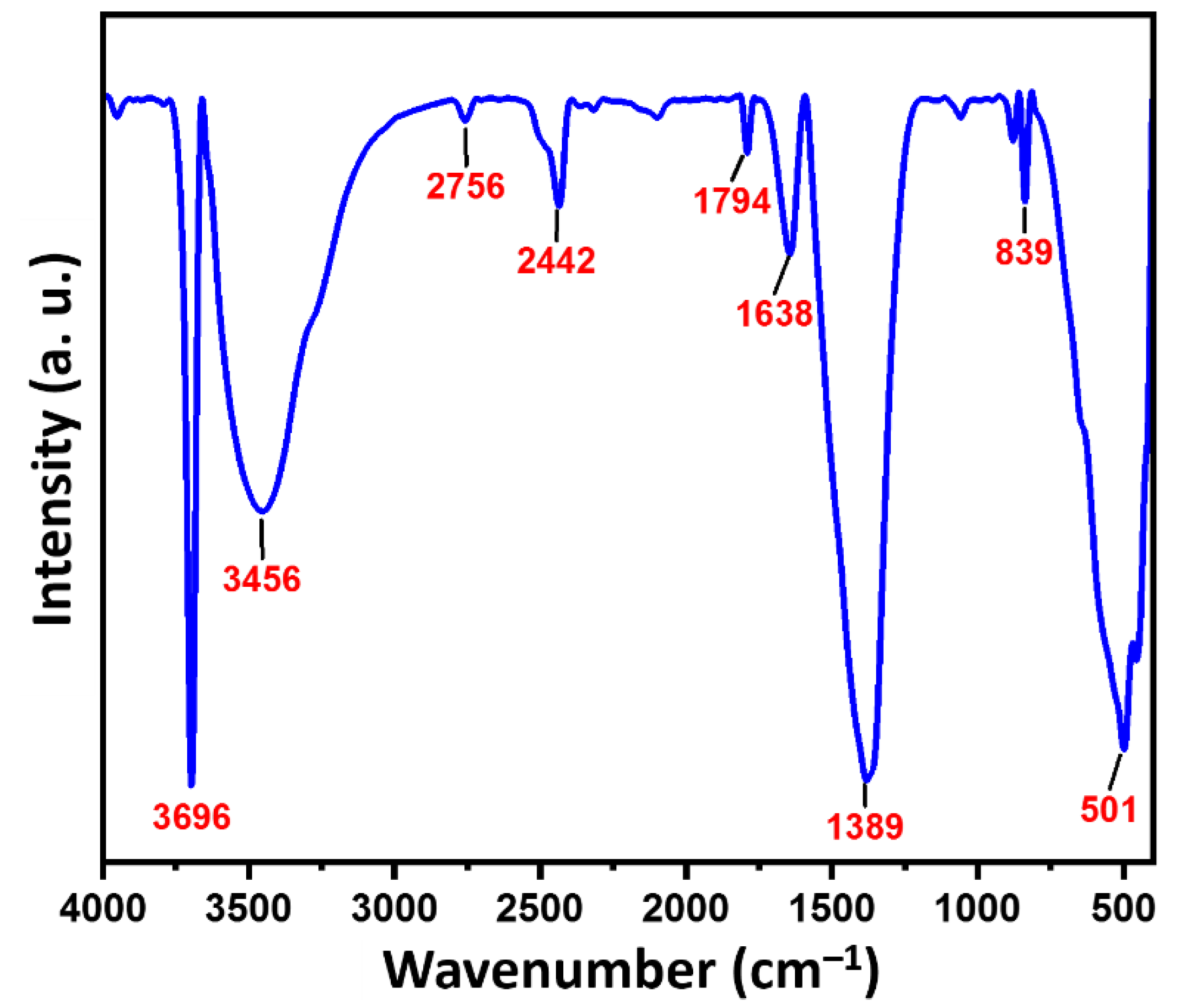
3.2. FTIR Analysis
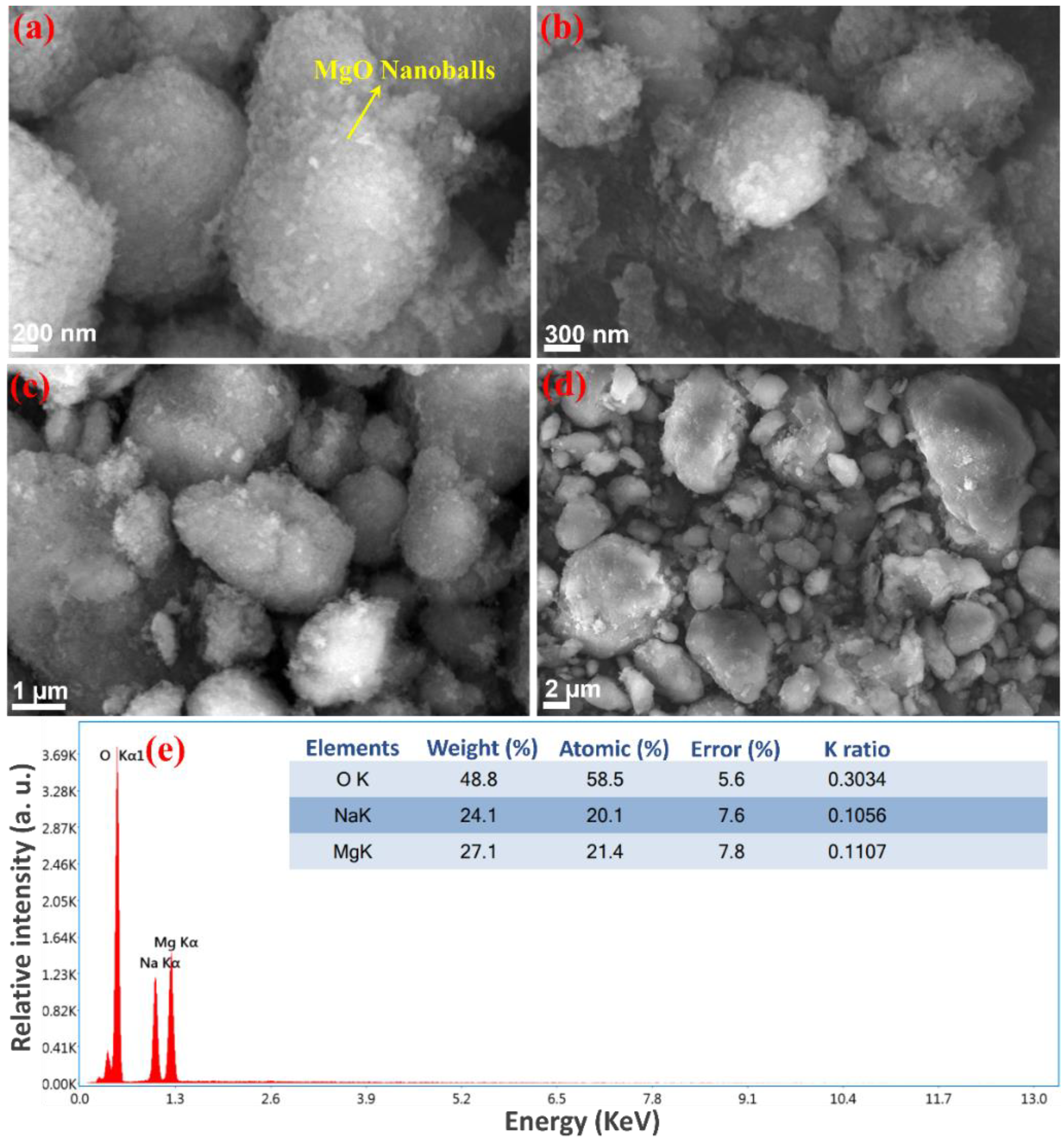
3.3. Morphology
3.4. Dynamic Light Scattering
3.5. Optical Properties
3.6. PL Spectra
3.7. Magnetic Studies
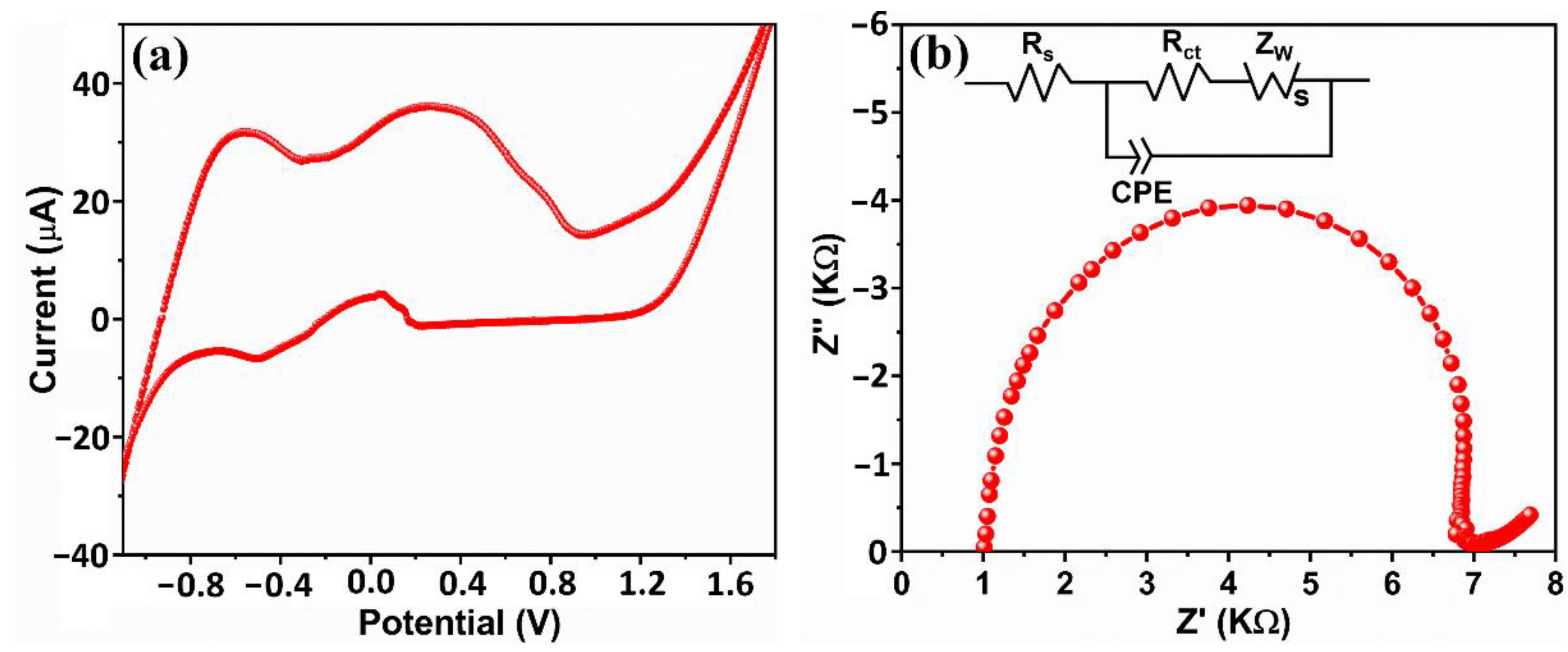
3.8. Electrochemical Studies
3.9. Photocatalytic Activity Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Subhan, M.A.; Choudhury, K.P.; Neogi, N. Advances with Molecular Nanomaterials in Industrial Manufacturing Applications. Nanomanufacturing 2021, 1, 75–97. [Google Scholar] [CrossRef]
- Anita Lett, J.; Sagadevan, S.; Weldegebrieal, G.K.; Fatimah, I. Hydrothermal Synthesis and Photocatalytic Activity of NiO Nanoparticles under Visible Light Illumination. Bull. Chem. React. Eng. Catal. 2022, 17, 340–349. [Google Scholar] [CrossRef]
- Pradeev Raj, K.; Sadaiyandi, K.; Kennedy, A.; Sagadevan, S. Photocatalytic and antibacterial studies of indium-doped ZnO nanoparticles synthesized by co-precipitation technique. J. Mater. Sci. Mater. Electron. 2017, 28, 19025–19037. [Google Scholar] [CrossRef]
- Tharani, K.; Jegatha, C.A.; Sagadevan, S.; Nehru, L.C. Photocatalytic and antibacterial performance of iron oxide nanoparticles formed by the combustion method. Chem. Phys. Lett. 2021, 771, 138524. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Jauhari, M.H.; Maharani, A.A.A.P.; Sagadevan, S.; Oh, W.-C.; Doong, R.-A. Synthesis and control of the morphology of SnO2 nanoparticles via various concentrations of Tinospora cordifolia stem extract and reduction methods. Arab. J. Chem. 2022, 15, 103738. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Ashir Hameed, M.; Mohsin Abrar, M.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Sibhatu, A.K.; Weldegebrieal, G.K.; Sagadevan, S.; Tran, N.N.; Hessel, V. Photocatalytic activity of CuO nanoparticles for organic and inorganic pollutants removal in wastewater remediation. Chemosphere 2022, 300, 134623. [Google Scholar] [CrossRef]
- EL-Mekkawi, D.M.; Abdelwahab, N.A.; Mohamed, W.A.; Taha, N.A.; Abdel-Mottaleb, M.S. Solar photocatalytic treatment of industrial wastewater utilizing recycled polymeric disposals as TiO2 supports. J. Clean. Prod. 2020, 249, 119430. [Google Scholar]
- Fatimah, I.; Purwiandono, G.; Citradewi, P.W.; Sagadevan, S.; Oh, W.-C.; Doong, R.-A. Influencing Factors in the Synthesis of Photoactive Nanocomposites of ZnO/SiO2-Porous Heterostructures from Montmorillonite and the Study for Methyl Violet Photodegradation. Nanomaterials 2021, 11, 3427. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Sagadevan, S.; Oh, W. Enhanced photocatalytic degradation of Acid Blue dye using CdS/TiO2 nanocomposite. Sci. Rep. 2022, 12, 5759. [Google Scholar] [CrossRef]
- Bai, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar]
- Ramanujam, K.; Sundrarajan, M. Antibacterial effects of biosynthesized MgO nanoparticles using an ethanolic fruit extract of Emblica officinalis. J. Photochem. Photobiol. B Biol. 2014, 141, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Wadhawan, S.; Kumar, V.; Mehta, S. Colorimetric sensing of Fe3+ ions in aqueous solution using Magnesium oxide nanoparticles synthesized using green approach. Chem. Phys. Lett. 2018, 706, 53–61. [Google Scholar] [CrossRef]
- Salem, J.K.; El-Nahhal, I.M.; Hammad, T.M.; Kuhn, S.; Sharekh, S.A.; El-Askalani, M.; Rolf, H. Optical and fluorescence properties of MgO nanoparticles in micellar solution of hydroxyethyl laurdimonium chloride. Chem. Phys. Lett. 2015, 636, 26–30. [Google Scholar] [CrossRef]
- Mangalampalli, B.; Dumala, N.; Grover, P. Allium cepa root tip assay in assessment of toxicity of magnesium oxide nanoparticles and microparticles. J. Environ. Sci. 2018, 66, 125–137. [Google Scholar] [CrossRef]
- Shukla, S.K.; Parashar, G.K.; Mishra, A.P.; Misra, P.; Yadav, B.C.; Shukla, R.K.; Bali, L.M.; Dubey, G.C. Nano-like magnesium oxide films and its significance in optical fiber humidity sensor. Sens. Actuators B 2004, 98, 5–11. [Google Scholar] [CrossRef]
- Camtakan, Z.; Erenturk, S.; Yusan, S. Magnesium oxide nanoparticles: Preparation, characterization, and uranium sorption properties. Environ. Prog. Sustain. Energy 2012, 31, 536–543. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J. On the synthesis and optical absorption studies of nano-size magnesium oxide powder. J. Phys. Chem. Solids 2008, 69, 2764–2772. [Google Scholar] [CrossRef]
- Veldurthi, S.; Shin, C.H.; Joo, O.S.; Jung, K.D. Synthesis of mesoporous MgO single crystals without templates. Microporous Mesoporous Mater. 2012, 152, 31–36. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Shi, F. Solvo-or hydrothermal fabrication and excellent carbon dioxide adsorption behaviors of magnesium oxide with multiple morphologies and porous structures. Mater. Chem. Phys. 2011, 128, 348–356. [Google Scholar] [CrossRef]
- Sundararajan, M.; Suresh, J.; Rajiv Gandhi, R. A comparative study on antibacterial properties of MgO nanoparticles prepared under different calcination temperature. Dig. J. Nanomater. Biostruct. 2012, 7, 983–989. [Google Scholar]
- Athar, T.; Hakeem, A.; Ahmed, W. Synthesis of MgO nanopowder via non aqueous sol-gel method. Adv. Sci. Lett. 2012, 7, 27–29. [Google Scholar] [CrossRef]
- Akbari, A.; Sabouri, Z.; Hosseini, H.A.; Hashemzadeh, A.; Khatami, M.; Darroudi, M. Effect of nickel oxide nanoparticles as a photocatalyst in dyes degradation and evaluation of effective parameters in their removal from aqueous environments. Inorg. Chem. Commun. 2020, 115, 107867. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, J.; Thakur, S.; Sharma, S.; Shrivastava, V. Antibacterial study of silver doped zinc oxide nanoparticles against Staphylococcus aureus and Bacillus subtilis. Drug Invent. Today 2013, 5, 50–54. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ahmed, A.I.; Mannaa, M.A. Preparation and characterization of SnO2 doped TiO2 nanoparticles: Effect of phase changes on the photocatalytic and catalytic activity. J. Sci. Adv. Mater. Devices 2019, 4, 400–412. [Google Scholar] [CrossRef]
- Khezrianjoo, S.; Lee, J.; Kim, K.-H.; Kumar, V. Eco-Toxicological and Kinetic Evaluation of TiO2 and ZnO Nanophotocatalysts in Degradation of Organic Dye. Catalysts 2019, 9, 871. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Xu, Y.; Su, Q.; Yang, R.; Wang, L.; Liu, B.; Shen, S.; Jiang, G.; Chen, W.; Wang, S. Hierarchical porous nanosheet-assembled MgO microrods with high adsorption capacity. Mater. Lett. 2014, 116, 332–336. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari, B.A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Pradeep, K.; Ruchika, A.; Kailas, L.; Wasewar, H.U.; Yoo, C. Status of adsorptive removal of dye from textile industry effluent. Desalination Water Treat. 2012, 50, 226–244. [Google Scholar] [CrossRef]
- Menon, P.; Singh, T.S.A.; Panic, N.; Nidheeshd, P.V. Electro-Fenton assisted sonication for removal of ammoniacal nitrogen and organic matter from dye intermediate industrial wastewater. Chemosphere 2021, 269, 128739. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Sergi, G.-S.; Enric, B. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar]
- San, M.S.; Rivero, M.J.; Ortiz, I. Unravelling the Mechanisms that Drive the Performance of Photocatalytic Hydrogen Production. Catalysts 2020, 10, 901. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Kumar, R.T.; Kennedy, L.J.; Vijaya, J.J. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO- micro and nanostructures. J. Alloys Compd. 2011, 509, 9809–9815. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Shamsuzzaman, Microwave-assisted MgO NP catalyzed one-pot multicomponent synthesis of polysubstituted steroidal pyridines. New J. Chem. 2018, 42, 184–197. [Google Scholar] [CrossRef]
- Nga, N.K.; Thuy, C.N.T.; Viet, P.H. Preparation and characterization of a chitosan/MgO composite for the effective removal of reactive blue 19 dye from aqueous solution. J. Sci. Adv. Mater. Devices 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Korbag, I.; Saleh, S.M. Studies on the formation of intermolecular interactions and structural characterization of polyvinyl alcohol/lignin film. Int. J. Environ. Stud. 2016, 73, 226–235. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B Biol. 2019, 190, 8–20. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Micheal, M.K.; Arasu, M.V.; Al-Dhabi, N.A. Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Mater. Lett. 2016, 166, 19–22. [Google Scholar]
- Hench, L.C.; West, J.K. Principles of Electronic Ceramics; John Wiley and Sons: New York, NY, USA, 1990. [Google Scholar]
- Maoz, B.M.; Tirosh, E.; Sadan, M.B.; Markovich, G. Defect-induced magnetism in chemically synthesized nanoscale sheets of MgO. Phys. Rev. B 2011, 83, 161201. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Hua, J.; Yang, C.; Zheng, Y.; Qin, H.; Suna, L.; Konga, X.; Jiang, M. First-principles study of magnetism driven by intrinsic defects in MgO. Solid State Commun. 2009, 149, 855–858. [Google Scholar] [CrossRef]
- Kumara, K.N.S.; Nagaswarupa, H.P.; Mahesh, K.R.V.; Prashantha, S.C.; Mylarappa, M.; Siddeshwara, D.M.K. Synthesis and characterization of nano ZnO and MgO powder by low temperature solution combustion method: Studies concerning electrochemical and photocatalytic behavior. Nanosyst. Phys. Chem. Math. 2016, 7, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Jain, U.; Pundir, C.S.; Gupta, S.; Chauhan, N. A novel electrochemical comparative sensing interface of MgO nanoparticles synthesized by different methods. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2017, 233, 753–762. [Google Scholar] [CrossRef]
- Mathialagan, A.; Manavalan, M.; Venkatachalam, K.; Mohammad, F.; Oh, W.C.; Sagadevan, S. Fabrication and physicochemical characterization of g-C3N4/ZnO composite with enhanced photocatalytic activity under visible light. Opt. Mater. 2020, 100, 109643. [Google Scholar] [CrossRef]
- Muthukumaran, M.; Prasath, P.V.; Kulandaivelu, R.; Sagadevan, S.; Mohammad, F.; Oh, W.C. Fabrication of nitrogen-rich graphitic carbon nitride/Cu2O (gC3N4@Cu2O) composite and its enhanced photocatalytic activity for organic pollutants degradation. J. Mater. Sci. Mater. Electron. 2020, 31, 2257–2268. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Velavan, R.; Batoo, K.M.; Emad; Raslan, H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Kumar, M.R.A.; Mahendra, B.; Nagaswarupa, H.P.; Surendra, B.S.; Ravikumar, C.R.; Shetty, K. Photocatalytic Studies of MgO Nano Powder; Synthesized by Green Mediated Route. Mater. Today: Proc. 2018, 5, 22221–22228. [Google Scholar]
- Bagheri GH, A.; Sabbaghanb, M.; Mirgani, Z. A comparative study on properties of synthesized MgO with different templates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1286–1291. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Amer, M.S.; Ahamed, M. Facile Synthesis, Characterization, Photocatalytic Activity, and Cytotoxicity of Ag-Doped MgO Nanoparticles. Nanomaterials 2021, 11, 2915. [Google Scholar] [CrossRef]




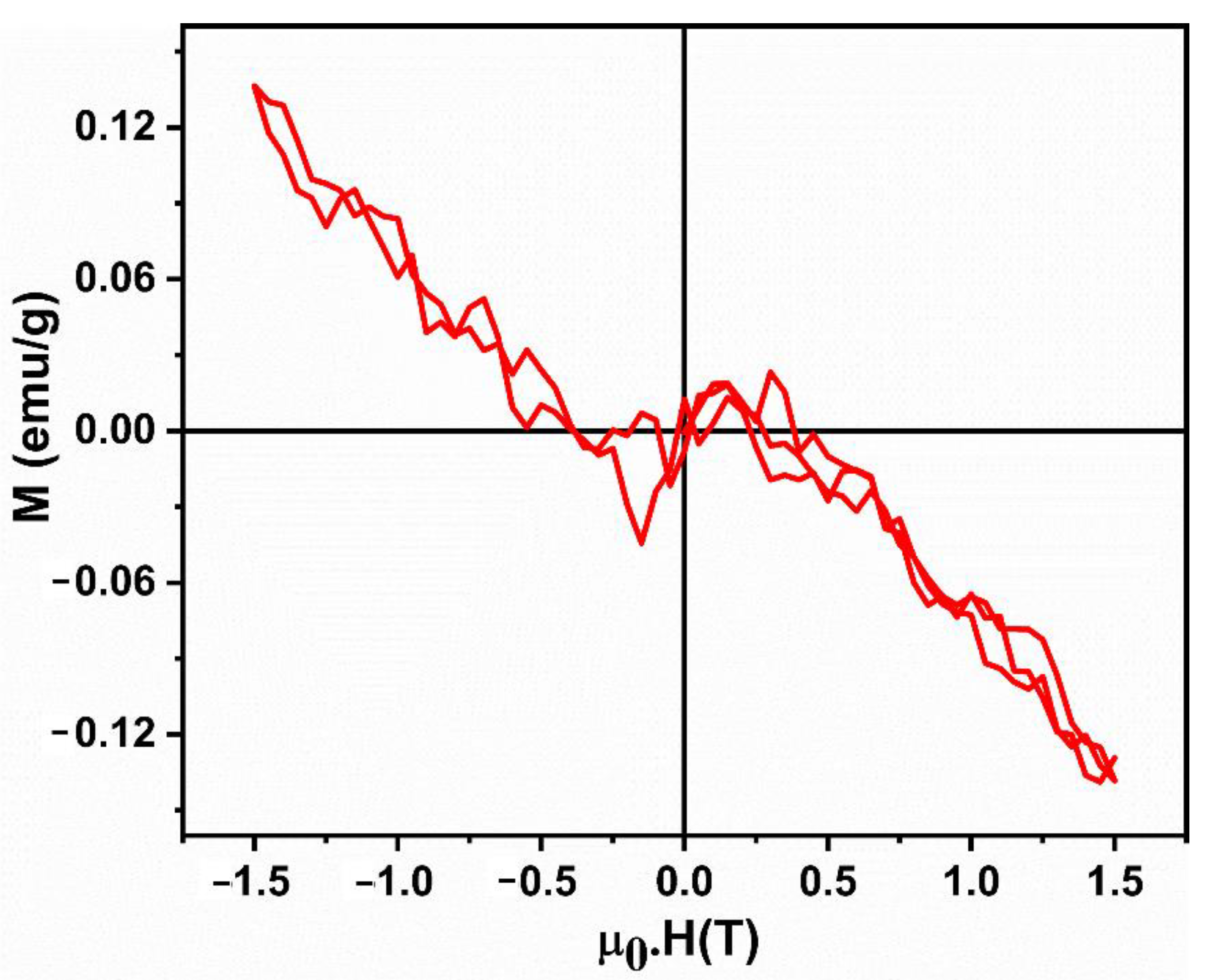

| Mr | Mmax | Hci | χ = M/H |
|---|---|---|---|
| 10−6 (emu/g) | (emu/g) | (G) | 10−8 (emu/gG) |
| 17.39 | −0.00021989 | 972.33 | −1.4659 |
| S.No. | Type of Nanocomposites | Synthesis Method | Contaminant (s) | Light | % Removal | Pseudo-First-Order Rate Constant | Reference |
|---|---|---|---|---|---|---|---|
| 1 | MgO nanostructures | microwave-assisted | MB | sunlight irradiation | 88% | 0.02086 min−1 | [42] |
| 2 | MgO nanostructures | hydrothermal | MB | sunlight irradiation | 92% | 0.02611 min−1 | [42] |
| 3 | MgO nanostructures | microwave-assisted | CR | sunlight irradiation | 82% | 0.00851 min−1 | [42] |
| 4 | MgO nanostructures | hydrothermal | CR | sunlight irradiation | 86% | 0.01189 min−1 | [42] |
| 5 | MgO Nanoparticles | combustion method | MB | UV irradiation. | 75% | - | [51] |
| 6 | MgO nano powders | bio mediated route | MG | sunlight irradiation | - | - | [52] |
| 7 | MgO nano powders | bio mediated route | MG | UV light | - | - | [52] |
| 8 | MgO nano powders | bio mediated route | Rh-B | sunlight irradiation | - | - | [52] |
| 9 | MgO nano powders | bio mediated route | Rh-B | UV light | - | - | [52] |
| 10 | MgO nano powders | IC dye | UV light irradiation | - | - | [53] | |
| 11 | MgO Nanoparticles | Green synthesis | MB | ultraviolet (UV) | 70% | - | [54] |
| 12 | MgO Nanoparticles | sol-gel method | MB | UV irradiation | 52% | 0.0038 min−1 | [55] |
| 13 | MgO Nanoparticles | Hydrothermal synthesis | MB | Visible | 27% | 0.00252 min−1 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagadevan, S.; Lett, J.A.; Fatimah, I.; Selvi, K.T.; Sivasankaran, R.P.; Weldegebrieal, G.K.; Oh, W.-C. Photocatalytic and Electrochemical Activity of Magnesium Oxide Nanoballs Synthesized via a Hydrothermal Route. Processes 2022, 10, 2098. https://doi.org/10.3390/pr10102098
Sagadevan S, Lett JA, Fatimah I, Selvi KT, Sivasankaran RP, Weldegebrieal GK, Oh W-C. Photocatalytic and Electrochemical Activity of Magnesium Oxide Nanoballs Synthesized via a Hydrothermal Route. Processes. 2022; 10(10):2098. https://doi.org/10.3390/pr10102098
Chicago/Turabian StyleSagadevan, Suresh, J. Anita Lett, Is Fatimah, K. Tamizh Selvi, Ramesh Poonchi Sivasankaran, Getu Kassegn Weldegebrieal, and Won-Chun Oh. 2022. "Photocatalytic and Electrochemical Activity of Magnesium Oxide Nanoballs Synthesized via a Hydrothermal Route" Processes 10, no. 10: 2098. https://doi.org/10.3390/pr10102098
APA StyleSagadevan, S., Lett, J. A., Fatimah, I., Selvi, K. T., Sivasankaran, R. P., Weldegebrieal, G. K., & Oh, W.-C. (2022). Photocatalytic and Electrochemical Activity of Magnesium Oxide Nanoballs Synthesized via a Hydrothermal Route. Processes, 10(10), 2098. https://doi.org/10.3390/pr10102098








