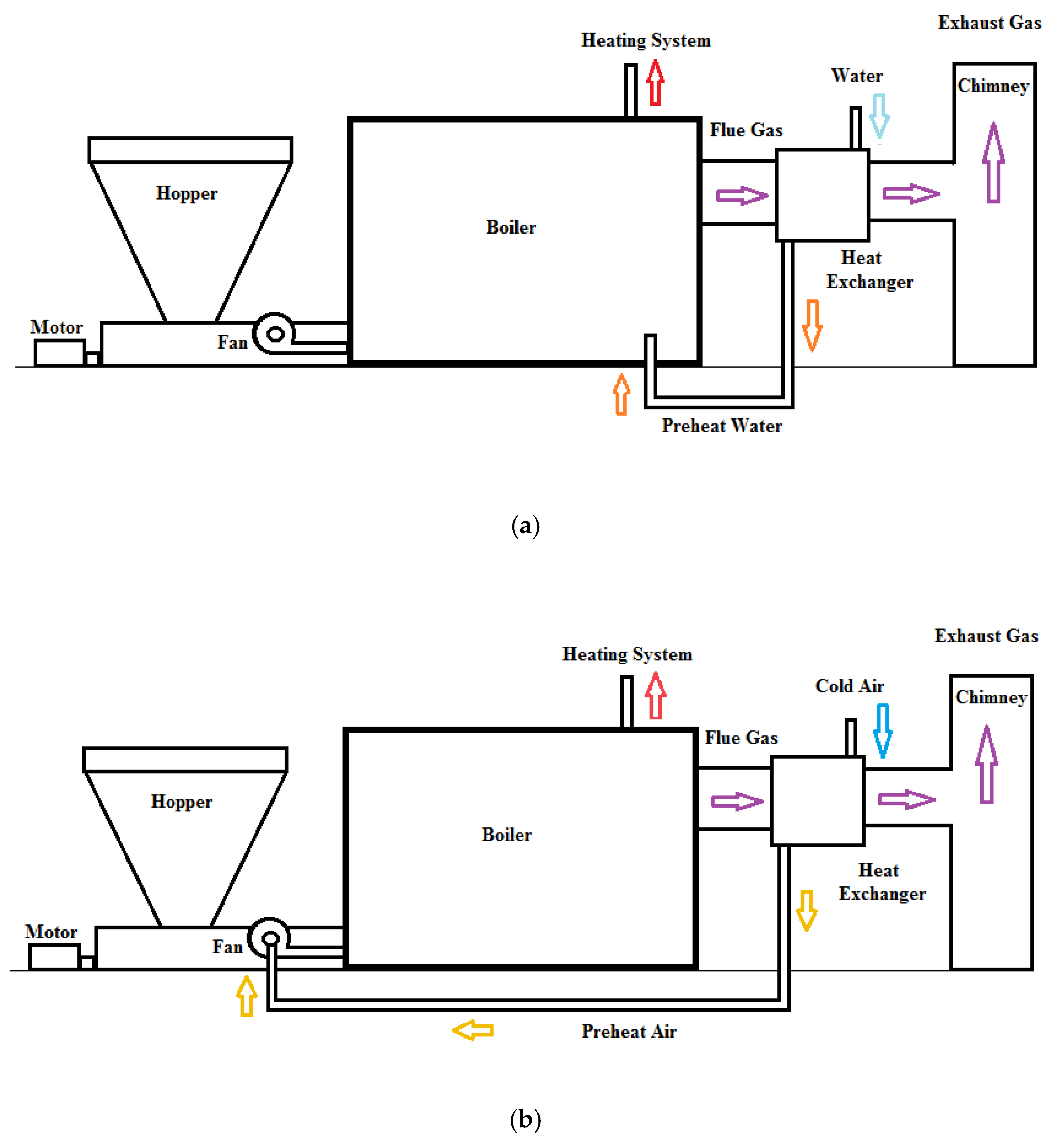
1. Introduction
One source of energy losses in buildings is those that occur during heating. The most widely used method for heating buildings is to obtain heat energy by burning fossil fuels. As a result of losses in boilers, not all of the energy obtained from fossil fuels can be used. Important losses occur in boiler chimneys as a result of fuel combustion. Considering the number of boilers used in various sectors, this loss has increased significantly in recent years [
1].
As a result of the reduction in energy resources, waste heat needs to be recovered. The advantages of waste heat recovery can be classified into two categories: direct and indirect. The advantages of direct heat recover are the reduction in consumption and cost, whereas the advantages of indirect heat recovery are the reduction in air pollution and the sizing of equipment, such as fans, chimneys and boiler air ducts [
2].
In the design of heat recovery units in boilers, important parameters include the amounts of carbon, hydrogen, oxygen and sulfur contained in the fuel. Van Krevelen graphs classify solid fuels based on the mole ratios of H/C and O/C, determining the quality of solid fuels. The fuel quality is important in chimney heat recovery. Apart from this, the dew point temperatures of the water and sulfuric acid vapor in the chimney are among other important parameters. In addition, outdoor temperature and relative humidity are also effective parameters [
3,
4].
In flue gas heat recovery applications, sulfur dioxide in the flue gas reacts chemically with water vapor to release sulfuric acid. When the water and sulfuric acid vapor in the flue gas come into contact with the cold surfaces in the heat exchanger and inside the chimney, if the temperature drops below the dew point, the water and sulfuric acid vapors condense, causing corrosion. The dew point temperature of sulfuric acid is higher than that of water vapor [
4]. These dew point temperatures, which are important in recovering heat from the boiler flue gas, present the problem of low-temperature corrosion [
5].
In coal-fired boilers, 4–8% of the heat content of the fuel is carried by the flue gas and can be converted into thermal energy [
6]. Boilers can generally lose 20% of their combustion energy through flue gas and can recover 50% of this lost energy according to operating conditions by using a flue gas heat exchanger [
7].
The change in the temperature of the boiler flue gases is a result of the heat exchange between the flue gases and a heated fluid, depending on the type of boiler. The temperature of the flue gases is also affected by the amount of supplied air, which is not involved in combustion and must be heated from ambient temperature to outlet temperature [
8].
The most common methods used to reduce the exhaust flue gas temperature and increase the waste heat recovery are preheating of the air supplied to the boiler, preheating of the water supplied to the boiler and obtaining hot water for use. To this end, a heat exchanger is placed in the chimney. Here, the heat transfer surface in the heat exchanger should be well-calculated. Increasing this surface area may not achieve the desired energy savings and may also increase the cost of the heat exchanger [
9].
According to a literature review, Jamil et al. [
1] et al. investigated the usability of a boiler flue heat recovery system to heat boiler return water and boiler feed air. To this end, they designed a recovery system consisting of an indirect contact condensation unit, a mechanical compression heat pump and a combination of two air preheaters. Thermodynamic analyses of the system were conducted. Sensitivity analysis was carried out to determine effects of different operating parameters on the performance of the chimney heat recovery system and the boiler. Terhan and Comakli [
2] conducted energy and exergy analyses for flue gases, which represent the largest heat losses in boilers, in the district heating system of a university comprising natural-gas-fired boilers. The ratio of flue gas energy to exergy losses in the boilers was determined. Engin et al. [
3] presented the results of an extensive experimental study on the combustion of low-grade Turkish lignites in a 30 kWh circulating fluidized bed combustor. Terhan [
4] analyzed the dew point temperatures of water and sulfuric acid vapors in flue gas by burning fuels such as coal, fuel oil, natural gas, LPG and wood in boilers in multiple cities in Turkey. Jin Y. [
5] et al. examined a waste heat recovery system with an organic liquid cycle integrating an organic Rankine cycle in a 660 MW supercritical coal-fired power plant. Wei et al. [
6] investigated a new system using the residual heat in sulfur-reduced flue gas using absorption technologies to reduce the waste flue gas temperature in the boiler at the Jinan-Beijiao coal-fired cogeneration power plant in China. The recovered heat was then used to heat the return water. Terhan and Comakli [
7] investigated the condensation of water vapor in flue gas to recover the latent heat of the exhaust flue gas of 60 MW natural-gas-fired boilers in a district heating system of a university. The design calculations of a flue gas condenser consisting of reverse-flow U-shaped stainless steel pipe bundles were performed with a computer program using the one-dimensional finite difference method. Bukowska et al. [
8] investigated chimney heat recovery proposals in bituminous coal-fired boilers with varying loads in Poland. The thermal losses of the boilers, the overall efficiency of the boiler plant, the exhaust gas temperature and the excess air factor were examined. In chimney heat recovery, a heat exchanger (economizer) was proposed to preheat the hot water supplied to a boiler. Xu et al. [
9] proposed a flue gas heat recovery system and an additional economizer system for coal-fired boilers. Direct use of flue gas energy and indirect flue gas heat recovery systems were investigated. Shamsi et al. [
10] et al. suggested a new waste heat and water recovery system that produces power in a thermal power plant, both working with the organic Rankine cycle and using a single working fluid consisting of cooling cycles. Kon and Caner [
11] investigated the heat recovery potential from flue gases of natural gas-fired boilers in a central heating system for four climatic regions of Turkey. The recovery energy potential of the flue gases was calculated according to the air excess coefficients and flue gas temperatures of the boilers. Men et al. [
12] proposed a new flue gas waste heat recovery system in Beijing to improve the thermal efficiency of a steam pump boiler with flue gas recirculation system and reduce oxynitride emissions. In the system, the oxidizing air is preheated and humidified before combustion in the boiler. Zhao et al. [
13] analyzed the waste heat potential of flue gas to increase the efficiency of a space heating system with natural gas and identified problems associated with the conventional space heating system. Wei et al. [
14] performed detailed calculations of acid condensation in coal-fired boilers. A detailed new calculation model for the acid dew point was proposed. Kon and Caner [
15] investigated the potential of flue gas heat recovery in heating installations in buildings due to the increase in the surface area and volume with an increase in the number of buildings to be heated. Xiang et al. [
16] developed a thermodynamic formula for acid dew point estimation to reduce the temperature of exhaust flue gas in boilers. Accordingly, a quasi-experimental prediction model was proposed. Blanco and Pena [
17] investigated the effects of acid dew point temperature on preheaters in a thermal power plant. The service life and maintenance cost of the equipment were examined in the transition to different fuels. To recover flue gas waste heat, a steam pump system consisting of a gas condenser, an air humidifier and a gas–water heat exchanger was installed by Wei et al. [
18]. Then, they investigated the heating and humidification of boiler oxidizing air by means of a condenser and humidifier and investigated flue gas dew point improvement. Lu et al. [
19] investigated a new gas-fired absorption heat pump, investigating the sensible and latent heats, i.e., the waste heat of flue gas, their recovery in the solution preheater and an intermediate evaporator. Chakrovorty et al. [
20] conducted research to reduce PM, SO
2, NO
x and CO
2 emissions and achieve waste heat recovery from a specially modified chimney for local rice boiling industries. Xiao et al. [
21] investigated the use of economizers for flue gas waste heat recovery in a coal-fired 600 MW ultra-supercritical power plant in China. The simulation results were examined to determine the effect of flue gas exhaust temperature on reducing the flue gas acid dew point in the presented economizer system. Kon and Yuksel [
22] investigated the flue gas heat recovery potential for a 600,000 kcal/h natural-gas-fired boiler in a seven-story public building in Balıkesir and recorded flue gas measurements. The authors determined that the chimney has the potential for heat recovery by obtaining waste heat for hot water. Xun et al. [
23] proposed a new type of low-pressure intermediate plate economizer for flue gas recovery in coal-fired boilers. The performance of this economizer was investigated by numerical simulations, and the results were compared experimentally. Niu et al. [
24] examined an absorption heat pump system and investigated its use in flue gas heat recovery with natural gas in northern China, examining the temperature reduction in heat exchangers.
Thiyagu et al. [
25] examined an economizer to heat the feed water supplied to the boiler and a heat exchanger to heat the air supplied to the boiler in order to recover flue gas heat. The heat exchanger was analyzed using ANSYS 14.5 software. Lepiksaar et al. [
26] investigated heat recovery through a flue gas condenser and the decrease in the return water temperature in a district heating system, estimating the amount of energy savings. A calculation model was developed for various return water temperatures in the district heating system. Abdullah B. [
27] B. Abdullah examined the precautions to be taken in boilers and burners and the factors causing internal cooling of the boiler by conducting a flue gas analysis. Fialko et al. [
28] studied a heat recovery system in which the exhaust gas is cooled below the dew point of water vapor and investigated a protection technology to prevent the formation of condensation in the exhaust gas ducts of boiler plants for high recovery of exhaust gas heat. Liu and Sun [
29] experimentally investigated a flue gas waste heat recovery system was experimentally, considering the acid corrosion of the exhaust gas of boilers in coal-fired power plants. They researched the exhaust gas temperature with varying flue gas flows, flue gas temperatures and ambient air temperatures. Suggestions were made for economizer and air heater properties in chimney heat recovery. Cui et al. [
30] analyzed an absorption heat pump for flue heat recovery of natural-gas-fired boilers. A two-stage waste heat recovery system was optimized, and the dew effect on chimney heat recovery was investigated. Terhan [
31] conducted a combustion analysis of coal fuels for multiple provinces in Turkey with the aim of preventing the risk of corrosion on the surfaces of a heat exchanger designed for the application of latent heat recovery from waste flue gas in boilers. The author determined in which regions of the designed heat exchanger the flue gas temperature reached the dew point temperatures of sulfuric acid and water vapor. The heat exchanger used for the designed air preheating consists of a reverse-flow, U-shaped stainless steel tube bundle. Zhao et al. [
32] examined the problems of district heating systems with natural gas boilers and analyzed the waste heat recovery potential of the chimney. They developed a new process to reduce the outlet temperature of the flue gas to about 20 °C and analyzed the economic performance of the proposed process. Yalçın [
33] calculated the cost of hot water or saturated steam for different air excess coefficients in boilers using natural gas and coal. Thermodynamic calculations were performed for the highest combustion temperature in the boiler in a continuous flow open system; the fuel, air and feed water entering the boiler; and the mass flow rate of the flue gases coming out of the boiler. Kon and Yuksel [
34] determined CO
2 emissions, water vapor and dew point temperature values of the combustion gases released by natural-gas-fired boilers in a heat center in a public building consisting of a seven-story main building and a single-story printing house in Balikesir province. To this end, the flue gases in heating boilers were measured. Differences between theoretical values and measured values were investigated. Kon and Caner [
35] examined the energy properties of new slurry fuels according to their Van Krevelen diagrams. The required stoichiometric air–fuel ratio in the burner was calculated during the combustion process.
The aim of the present study is to determine the flue gas heat recovery potential and its evaluation possibilities for and entire city using the flue gas measurements from boilers of buildings with district and central heating systems containing an average of 41 flats with a central heating system in the city center using coal provided by the Balikesir Municipality Environmental Protection and Control Department of Turkey. Measurements were conducted in February and March 2019. In the city center of Balikesir, the measurements were taken in the district and central heating systems in 12 neighborhoods with central and district heating systems fueled by coal. Because Balikesir has a generally mild climate, it was assumed that the heating system works 14 h a day. In the study, Kütahya Tunçbilek (washed), Kütahya Ömerler (washed), Kütahya Seyitomer-Höyükaltı, Manisa Imbat (washed), Bursa Orhaneli, Bursa Keles and Çanakkale Çan lignite coals from the surrounding cities for heating purposes were used as fuel. We determined the flue gas heat recovery potential of the boilers of these seven types of coal-fired buildings with district and central heating. To determine the flue gas heat recovery potential in lignite coal-burning boilers, we considered the acidification temperature and the amounts of carbon, hydrogen, oxygen and sulfur with respect to the fuel and combustion characteristics of the lignite coal, as well as their ratios to one another. For the flue gas heat recovery evaluation of the boilers, energy savings were achieved by using heat exchangers placed in the flue to heat the feed water supplied to the boiler and to heat the combustion air supplied to the boiler. We determined energy-saving potential for the whole city based on these investigations and research. Buildings with central and district heating systems can benefit from flue gas heat, which has the potential to be used and wasted, therefore achieving energy savings. In solid fuel boilers, the properties and content of solid fuel are important parameters for flue gas recovery. In this study we investigated the flue gas heat recovery potential and usage possibilities in buildings using lignite coal in central and district heating systems.
3. Results and Discussions
We examined the chemical compositions for flue gas heat recovery of seven most used types of lignite coal in Balikesir, which are burned in boilers in central and district heating systems. The highest C ratio of 70.58% is associated with Kütahya Tunçbilek (washed) coal, and the lowest C ratio is 39.21%, corresponding to Çanakkale Çan coal. The highest H ratio is associated with Bursa Orhaneli coal (4.74%) and the lowest H ratio corresponds to Çanakkale Çan coal (2.86%). The highest O rate is associated with Bursa Orhaneli coal (13.50%), and the lowest O rate corresponds to Kütahya Ömerler (washed) coal (5.48%). The highest S ratio is associated with Çanakkale Çan coal (5.69%), and the lowest S ratio corresponds to Manisa Imbat (washed) coal (1.01%). In terms of the compositions of the seven investigated coal types, Kütahya Tunçbilek (washed) coal contains the most C (5.882), and Çanakkale Çan coal contains the least C (3.268). When the H ratio is considered, the highest ratio is associated with Bursa Orhaneli coal (4.740), with the lowest H ratio corresponding to Çanakkale Çan coal (2.860). The highest O rate is associated with Bursa Orhaneli coal (0.844), and the lowest O rate corresponds to Kütahya Ömerler (washed) coal (0.343). The highest S ratio was found in Çanakkale Çan coal (0.178), and the lowest S ratio corresponds to Manisa Imbat (washed) coal (0.032). The highest H/C ratio is associated with Bursa Keles coal (0.945), and the lowest H/C ratio corresponds to was Kütahya Tunçbilek (washed) coal (0.700). The highest O/C ratio was calculated in Bursa Keles coal (0.170), and the lowest O/C ratio corresponds to Kütahya Ömerler (washed) coal (0.059). H/C and O/C are the ratios used to determine the quality of coal as fuel. According to the literature, the fuels with the highest H/C and O/C ratios are those that produce the most heat when burned. The highest water vapor dew point temperature was determined at 19.842 °C in Bursa Keles coal, and the lowest at 19.829 °C in Kütahya Tunçbilek (washed) coal. The water vapor dew point temperature is directly proportional to the H/C and O/C ratios. The water vapor dew point increased with increased carbon content. Carbon is the most important burning element in fuels. Sulfur causes acidification, which damages the flue. Therefore, the acidification temperature of coal-fired boilers is an important factor in the flue. When the temperature drops below this threshold, corrosion and other negative effects are caused in the flue. In the present study, flue gas heat recovery was calculated by reducing the flue gas temperature to the acidification temperature. In the absence of sulfur content in fuels such as natural gas, the flue temperature can be increased to the dew point temperature, which is much lower. Thus, a much higher rate of flue gas heat recovery can be achieved in natural-gas-fired boilers. The highest S/C ratio was calculated in Çanakkale Çan coal (0.054), and the lowest S/C ratio corresponded to Manisa Imbat (washed) (0.006). The highest S/O ratio was found in Çanakkale Çan coal (0.372), and the lowest S/O ratio corresponded to Manisa Imbat (washed) coal (0.044). Therefore, the highest acid dew point temperature was detected in Çanakkale Çan coal (169.713 °C) and the lowest in Manisa Imbat (washed) coal (143.526 °C). The acid dew point temperature of coal with high S/C and S/O ratios is directly proportional. Values related to water vapor dew point temperature and H/C and O/C ratios, as well as acid dew point temperature and S/H and S/O ratios are presented in
Figure 7.
The results of this study can serve as a basis for future studies to determine both the emission and heat recovery potential of the flue gas of boilers, depending on the characteristics of the solid fuel used for central and district heating systems. The recovery potential from flue gases of boilers used in industry has been investigated in the literature. This preliminary study can be extended to settlements that burn solid fuel through central and district heating systems. In the present study, we demonstrated that energy saving can be achieved not only in big cities but also in buildings with a smaller central heating systems. The use of natural gas is increasing in buildings with central and district heating systems, such as in the city of Balikesir. Therefore, in future studies, the flue gas heat recovery potential for systems using natural gas can be investigated. The use of solid, liquid and gas fuels can be compared in terms of flue gas recovery. For settlements such as smaller towns, the potential of flue gas recovery can be investigated for different fuel types.
The highest flue efficiency loss was calculated in Bursa Keles coal (1.734%), and the lowest fuel efficiency loss corresponded to Kütahya Tunçbilek (washed) coal (0.921%). The flue efficiency loss depends on the H/C and O/C ratios of the coal. Coals with high H/C and O/C ratios exhibit high flue efficiency loss. The heating value is inversely proportional to the flue loss. Bursa Keles coal has the lowest heating value of 2.972 kWh, and Kütahya Tunçbilek (washed) coal has the highest heating value of 6.025 kWh. The heating values of the coals depend on the amount of coal consumption in kilograms per second in the central and district heating system, as well as the flue loss values in the boilers after the coal is burned (
Figure 8).
The most fuel is required when burning Bursa Keles coal (739 kg/h), and the least among of fuel is required when burning Kütahya Tunçbilek (washed) coal. Bursa Keles coal has the highest flue gas heat recovery potential of 4,027,889 kWh, with lowest flue gas heat recovery potential corresponding to Kütahya Tunçbilek (washed) coal (2,138,920 kWh). More flue loss occurs in coals with lower heating values and high H/C and O/C ratios, but with more flue gas heat recovery potential. In coals with lower heating values and low H/C and O/C ratios, a large portion of the coal is converted into heat energy via burning in boilers in central and district heating systems. On the other hand, in coals with lower heating values and high H/C and O/C ratios, some of the heat energy is lost as flue loss as a result of combustion, as not all of the coal is burned in the boilers, and smooth combustion does not occur. Changes in H/C, O/C, S/C and S/H ratios in terms of flue gas recovery energy-saving potential of the seven investigates lignite coal types are shown in
Figure 9. Changes in H/C, O/C, S/C and S/H ratios of lignite coals due to flue loss in boilers in apartment buildings with coal-fired district and central heating systems are shown in
Figure 10. The changes in the S/H ratio depending on the S/C ratio and changes in the O/C ratio depending on the H/C ratio of different lignite coal types are shown in
Figure 11.
In order to increase the efficiency of central and district heating systems in apartment buildings by 1%, it is necessary to increase (preheated air) the temperature of the combustion air supplied to the boiler by 20 °C. The efficiency of the heating system will increase by 1% with every 20 °C increase in the temperature of the combustion air supplied to the boiler. Thus, if the combustion air temperature is increased by 40 °C, the efficiency will be increased by 2%, and with an increase of 60 °C, the efficiency will be increased by 3% [
44]. The most preheated air can be generated using (14,307 kg/h), with the least amount of preheated air generated using Kütahya Tunçbilek (washed) coal (7597 kg/h) if the temperature of the combustion air is increased by 20 °C. The highest (7153 kg/s) and lowest (3799 kg/s) air volume can be generated using Bursa Keles coal and Kütahya Tunçbilek (washed) coal, respectively, if the combustion air is increased by 40 °C. The highest (4769 kg/s) and lowest (2533 kg/s) air volume can be generated using Bursa Keles coal and Kütahya Tunçbilek (washed) coal, respectively, if the temperature of the combustion air is increased by 60 °C. These values are presented in
Figure 12.
According to measurements of coal-burning district and central heating systems, the temperature of the feed used to supply water to the boiler can be increased by flue gas energy recovery for the whole Balikesir city center. An increase of 6 °C in the preheated water temperature results in a 1% savings in fuel consumption [
44]. If the temperature of the preheated water supplied to the boiler is increased by 6 °C, the highest (11,455 kg/h) and lowest (6083 kg/h) air volume can be generated using Bursa Keles coal and Kütahya Tunçbilek (washed) coal, respectively. The highest (5728 kg/s) and lowest (3042 kg/s) air volume can be generated using the same coal types if the temperature of the combustion air is increased by 12 °C. The highest (3818 kg/s) and lowest (2028 kg/s) air volume can be generated using same coal types if the temperature of the combustion air is increased by 18 °C. These values are shown in
Figure 13.
4. Conclusions
In the present study, we investigated the flue gas heat recovery potential for Balikesir city center by considering apartment building with central and district heating systems comprising coal-burning boilers. This flue gas heat recovery potential can be used to increase the temperature (preheated air) of the combustion air supplied to the boiler and to increase the temperature of the preheated water supplied to the boiler and taken from the main lines. With heat recovery from flue, the amount of pollutants and emissions released into the atmosphere can be reduced. In this respect, the proposed flue heat recovery system is an environmentally friendly and energy-saving alternative. The following results were obtained in the present study.
As the amount of coal used in boilers in central and district heating system increases, heat loss from the flue gas increases. As the H/C and O/C ratios increase, the percentage of flue loss also increases. As the lower heating value increases, the flue loss decreases. As the H/C and O/C ratios increase, the flue gas heat recovery potential increases. As the lower heating value increases, the flue gas heat recovery potential decreases. S/C and S/H ratios were found to have an indirect effect on flue gas heat recovery without a potential direct effect. The highest flue gas heat recovery potential was calculated in Bursa Keles coal (287,706 kW) and the lowest in Kütahya Tunçbilek (washed) coal (152,780 kW). Bursa Keles coal has the lowest calorific value, whereas Kütahya Tunçbilek (washed) coal has the highest calorific value. The lower heating value of coal is inversely proportional to the flue gas heat recovery potential.
According to measurements conducted by Balikesir Municipality Environmental Protection and Control Department in February and March 2019, the excess air coefficient was much higher than normal, increasing the chimney temperature, indicating that the proposed flue heat recovery system should be used. The proposed system is important in terms of energy saving.
In future studies, we will determine the most suitable design parameters for heat exchangers to be placed in chimneys for flue gas heat recovery. Then, we will examine the tube number, tube diameter, tube shape, tube material, etc., of the heat exchanger.