Comparative Evaluation of Marking Effects of Two Fluorescent Chemicals, Alizarin Red S and Calcein, on Black Sea Bream (Acanthopagrus schlegelii)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Temporary Rearing of Test Fish
2.2. Alizarin Red S (ARS) and Calcein (CAL) Immersion Marking
2.3. Growth Experiment
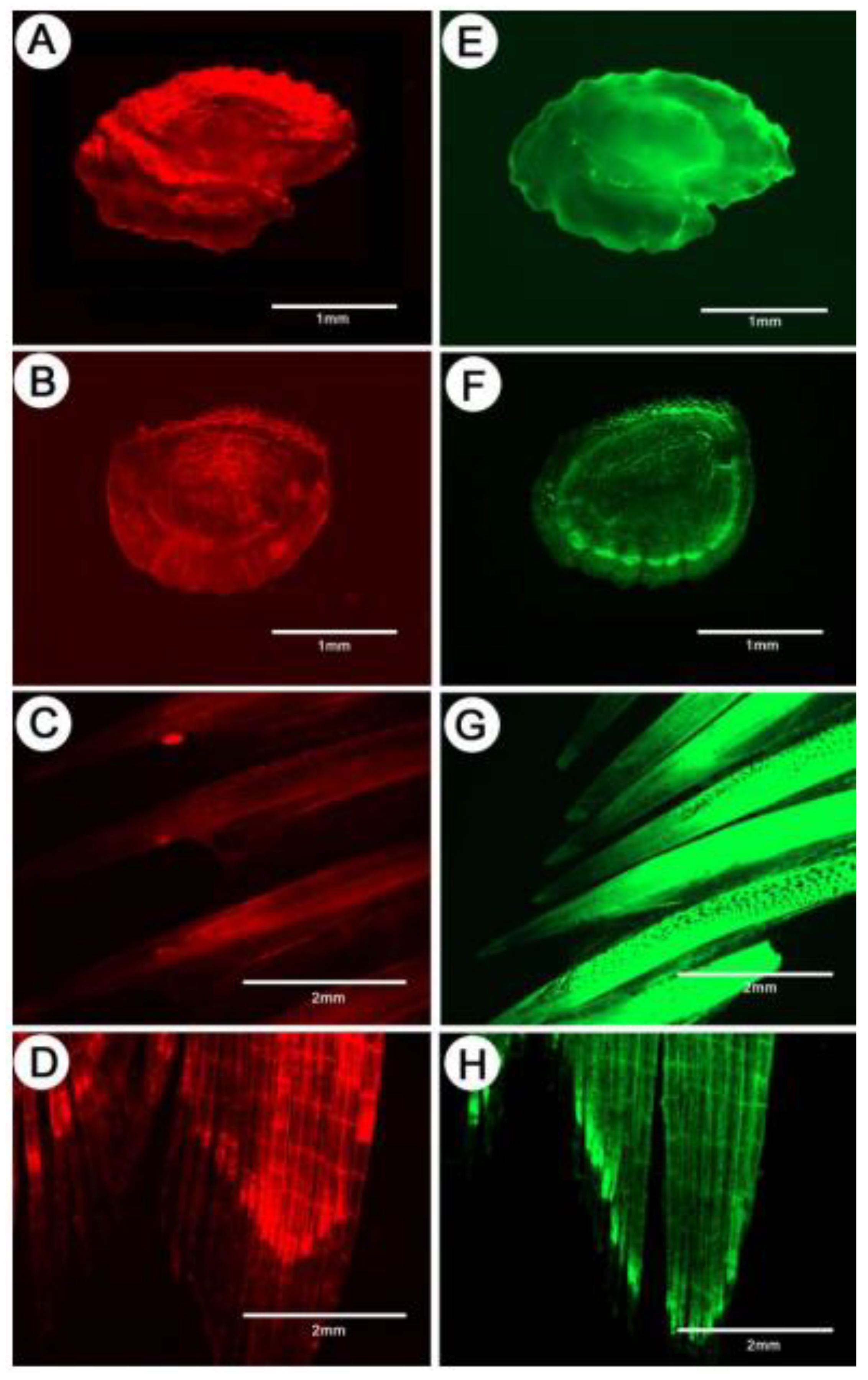
2.4. Sampling and Mark Detection
2.5. Data Analysis
3. Results and Analysis
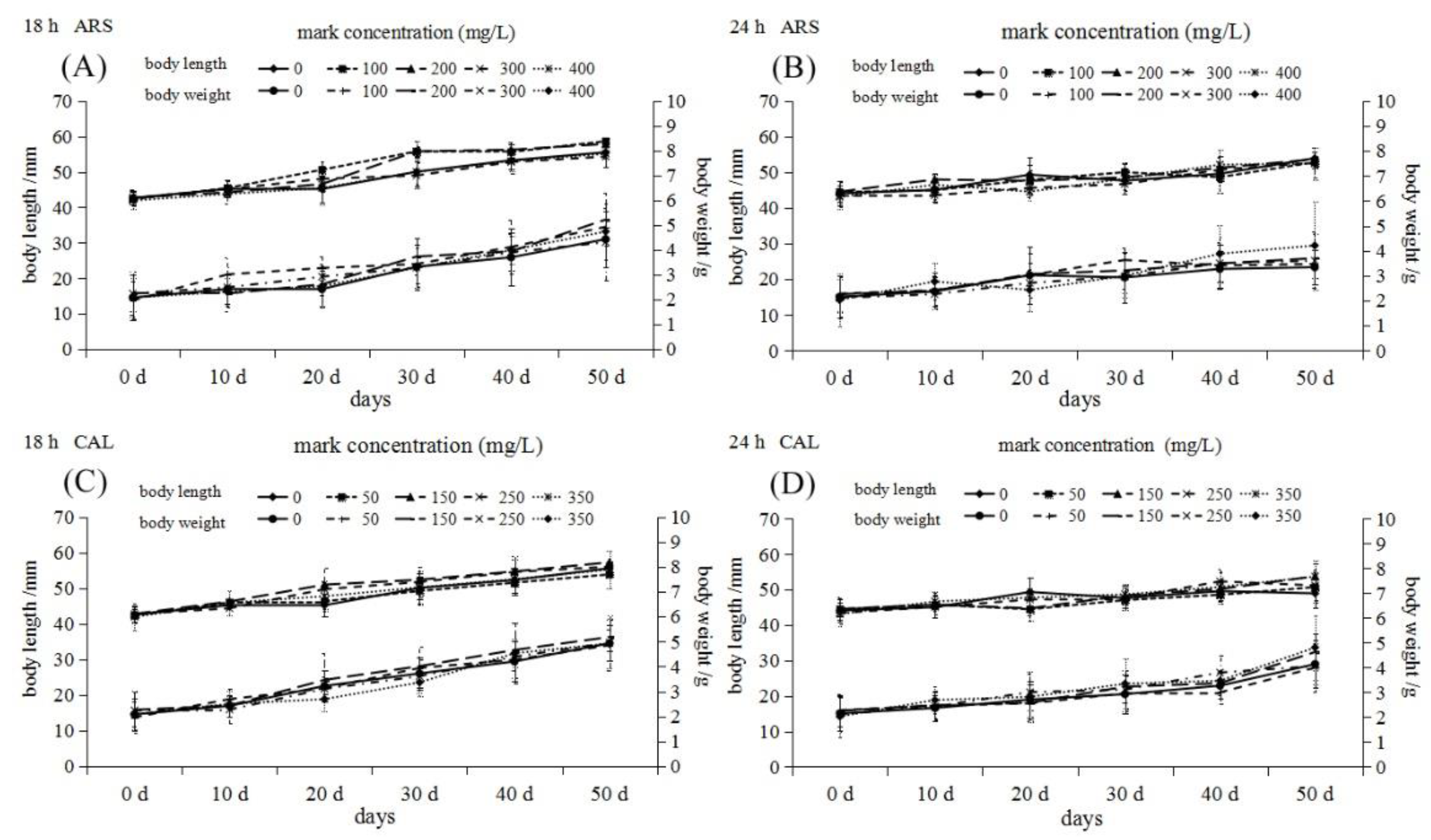
3.1. Mortality after Immersion and Growth after Marking
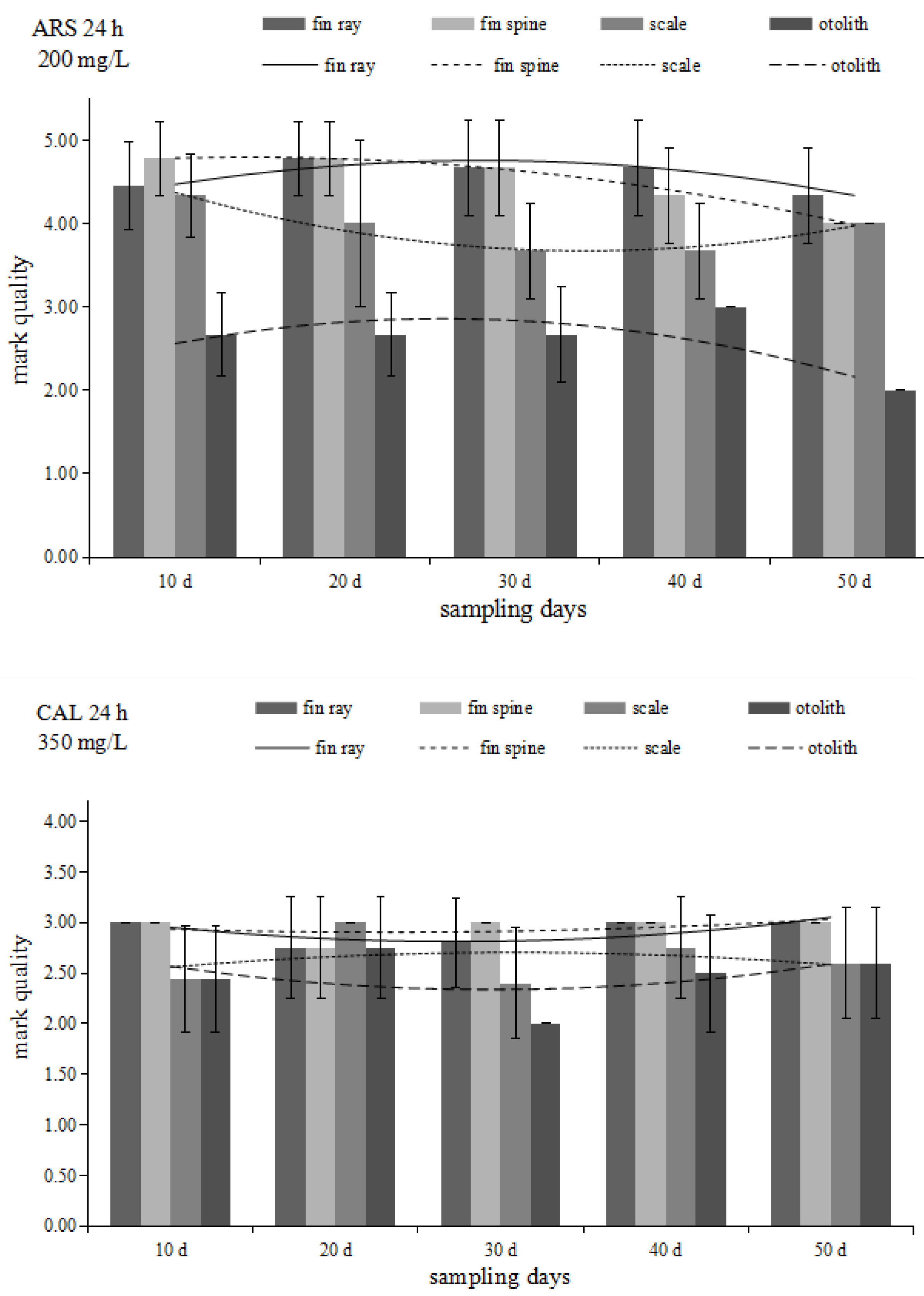
3.2. Mark Quality
3.2.1. Comparison of Mark Quality between Different Sampling Tissues
3.2.2. Comparison of Mark Quality between Immersion Times
3.2.3. Comparison of Mark Quality among Immersion Concentrations
3.2.4. Changes in Mark Quality with Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith-Vaniz, W.F. Reef Fishes of Hong Kong. BioOne 2001, 2001, 577–578. [Google Scholar] [CrossRef]
- Tang, J.H.; Cheng, M.H. Experiments on propagation and release of Sparus macrocephalus in the coastal waters of Jiangsu and Zhejiang Province. J. Shanghai Fish. Univ. 1998, 7, 167–171. [Google Scholar]
- Sadovy, Y.; Cornish, A.S. Reef fishes of Hong Kong; Hong Kong University Press: Hong Kong, China, 2000; p. 321. [Google Scholar]
- Gonzalez, E.B.; Umino, T.; Nagasawa, K. Stock enhancement programme for black sea bream, Acanthopagrus schlegelii (Bleeker), in Hiroshima Bay, Japan: A review. Aquac. Res. 2010, 39, 1307–1315. [Google Scholar] [CrossRef]
- Xu, L.J. Making Juvenile Zebrafish with Fluorescent Substances and Its Effects on Antioxidases; Southwest University: Chongqing, China, 2012. [Google Scholar]
- Bashey, F. A Comparison of the suitability of alizarin red S and calcein for inducing a nonlethally detectable mark in juvenile guppies. Trans. Am. Fish. Soc. 2004, 133, 1516–1523. [Google Scholar] [CrossRef]
- Lü, H.J.; Fu, M.; Xi, D.; Zhang, Z.X. Experimental evaluation of calcein and alizarin red S for immersion marking of silver carp Hypophthalmichthys molitrix (Valenciennes, 1844). J. Appl. Ichthyol. 2016, 32, 83–91. [Google Scholar] [CrossRef]
- Crook, D.A.; O’Mahony, D.J.; Sanger, A.C.; Munro, A.R.; Gillanders, B.M.; Thurstan, S. Development and evaluation of methods for osmotic induction marking of golden perch Macquaria ambigua with calcein and alizarin reds. N. Am. J. Fish. Manag. 2009, 29, 279–287. [Google Scholar] [CrossRef]
- Morales-Nin, B.; Grau, A.; Pérez-Mayol, S.; Gil, M.M. Marking of otoliths, age validation and growth of Argyrosomus regius juveniles (Sciaenidae). Fish. Res. 2010, 6, 76–80. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Jiang, T.; Chen, X.b.; Liu, H.b.; Yang, J. Effect evaluation of alizarin complexone marking for otoliths of juvenile black carp (Mylopharyngodon piceus). South China Fish. Sci. 2021, 17, 93–100. [Google Scholar]
- Clear, N.P.; Gunn, J.S.; Rees, A.J. Direct validation of annual increments in the otoliths of juvenile southern bluefifin tuna, Thunnus maccoyii, by means of a large-scale mark–recapture experiment with strontium chloride. Fish. Bull. 2000, 98, 25–40. [Google Scholar]
- Blom, G.; Nordeide, J.T.; Svasand, T.; Borge, A. Application of two flfluorescent chemicals, alizarin complexone and alizarin red S, to mark otoliths of Atlantic cod, Gadus morhua L. Aquac. Fish. Manag. 1994, 25, 229–243. [Google Scholar]
- Lü, H.J.; Fu, M.I.; Zhang, Z.X.; Su, S.Q.; Yao, W.Z. Marking fish with fluorochrome dyes. Rev. Fish. Sci. Aquac. 2020, 28, 117–135. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.M.; Zhang, P.D.; Nwafili, S.A. The use of alizarin red S and alizarin complexone for immersion marking Japanese flounder Paralichthys olivaceus. Fish. Res. 2009, 98, 67–74. [Google Scholar] [CrossRef]
- Spires, J.E.; Dungan, C.F.; North, E.W. Marking the shells of pediveliger eastern oysters Crassostrea virginica, with a calcein fluorochrome dye. J. Shellfish. Res. 2022, 40, 479–487. [Google Scholar] [CrossRef]
- Moran, A.L. Calcein as a marker in experimental studies newly-hatched gastropods. Mar. Biol. 2000, 137, 893–898. [Google Scholar] [CrossRef]
- Chen, P.M.; Yuan, H.R.; Jia, X.P.; Qin, C.X.; Cai, W.G.; Yu, J.; Su, L.M.; Li, X.G.; Zhou, Y.B. Changes in fishery resources of Yangmeikeng artificial reef area in Daya Bay. South China Fish. Sci. 2013, 9, 100–108. [Google Scholar]
- Liu, Y.; Sun, D.R.; Geng, Q.; Yang, C.P.; Zhao, J.; Duan, Y. Comparative study on immersion marking with alizarin red S and calcein for black porgy Acanthopagrus schlegelii of different sizes. South China Fish. Sci. 2016, 12, 17–24. [Google Scholar]
- Lv, H.J. Experimental Studies of Marking Techniques and Mark-Recapture for Juvenile Black Rockfish (Sebasews schlegelii) and Japanese Flounder (Paralichthys olivaceus); Ocean University of China: Qingdao, China, 2013. [Google Scholar]
- Katayama, S.; Isshiki, T. Variation in otolith macrostructure of Japanese flounder (Paralichthys olivaceus): A method to discriminate between wild and released fish. J. Sea Res 2007, 57, 180–186. [Google Scholar] [CrossRef]
- Xu, C.; Chen, G.Z.; Lin, X.T.; Yao, D.Z.; Zhao, T. Otolith tagging and daily increments validation with fluoresecent substance in larval Channa asiatica. J. Hydro. 2012, 33, 110–114. [Google Scholar]
- Daniel, W.B.; Robert, G.S. A simple method for marking fish otoliths with alizarin compounds. T. Am. Fish. Soc. 1996, 125, 146–149. [Google Scholar]
- Poczyczyński, K.K.; Kozłowski, J.; Martyniak, A. Marking and return method for evaluating the effects of stocking larval vendace, Coregonus albula (L.), into Lake Wigry in 2000–2001. Arch. Pol. Fish. 2011, 19, 259–265. [Google Scholar] [CrossRef]
- Shen, H.; Wan, X.H.; Wang, L.B.; Zhang, Z.Y.; Yao, G.Q.; Hu, C.M.; Ling, Y. Comparative studies of behaviors of the predation of different fishes and hedging of Exopalaemon carinicauda Holthuis. J. Aquac. 2010, 31, 40–42. [Google Scholar]
- Hang, X.R.; Zhuang, P. Status and practice of fish behavioral studies. Freshw. Fish 2002, 32, 53–56. [Google Scholar]
- Wu, C.W.; Xu, M.Y.; Hu, C.C. Study on the behavioral characteristics of fishes in the deep water sea cage. J. Fish. China 2006, 30, 481–488. [Google Scholar]
- Simon, J.; Dörner, H. Marking the European eel with oxytetracycline, alizarin red and coded wire tags: An evaluation of methods. J. Fish Biol. 2005, 67, 1486–1491. [Google Scholar] [CrossRef]
- Jerre, W.M. Immersion of larval Atlantic salmon in calcein solutions to induce a non-lethally detectable mark. N. Am. J. Fish. Manag. 1997, 17, 751–756. [Google Scholar]
- Monaghan, J. Notes: Comparison of calcein and tetracycline as chemical markers in summer flounder. T. Am. Fish. Soc. 1993, 122, 298–301. [Google Scholar] [CrossRef]
- Le, J.B.; Wei, Q.W.; Sun, Q.L.; Li, L.X.; Gan, F. Alizarin marking of otolith at eyed eggs and larvae stages in Brachymystax lenok tsinlingensis. Freshw. Fish 2011, 41, 10–16. [Google Scholar]
| Light Source | Wavelength | |
|---|---|---|
| Excitation Filter | Barrier Filter | |
| DAPI | 357/44 | 447/60 |
| GFP | 470/22 | 510/42 |
| RFP | 531/40 | 593/40 |
| Concentration mg/L | Marking Duration (h) | Number of Deaths | Total Mortality (%) | |
|---|---|---|---|---|
| Deaths during Marking | Deaths 48 h after Marking | |||
| 0 | 24 | 45 | 3 | 26.67 |
| 18 | 44 | 5 | 27.22 | |
| 100 | 24 | 9 | 15 | 13.33 |
| 18 | 5 | 2 | 3.89 | |
| 200 | 24 | 4 | 4 | 4.44 |
| 18 | 0 | 0 | 0.00 | |
| 300 | 24 | 4 | 1 | 2.78 |
| 18 | 0 | 1 | 0.56 | |
| 400 | 24 | 0 | 1 | 0.56 |
| 18 | 0 | 1 | 0.56 | |
| Concentration mg/L | Marking Duration (h) | Number of Deaths | Total Mortality (%) | |
|---|---|---|---|---|
| Deaths during Marking | Deaths 48 h after Marking | |||
| 0 | 24 | 45 | 3 | 26.67 |
| 18 | 44 | 5 | 27.22 | |
| 50 | 24 | 23 | 1 | 13.33 |
| 18 | 13 | 1 | 7.78 | |
| 150 | 24 | 28 | 5 | 18.33 |
| 18 | 15 | 1 | 8.89 | |
| 250 | 24 | 14 | 4 | 10.00 |
| 18 | 4 | 2 | 3.33 | |
| 350 | 24 | 12 | 3 | 8.33 |
| 18 | 4 | 2 | 3.33 | |
| Time | Concentration mg/L | 24 h | 18 h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fin Spine (P) | Fin Ray (P) | Scale | Otolith | Fin Spine (P) | Fin Ray (P) | Scale | Otolith | ||
| 10 d | 100 | 4.78 ± 0.44 | 5.00 ± 0.00 | 3.67 ± 0.71 | 2.44 ± 0.53 | 4.00 ± 0.00 | 4.25 ± 0.50 | 3.50 ± 1.00 | 3.00 ± 0.00 |
| 200 | 4.44 ± 0.53 | 4.78 ± 0.44 | 4.33 ± 0.50 | 2.67 ± 0.50 | 4.00 ± 0.00 | 4.50 ± 0.58 | 4.00 ± 0.00 | 2.75 ± 0.50 | |
| 300 | 4.78 ± 0.44 | 4.78 ± 0.44 | 4.33 ± 0.50 | 2.33 ± 0.50 | 4.00 ± 0.00 | 4.00 ± 0.00 | 3.75 ± 0.50 | 2.50 ± 0.58 | |
| 400 | 4.78 ± 0.44 | 4.78 ± 0.44 | 4.22 ± 0.67 | 2.44 ± 0.53 | 4.00 ± 0.00 | 4.00 ± 0.00 | 3.25 ± 0.50 | 2.25 ± 0.50 | |
| 20 d | 100 | 4.78 ± 0.44 | 4.89 ± 0.33 | 4.00 ± 0.71 | 2.64 ± 0.50 | 4.00 ± 0.00 | 4.40 ± 0.55 | 2.20 ± 0.45 | 2.00 ± 0.00 |
| 200 | 4.78 ± 0.44 | 4.78 ± 0.44 | 4.00 ± 1.00 | 2.67 ± 0.50 | 3.25 ± 0.96 | 3.50 ± 1.00 | 3.00 ± 1.15 | 1.75 ± 0.50 | |
| 300 | 4.78 ± 0.44 | 4.89 ± 0.33 | 3.67 ± 0.87 | 2.56 ± 0.53 | 4.00 ± 0.00 | 4.00 ± 0.00 | 2.00 ± 0.00 | 1.40 ± 0.55 | |
| 400 | 4.44 ± 0.53 | 4.44 ± 0.53 | 3.67 ± 0.71 | 2.78 ± 0.44 | 3.20 ± 0.84 | 3.40 ± 0.89 | 2.40 ± 0.89 | 2.00±0.00 | |
| 30 d | 100 | 4.40 ± 0.55 | 4.60 ± 0.55 | 3.80 ± 1.10 | 2.40 ± 0.55 | 3.80 ± 0.45 | 3.60 ± 0.55 | 2.00 ± 0.00 | 1.80 ± 0.45 |
| 200 | 4.67 ± 0.58 | 4.67 ± 0.58 | 3.67 ± 0.58 | 2.67 ± 0.58 | 4.00 ± 0.00 | 4.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | |
| 300 | 4.80 ± 0.45 | 4.60 ± 0.55 | 4.00 ± 0.00 | 2.40 ± 0.55 | 4.00 ± 0.00 | 4.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | |
| 400 | 4.60 ± 0.55 | 4.40 ± 0.55 | 4.00 ± 0.71 | 2.40 ± 0.55 | 4.20 ± 0.45 | 4.20 ± 0.45 | 2.40 ± 0.89 | 1.80 ± 0.45 | |
| 40 d | 100 | 3.50 ± 0.58 | 4.25 ± 0.50 | 2.75 ± 0.50 | 2.25 ± 0.50 | 3.80 ± 0.45 | 3.60 ± 0.55 | 2.00 ± 0.00 | 2.00 ± 0.00 |
| 200 | 4.67 ± 0.58 | 4.33 ± 0.58 | 3.67 ± 0.58 | 3.00 ± 0.00 | 3.67 ± 0.58 | 3.67 ± 0.58 | 2.00 ± 0.00 | 2.00 ± 0.00 | |
| 300 | 3.67 ± 1.61 | 3.61 ± 1.56 | 2.86 ± 1.24 | 2.43 ± 1.11 | 4.00 ± 0.00 | 3.80 ± 0.45 | 2.00 ± 0.00 | 2.00 ± 0.00 | |
| 400 | 4.12 ± 0.98 | 4.11 ± 1.00 | 3.10 ± 0.77 | 2.69 ± 0.62 | 4.00 ± 0.00 | 4.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | |
| 50 d | 100 | 3.67 ± 0.58 | 3.67 ± 0.58 | 3.33 ± 0.58 | 2.33 ± 0.58 | 4.00 ± 0.00 | 3.67 ± 0.58 | 2.00 ± 0.00 | 2.00 ± 0.00 |
| 200 | 4.33 ± 0.58 | 4.00 ± 0.00 | 4.00 ± 0.00 | 2.00 ± 0.00 | 3.80 ± 0.45 | 3.80 ± 0.45 | 2.00 ± 0.00 | 2.00 ± 0.00 | |
| 300 | 3.67 ± 0.58 | 3.67 ± 0.58 | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.60 ± 0.89 | 3.60 ± 0.89 | 2.00 ± 0.00 | 2.00 ± 0.00 | |
| 400 | 4.00 ± 0.63 | 4.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.80 ± 0.45 | 3.60 ± 0.55 | 2.00 ± 0.00 | 2.00 ± 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Guo, Y.; Liu, M.; Shan, B.; Wang, L.; Yu, W.; Yang, C.; Sun, D. Comparative Evaluation of Marking Effects of Two Fluorescent Chemicals, Alizarin Red S and Calcein, on Black Sea Bream (Acanthopagrus schlegelii). Processes 2022, 10, 2041. https://doi.org/10.3390/pr10102041
Liu Y, Guo Y, Liu M, Shan B, Wang L, Yu W, Yang C, Sun D. Comparative Evaluation of Marking Effects of Two Fluorescent Chemicals, Alizarin Red S and Calcein, on Black Sea Bream (Acanthopagrus schlegelii). Processes. 2022; 10(10):2041. https://doi.org/10.3390/pr10102041
Chicago/Turabian StyleLiu, Yan, Yunfeng Guo, Manting Liu, Binbin Shan, Liangming Wang, Wei Yu, Changping Yang, and Dianrong Sun. 2022. "Comparative Evaluation of Marking Effects of Two Fluorescent Chemicals, Alizarin Red S and Calcein, on Black Sea Bream (Acanthopagrus schlegelii)" Processes 10, no. 10: 2041. https://doi.org/10.3390/pr10102041
APA StyleLiu, Y., Guo, Y., Liu, M., Shan, B., Wang, L., Yu, W., Yang, C., & Sun, D. (2022). Comparative Evaluation of Marking Effects of Two Fluorescent Chemicals, Alizarin Red S and Calcein, on Black Sea Bream (Acanthopagrus schlegelii). Processes, 10(10), 2041. https://doi.org/10.3390/pr10102041







