The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review
Abstract
1. Introduction
2. Fruit Losses and Waste
3. Bioactive Compounds in Fruit Waste
4. Dietary Fiber in Fruit Waste
5. Phenolic Compounds
6. Fruit Waste as a Source of Flavoring Agent
7. Important Enzymes in Fruit Waste
7.1. Amylases
7.2. Cellulases
7.3. Pectinases
7.4. Invertase
7.5. Other Enzymes
8. Organic Acids
| Fruit Waste | Value-Added Products Enzymes | Microorganisms Used | Organic Acid | Flavor | References |
|---|---|---|---|---|---|
| Banana | Amylases Cellulases Laccases, xylanases Lipases | Bacillus megaterium, pseudomonas fluorescence, penicillium putida, cellulomonas carte, Bacillus subtilis, Bacillus sp., Aspergillus niger, Aspergillus spp. MPS-002, Phylostica spp. MPS-001, Trametes pubescens, Bacillus sp., Aspergillus niger, Penicillium Citrinum, Aspergillus foetidus | Glutamic, aspartic, glutaric, quinic, glyceric, glycolic, and succinic acids plus several keto acids | Vanillin in banana peel is used as an aroma and flavoring agent in the food industry | [139,140,141,142,143] |
| Mango | Cellulases | Fusarium solani, Aspergillus niger | Decanal, 1-octen-3-one, nonanal, limonene, β-damascenone, and 2-nonenal | [141,143,144] | |
| Apple | ethyl butyrate Laccases Pectinases Xylanases | Trametes hirsute, Lentinus edodes, Aspergillus foetidus, Trichoderma harzianum 1073 D3 | Citric acid | Ethyl acetate | [141,143] |
| Orange/lemon | Invertases Lipases Pectinases α-Amylases | Aspergillus flavus, Trametes hirsute, Pleurotus sp., Chaloropsis thielarioides, Colletotrichum Gloesporioides, Bacillus sp., Aspergillus niger, Penicillium Citrinum, Aspergillus foetidus, Aspergillus niger | Citral, Limonene | [141,143] | |
| Pineapple | Invertases Pectinases | Aspergillus flavus, Penicillium chrysogenum, Aspergillus foetidus, Trichoderma koeningi | Citric acid, Acetic acid | Some aroma compounds were found in the volatiles of pineapple fruit. These compounds include esters, aldehydes, alcohols, acids, lactones | [141,143,145] |
| Pomegranate | Invertases | Aspergillus flavus | Primarily citric and malic acids | Glucose and fructose | [141,143,146] |
| Kiwifruit | Laccases | Trametes hirsute | Quinic acid, citric acid, malic acid and tartaric acid | (E)-2-hexenal and hexanal | [141,143,147,148] |
| Grapes | Laccases Pectinases Cellulases Xylanases | Trametes hirsute, Aspergillus foetidus, Aspergillus awamori | Tartaric and malic acids | Volatile thiols | [141,143,149] |
| Watermelon | Xylanases | Trichoderma harzianum 1073 D3, Trichoderma sp. | malic acid, citric acid, and oxalic acid | --- | [143] |
| Papaya | --- | --- | Acetic acid | ---- | [143] |
9. Bioactive Compounds Extraction
9.1. Conventional Extraction Techniques
9.2. Novel Extraction Techniques
9.2.1. Solid–Liquid Extraction (SLE)
9.2.2. Solvent Extraction Technique (SET)
9.2.3. Enzyme-Assisted Extraction (EAE)
9.2.4. Fermentation
9.2.5. Pulsed Electric Field (PEF)
9.2.6. Microwave-Assisted Extraction (MAE)
9.2.7. Ultrasound-Assisted Extraction (UAE)
9.2.8. Supercritical Fluid Extraction (SFE)
9.2.9. Subcritical Water Extraction (SWE)
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrera, E.L.; Hertel, T. Global food waste across the income spectrum: Implications for food prices, production and resource use. Food Policy 2021, 98, 101874. [Google Scholar] [CrossRef]
- Lins, M.; Puppin Zandonadi, R.; Raposo, A.; Ginani, V.C. Food waste on foodservice: An overview through the perspective of sustainable dimensions. Foods 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Deshi, V.; Ozturk, B.; Siddiqui, M.W. Fresh-cut fruits and vegetables: Quality issues and safety concerns. In Fresh-Cut Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of microalgae for biomaterial production and environmental applications at biorefineries. Sustainability 2021, 13, 3063. [Google Scholar] [CrossRef]
- Nedović, V.A.; Mantzouridou, F.T.; Đorđević, V.B.; Kaluševič, A.M.; Nenadis, N.; Bugarski, B. Isolation, purification and encapsulation techniques for Bioactive Compounds from agricultural and Food production Waste. In Utilisation of Bioactive Compounds from Agricultural and Food Waste; CRC Press: Boca Raton, FL, USA, 2017; pp. 159–194. [Google Scholar]
- Kavitha, S.; Kannah, R.Y.; Kumar, G.; Gunasekaran, M.; Banu, J.R. Introduction: Sources and characterization of food waste and food industry wastes. In Food Waste to Valuable Resources; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–13. [Google Scholar]
- Jensen, H.; Elleby, C.; Domínguez, I.; Chatzopoulos, T.; Charlebois, P. Insect-based protein feed: From fork to farm. J. Insects Food Feed 2021, 7, 1219–1233. [Google Scholar] [CrossRef]
- Liu, X.; Dou, Y.; Guan, D.; Hewings, G.; Wang, S. Forecasting China’s Food Grain Demand 2021–2050 with Attention to Balanced Dietary and Fertility Policies. Lancet, 2021; preprint. [Google Scholar] [CrossRef]
- Garcia, D.J.; Lovett, B.M.; You, F. Considering agricultural wastes and ecosystem services in Food-Energy-Water-Waste Nexus system design. J. Clean. Prod. 2019, 228, 941–955. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous yeasts as cell factories for the sustainable production of microbial lipids by the valorization of agri-food wastes. Fermentation 2021, 7, 50. [Google Scholar] [CrossRef]
- Kumar, N.; Daniloski, D.; D’cunha, N.M.; Naumovski, N.; Petkoska, A.T. Pomegranate peel extract—A natural bioactive addition to novel active edible packaging. Food Res. Int. 2022, 156, 111378. [Google Scholar] [CrossRef]
- Krahe, J.; Krahe, M.A.; Naumovski, N. The implications of post-harvest storage time and temperature on the phytochemical composition and quality of Japanese-styled green tea grown in Australia: A food loss and waste recovery opportunity. Beverages 2021, 7, 25. [Google Scholar] [CrossRef]
- Limaye, L.; Patil, R.; Ranadive, P.; Kamath, G. Application of potent actinomycete strains for bio-degradation of domestic agro-waste by composting and treatment of pulp-paper mill effluent. Adv. Microbiol. 2017, 7, 94–108. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Figueroa-Torres, G.; Azapagic, A. The extent of food waste generation in the UK and its environmental impacts. Sustain. Prod. Consum. 2021, 26, 532–547. [Google Scholar] [CrossRef]
- Guarnieri, P.; de Aguiar, R.C.; Thomé, K.M.; Watanabe, E.A.d.M. The Role of Logistics in Food Waste Reduction in Wholesalers and Small Retailers of Fruits and Vegetables: A Multiple Case Study. Logistics 2021, 5, 77. [Google Scholar] [CrossRef]
- Tollington, S.; Kareemun, Z.; Augustin, A.; Lallchand, K.; Tatayah, V.; Zimmermann, A. Quantifying the damage caused by fruit bats to backyard lychee trees in Mauritius and evaluating the benefits of protective netting. PLoS ONE 2019, 14, e0220955. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Vigneshwar, S.S.; Swetha, A.; Gopinath, K.P.; Goutham, R.; Pal, R.; Arun, J.; SundarRajan, P.; Bhatnagar, A.; Chi, N.T.L.; Pugazhendhi, A. Bioprocessing of biowaste derived from food supply chain side—Streams for extraction of value added bioproducts through biorefinery approach. Food Chem. Toxicol. 2022, 165, 113184. [Google Scholar] [CrossRef] [PubMed]
- Eixenberger, D.; Carballo-Arce, A.-F.; Vega-Baudrit, J.-R.; Trimino-Vazquez, H.; Villegas-Peñaranda, L.R.; Stöbener, A.; Aguilar, F.; Mora-Villalobos, J.-A.; Sandoval-Barrantes, M.; Bubenheim, P. Tropical agroindustrial biowaste revalorization through integrative biorefineries—Review part II: Pineapple, sugarcane and banana by-products in Costa Rica. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Ali, S.Y.; Hossain, M.; Zakaria, M.; Hoque, M.; Ahiduzzaman, M. Postharvest Losses of Mangoes at Different Stages from Harvesting to Consumption. Int. J. Bus. Soc. Sci. Res 2019, 7, 21–26. [Google Scholar]
- Abi Tarabay, P.; Chahine-Tsouvalakis, H.; Tawk, S.T.; Nemer, N.; Habib, W. Reduction of food losses in Lebanese apple through good harvesting and postharvest practices. Ann. Agric. Sci. 2018, 63, 207–213. [Google Scholar] [CrossRef]
- Mebratie, M.A.; Haji, J.; Woldetsadik, K.; Ayalew, A.; Ambo, E. Determinants of postharvest banana loss in the marketing chain of central Ethiopia. Food Sci. Qual. Manag. 2015, 37, 52–63. [Google Scholar]
- Omayio, D.G.; Abong, G.O.; Okoth, M.W.; Gachuiri, C.K.; Mwang’ombe, A.W. Current status of guava (Psidium Guajava L.) production, utilization, processing and preservation in Kenya: A review. Curr. Agric. Res. J. 2019, 7, 318. [Google Scholar] [CrossRef]
- Kansiime, M.K.; Rwomushana, I.; Mugambi, I.; Makale, F.; Lamontagne-Godwin, J.; Chacha, D.; Kibwage, P.; Oluyali, J.; Day, R. Crop losses and economic impact associated with papaya mealybug (Paracoccus marginatus) infestation in Kenya. Int. J. Pest Manag. 2020, 1–14. [Google Scholar] [CrossRef]
- Ningombam, S.; Noel, A.; Singh, J. Post-harvest losses of pineapple at various stages of handling from the farm level up to the consumer in Manipur. Int. J. Agric. Sci. 2019, 11, 9235–9237. [Google Scholar]
- Rajabi, S.; Lashgarara, F.; Omidi, M.; Hosseini, S.J.F. Quantifying the grapes losses and waste in various stages of supply chain. Biol. Forum 2015, 7, 225–229. [Google Scholar]
- Thapa, S.; Sapkota, S.; Adhikari, D. Effect of different postharvest treatments on prolonging shelf life and maintaining quality of sweet orange (Citrus sinensis Osbeck.). Sustain. Food Agric. 2020, 1, 69–75. [Google Scholar] [CrossRef]
- Profits, Fruits. Fruitprofits: Your Berry Expert. Available online: https://fruitprofits.com/berries/16-b.html (accessed on 15 July 2021).
- Khan, M.; Rahim, T.; Naeem, M.; Shah, M.; Bakhtiar, Y.; Tahir, M. Post harvest economic losses in peach produce in district Swat. Sarhad J. Agric 2008, 24, 705–711. [Google Scholar]
- Uzundumlu, A.S.; Karabacak, T.; Ali, A. Apricot production forecast of the leading countries in the period of 2018–2025. Emir. J. Food Agric. 2021, 33, 682–690. [Google Scholar] [CrossRef]
- Khalid, W.; Ali, A.; Arshad, M.S.; Afzal, F.; Akram, R.; Siddeeg, A.; Kousar, S.; Rahim, M.A.; Aziz, A.; Maqbool, Z. Nutrients and bioactive compounds of Sorghum bicolor L. used to prepare functional foods: A review on the efficacy against different chronic disorders. Int. J. Food Prop. 2022, 25, 1045–1062. [Google Scholar] [CrossRef]
- Ahmed, M.; Ali, A.; Sarfraz, A.; Hong, Q.; Boran, H. Effect of Freeze-Drying on Apple Pomace and Pomegranate Peel Powders Used as a Source of Bioactive Ingredients for the Development of Functional Yogurt. J. Food Qual. 2022, 2022, 3327401. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Hussain, A.; Naumovski, N.; Ranjha, M.M.A.N.; Ahmad, N.; Karrar, E.; Xu, B.; Ibrahim, S.A. A Narrative Review of Recent Advances in Rapid Assessment of Anthocyanins in Agricultural and Food Products. Front. Nutr. 2022, 9, 1352. [Google Scholar] [CrossRef]
- Inamuddin, A.; Asiri, A.; Suvardhan, K. Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and human health—A focus on oxidative stress, inflammation and disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Ghosh, S.K. and Its Products From Laboratory to Patient Bedside in Medical Science: An Emerging Aspect. In Advances in Trichoderma Biology for Agricultural Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 499–544. [Google Scholar]
- Ali, A.; Manzoor, M.F.; Ahmad, N.; Aadil, R.M.; Qin, H.; Siddique, R.; Riaz, S.; Ahmad, A.; Korma, S.A.; Khalid, W. The Burden of Cancer, Government Strategic Policies, and Challenges in Pakistan: A Comprehensive Review. Front. Nutr. 2022, 9, 940514. [Google Scholar] [CrossRef]
- Speer, H.; McKune, A.J. Aging under Pressure: The Roles of Reactive Oxygen and Nitrogen Species (RONS) Production and Aging Skeletal Muscle in Endothelial Function and Hypertension—From Biological Processes to Potential Interventions. Antioxidants 2021, 10, 1247. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hu, J.; Gao, H.; Chen, H.; Fang, X.; Mu, H.; Han, Y.; Liu, R. The potential cholesterol-lowering and prebiotic effects of bamboo shoot dietary fibers and their structural characteristics. Food Chem. 2020, 332, 127372. [Google Scholar] [CrossRef]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Ahmed, N.; Ali, A.; Riaz, S.; Ahmad, A.; Aqib, M. Vegetable proteins: Nutritional value, sustainability, and future perspectives. In Vegetable Crops-Health Benefits and Cultivation; IntechOpen: London, UK, 2021. [Google Scholar]
- Praveen, M.A.; Parvathy, K.K.; Balasubramanian, P.; Jayabalan, R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M.; Miliar, Y.; Frolenkova, S. Enhanced phenolic compounds extraction from apricot pomace by natural deep eutectic solvent combined with ultrasonic-assisted extraction. J. Chem. Technol. Met. 2021, 56, 919–931. [Google Scholar]
- Sim, Y.Y.; Ong, W.T.J.; Nyam, K.L. Effect of various solvents on the pulsed ultrasonic assisted extraction of phenolic compounds from Hibiscus cannabinus L. leaves. Ind. Crops Prod. 2019, 140, 111708. [Google Scholar] [CrossRef]
- Espro, C.; Paone, E.; Mauriello, F.; Gotti, R.; Uliassi, E.; Bolognesi, M.L.; Rodríguez-Padrón, D.; Luque, R. Sustainable production of pharmaceutical, nutraceutical and bioactive compounds from biomass and waste. Chem. Soc. Rev. 2021, 50, 11191–11207. [Google Scholar] [CrossRef]
- Rodriguez Garcia, S.L.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Rajput, H.; Srivastava, P.; Sharma, R. Citrus Fruits and Their By-Products, Power House of Remedial Antioxidants. In Antioxidant-Based Therapies for Disease Prevention and Management; Apple Academic Press: Palm Bay, FL, USA, 2021; pp. 145–181. [Google Scholar]
- Rysová, J.; Šmídová, Z. Effect of salt content reduction on food processing technology. Foods 2021, 10, 2237. [Google Scholar] [CrossRef]
- Kadzińska, J.; Janowicz, M.; Kalisz, S.; Bryś, J.; Lenart, A. An overview of fruit and vegetable edible packaging materials. Packag. Technol. Sci. 2019, 32, 483–495. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.; Gani, A.; Nazir, S.; Jhan, F. Pectin recovery from apple pomace: Physico-chemical and functional variation based on methyl-esterification. Int. J. Food Sci. Technol. 2021, 56, 4669–4679. [Google Scholar] [CrossRef]
- Alba, K.; Macnaughtan, W.; Laws, A.; Foster, T.J.; Campbell, G.; Kontogiorgos, V. Fractionation and characterisation of dietary fibre from blackcurrant pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Ajila, C.; Leelavathi, K.; Rao, U.P. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Ajila, C.; Bhat, S.; Rao, U.P. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Sudha, M.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Wachirasiri, P.; Julakarangka, S.; Wanlapa, S. The effects of banana peel preparations on the properties of banana peel dietary fibre concentrate. Songklanakarin J. Sci. Technol. 2009, 31, 605–611. [Google Scholar]
- Zaini, H.B.M.; Sintang, M.D.B.; Pindi, W. The roles of banana peel powders to alter technological functionality, sensory and nutritional quality of chicken sausage. Food Sci. Nutr. 2020, 8, 5497–5507. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Rincón, M.; Pulido, R.; Saura-Calixto, F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J. Agric. Food Chem. 2001, 49, 5489–5493. [Google Scholar] [CrossRef]
- Pavithra, C.; Devi, S.S.; Suneetha, W.; Rani, C.V.D. Nutritional properties of papaya peel. Pharma Innov. J. 2017, 6, 170–173. [Google Scholar]
- Selani, M.M.; Brazaca, S.G.C.; dos Santos Dias, C.T.; Ratnayake, W.S.; Flores, R.A.; Bianchini, A. Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 2014, 163, 23–30. [Google Scholar] [CrossRef]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Nichelle, S.M.; Minuzzi, N.M.; Teixeira, C.F.; da Cruz, I.B.M.; Júnior, M.R.M.; Emanuelli, T. Dietary fiber and fiber-bound polyphenols of grape peel powder promote GSH recycling and prevent apoptosis in the colon of rats with TNBS-induced colitis. J. Funct. Foods 2020, 64, 103644. [Google Scholar] [CrossRef]
- Khanpit, V.V.; Tajane, S.P.; Mandavgane, S.A. Orange waste peel to high value soluble dietary fiber concentrate: Comparison of conversion methods and their environmental impact. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- McKEE, L.H.; Latner, T. Underutilized sources of dietary fiber: A review. Plant Foods Hum. Nutr. 2000, 55, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Kasapoğlu, E.D.; Kahraman, S.; Törnük, F. Apricot juice processing byproducts as sources of value-added compounds for food industry. Eur. Food Sci. Eng. 2020, 1, 18–23. [Google Scholar]
- Al-Sayed, H.M.; Ahmed, A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Siddique, R.; Hussain, A.; Ahmad, N.; Rehman, A.; Siddeeg, A.; Alfarga, A.; Alshammari, G.M.; Yahya, M.A. Thermosonication effect on bioactive compounds, enzymes activity, particle size, microbial load, and sensory properties of almond (Prunus dulcis) milk. Ultrason. Sonochemistry 2021, 78, 105705. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Xu, B.; Khan, S.; Shukat, R.; Ahmad, N.; Imran, M.; Rehman, A.; Karrar, E.; Aadil, R.M.; Korma, S.A. Impact of high-intensity thermosonication treatment on spinach juice: Bioactive compounds, rheological, microbial, and enzymatic activities. Ultrason. Sonochemistry 2021, 78, 105740. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Hussain, A.; Tazeddinova, D.; Abylgazinova, A.; Xu, B. Assessing the Nutritional-Value-Based Therapeutic Potentials and Non-Destructive Approaches for Mulberry Fruit Assessment: An Overview. Comput. Intell. Neurosci. 2022, 2022, 6531483. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Manzoor, A.; Kalsoom, A. Food based phytochemical luteolin their derivatives, sources and medicinal benefits. Int. J. Agric. Life Sci.-IJAL 2017, 3, 11. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (dys) function and insulin resistance: From pathophysiological molecular mechanisms to the impact of diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Kong, X.; Cheng, R.; Wang, J.; Fang, Y.; Hwang, K.C. Nanomedicines inhibiting tumor metastasis and recurrence and their clinical applications. Nano Today 2021, 36, 101004. [Google Scholar] [CrossRef]
- Shehzad, M.; Rasheed, H.; Naqvi, S.A.; Al-Khayri, J.M.; Lorenzo, J.M.; Alaghbari, M.A.; Manzoor, M.F.; Aadil, R.M. Therapeutic potential of date palm against human infertility: A review. Metabolites 2021, 11, 408. [Google Scholar] [CrossRef]
- Borsacchi, L.; Pinelli, P. Sustainable and innovative practices of small and medium-sized enterprises in the water and waste management sector. In Innovation Strategies in Environmental Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 255–290. [Google Scholar]
- Olas, B. Honey and its phenolic compounds as an effective natural medicine for cardiovascular diseases in humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef]
- Ali, A.; Ain, Q.; Saeed, A.; Khalid, W.; Ahmed, M.; Bostani, A. Bio-Molecular Characteristics of Whey Proteins with Relation to Inflammation; IntechOpen: London, UK, 2021. [Google Scholar]
- Ma, Y.; Xiao, Y.; Zhao, Y.; Bei, Y.; Hu, L.; Zhou, Y.; Jia, P. Biomass based polyols and biomass based polyurethane materials as a route towards sustainability. React. Funct. Polym. 2022, 175, 105285. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. Rapid, enhanced and eco-friendly recovery of punicalagin from fresh waste pomegranate peels via aqueous ball milling. J. Clean. Prod. 2019, 228, 1238–1247. [Google Scholar] [CrossRef]
- El-Feky, A.M.; eL Batanony, M. Potential Phytoconstituents of Some Fruit and Vegetable Peels Against Oxidative Damage, Inflammatory and Cytotoxic Diseases. Egypt. J. Chem. 2022, 65, 261–272. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Fernandez-Lafuente, R.; Berenguer-Murcia, Á.; Meza-Gordillo, R.; Gutiérrez, L.-F.; Pacheco, N.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T. Phenolic compounds in mango fruit: A review. J. Food Meas. Charact. 2022, 16, 619–636. [Google Scholar] [CrossRef]
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: Assessment, analysis and application. J. Food Sci. Technol. 2016, 53, 1727–1738. [Google Scholar] [CrossRef]
- Zaini, H.M.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana peels as a bioactive ingredient and its potential application in the food industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Naseer, S.; Hussain, S.; Naeem, N.; Pervaiz, M.; Rahman, M. The phytochemistry and medicinal value of Psidium guajava (guava). Clin. Phytoscience 2018, 4, 32. [Google Scholar] [CrossRef]
- Sharma, A.; Bachheti, A.; Sharma, P.; Bachheti, R.K.; Husen, A. Phytochemistry, pharmacological activities, nanoparticle fabrication, commercial products and waste utilization of Carica papaya L.: A comprehensive review. Curr. Res. Biotechnol. 2020, 2, 145–160. [Google Scholar] [CrossRef]
- Kumar, A. Utilization of bioactive components present in pineapple waste: A review. Pharma Innov. J. 2021, 10, 954–961. [Google Scholar]
- Moro, K.I.B.; Bender, A.B.B.; da Silva, L.P.; Penna, N.G. Green extraction methods and microencapsulation technologies of phenolic compounds from grape pomace: A Review. Food Bioprocess Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus persica): Phytochemicals and health benefits. Food Rev. Int. 2020, 38, 1703–1734. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Cipriano, L.; Nazzaro, F. Apricots: Biochemistry and functional properties. Curr. Opin. Food Sci. 2018, 19, 23–29. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aadil, R.M. An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends Food Sci. Technol. 2021, 114, 275–291. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D. Characteristics and pro-health properties of mini kiwi (Actinidia arguta). Hortic. Environ. Biotechnol. 2019, 60, 217–225. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.L.; Oksman-Caldentey, K.M. The action of berry phenolics against human intestinal pathogens. Biofactors 2005, 23, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A food additive with multiple biological activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Haleva-Toledo, E.; Naim, M.; Zehavi, U.; Rouseff, R.L. Effects of L-cysteine and N-acetyl-L-cysteine on 4-hydroxy-2, 5-dimethyl-3 (2 H)-furanone (furaneol), 5-(hydroxymethyl) furfural, and 5-methylfurfural formation and browning in buffer solutions containing either rhamnose or glucose and arginine. J. Agric. Food Chem. 1999, 47, 4140–4145. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Said-Al Ahl, H.A.; Tkachenko, K.G.; Bratovcic, A.; Szczepanek, M.; Rodriguez, R.M. Sustainable and Environmentally Friendly Essential Oils Extracted from Pineapple Waste. Biointerface Res. Appl. Chem. 2021, 12, 6833–6844. [Google Scholar]
- Pattnaik, M.; Pandey, P.; Martin, G.J.; Mishra, H.N.; Ashokkumar, M. Innovative technologies for extraction and microencapsulation of bioactives from plant-based food waste and their applications in functional food development. Foods 2021, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Meshram, A.; Singhal, G.; Bhagyawant, S.S.; Srivastava, N. Plant-derived enzymes: A treasure for food biotechnology. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 483–502. [Google Scholar]
- Chandra, V.; Tiwari, A.; Pant, K.K.; Bhatt, R. Animal Cell Culture: Basics and Applications. In Industrial Microbiology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 691–719. [Google Scholar]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Rana, N.; Walia, A.; Gaur, A. α-Amylases from microbial sources and its potential applications in various industries. Natl. Acad. Sci. Lett. 2013, 36, 9–17. [Google Scholar] [CrossRef]
- Mushtaq, Q.; Irfan, M.; Tabssum, F.; Iqbal Qazi, J. Potato peels: A potential food waste for amylase production. J. Food Process Eng. 2017, 40, e12512. [Google Scholar] [CrossRef]
- Erdal, S.; Taskin, M. Production of α-amylase by Penicillium expansum MT-1 in solid-state fermentation using waste Loquat (Eriobotrya japonica Lindley) kernels as substrate. Rom. Biotechnol. Lett. 2010, 15, 5342–5350. [Google Scholar]
- Mahmoud, K. Statistical optimization of cultural conditions of an halophilic alpha-amylase production by halophilic Streptomyces sp. grown on orange waste powder. Biocatal. Agric. Biotechnol. 2015, 4, 685–693. [Google Scholar]
- Selvam, K.; Selvankumar, T.; Rajiniganth, R.; Srinivasan, P.; Sudhakar, C.; Senthilkumar, B.; Govarthanan, M. Enhanced production of amylase from Bacillus sp. using groundnut shell and cassava waste as a substrate under process optimization: Waste to wealth approach. Biocatal. Agric. Biotechnol. 2016, 7, 250–256. [Google Scholar] [CrossRef]
- Acourene, S.; Amourache, L.; Djafri, K.; Bekal, S. Date wastes as substrate for the production of α-amylase and invertase. Iran. J. Biotechnol. 2014, 12, 41–49. [Google Scholar]
- Ousaadi, M.I.; Merouane, F.; Berkani, M.; Almomani, F.; Vasseghian, Y.; Kitouni, M. Valorization and optimization of agro-industrial orange waste for the production of enzyme by halophilic Streptomyces sp. Environ. Res. 2021, 201, 111494. [Google Scholar] [CrossRef]
- Binod, P.; Gnansounou, E.; Sindhu, R.; Pandey, A. Enzymes for second generation biofuels: Recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 5, 317–325. [Google Scholar] [CrossRef]
- Banerjee, S.; Maiti, T.K.; Roy, R.N. Enzyme producing insect gut microbes: An unexplored biotechnological aspect. Crit. Rev. Biotechnol. 2022, 42, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Nag, P.; Ghati, A.; Chakraborty, D.; Maximiano, M.R.; Franco, O.L.; Mandal, A.K.; Kuila, A. Employment of the CRISPR/Cas9 system to improve cellulase production in Trichoderma reesei. Biotechnol. Adv. 2022, 108022. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J.C. Microbial cellulases: Engineering, production and applications. Renew. Sustain. Energy Rev. 2014, 33, 188–203. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Li, J.; Zhao, P.; Peng, M. Banana peel: A novel substrate for cellulase production under solid-state fermentation. Afr. J. Biotechnol. 2011, 10, 17887–17890. [Google Scholar]
- Ezike, T.C. Production and Characterization of Pectinases Obtained from Aspergillusniger under Submerged Fermentation System using Pectin Extracted from Orange Peels as Carbon Source. Ph.D. Thesis, University of Nigeria Nsukka, Nsukka, Nigeria, 2012. [Google Scholar]
- de Souza, T.S.; Kawaguti, H.Y. Cellulases, hemicellulases, and pectinases: Applications in the food and beverage industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Satapathy, S.; Soren, J.P.; Mondal, K.C.; Srivastava, S.; Pradhan, C.; Sahoo, S.L.; Thatoi, H.; Rout, J.R. Industrially relevant pectinase production from Aspergillus parvisclerotigenus KX928754 using apple pomace as the promising substrate. J. Taibah Univ. Sci. 2021, 15, 347–356. [Google Scholar] [CrossRef]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef]
- Veana, F.; Flores-Gallegos, A.C.; Gonzalez-Montemayor, A.M.; Michel-Michel, M.; Lopez-Lopez, L.; Aguilar-Zarate, P.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R. Invertase: An enzyme with importance in confectionery food industry. In Enzymes in Food Technology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 187–212. [Google Scholar]
- Siemens, J.; GONZÁLEZ, M.C.; Wolf, S.; Hofmann, C.; Greiner, S.; Du, Y.; Rausch, T.; Roitsch, T.; Ludwig-Müller, J. Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Mol. Plant Pathol. 2011, 12, 247–262. [Google Scholar] [CrossRef]
- Rasbold, L.M.; Delai, V.M.; da Cruz Kerber, C.M.; Simões, M.R.; Heinen, P.R.; da Conceição Silva, J.L.; de Cássia Garcia Simão, R.; Kadowaki, M.K.; Maller, A. Production, immobilization and application of invertase from new wild strain Cunninghamella echinulata PA3S12MM. J. Appl. Microbiol. 2022, 132, 2832–2843. [Google Scholar] [CrossRef]
- Kumar, A. Aspergillus nidulans: A potential resource of the production of the native and heterologous enzymes for industrial applications. Int. J. Microbiol. 2020, 2020, 8894215. [Google Scholar] [CrossRef]
- Basheer, S.M.; Chellappan, S.; Sabu, A. Enzymes in fruit and vegetable processing. In Value-Addition in Food Products and Processing Through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–110. [Google Scholar]
- Dahiya, S.; Bajaj, B.K.; Kumar, A.; Tiwari, S.K.; Singh, B. A review on biotechnological potential of multifarious enzymes in bread making. Process Biochem. 2020, 99, 290–306. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, S.; Bhatnagar, A. Potential of fungal laccase in decolorization of synthetic dyes. Microb. Wastewater Treat. 2019, 127–151. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Suriya, J.; Krishnan, M.; Manivasagan, P.; Kim, S.-K. Production of enzymes from agricultural wastes and their potential industrial applications. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 125–148. [Google Scholar]
- Singh, R.; Singh, T.; Larroche, C. Biotechnological applications of inulin-rich feedstocks. Bioresour. Technol. 2019, 273, 641–653. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yamini, D.; Ambika, V.; Sravya Sowdamini, N. Trends in inulinase production—A review. Crit. Rev. Biotechnol. 2009, 29, 67–77. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Yamashita, H. Koji starter and Koji world in Japan. J. Fungi 2021, 7, 569. [Google Scholar] [CrossRef]
- Reena, R.; Sindhu, R.; Balakumaran, P.A.; Pandey, A.; Awasthi, M.K.; Binod, P. Insight into citric acid: A versatile organic acid. Fuel 2022, 327, 125181. [Google Scholar] [CrossRef]
- Dhillon, G.; Brar, S.; Verma, M.; Tyagi, R. Enhanced solid-state citric acid bio-production using apple pomace waste through surface response methodology. J. Appl. Microbiol. 2011, 110, 1045–1055. [Google Scholar] [CrossRef]
- Prabha, M.; Rangaiah, G. Citric acid production using Ananas comosus and its waste with the effect of alcohols. Intl. J. Curr. Microbiol. Appl. 2014, 3, 747–754. [Google Scholar]
- Anyasi, T.; Jideani, A.; Edokpayi, J.; Anokwuru, C. Application of organic acids in food preservation. In Organic Acids, Characteristics, Properties and Synthesis; Vargas, C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 1–47. [Google Scholar]
- Ricci, A.; Diaz, A.B.; Caro, I.; Bernini, V.; Galaverna, G.; Lazzi, C.; Blandino, A. Orange peels: From by-product to resource through lactic acid fermentation. J. Sci. Food Agric. 2019, 99, 6761–6767. [Google Scholar] [CrossRef]
- Vijayakumar, J.; Aravindan, R.; Viruthagiri, T. Recent trends in the production, purification and application of lactic acid. Chem. Biochem. Eng. Q. 2008, 22, 245–264. [Google Scholar]
- Rodrigues, C.; Vandenberghe, L.; Woiciechowski, A.; de Oliveira, J.; Letti, L.; Soccol, C. Production and application of lactic acid. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 543–556. [Google Scholar]
- Farooq, M.A.; Ali, S.; Hassan, A.; Tahir, H.M.; Mumtaz, S.; Mumtaz, S. Biosynthesis and industrial applications of α-amylase: A review. Arch. Microbiol. 2021, 203, 1281–1292. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Alhazmi, A.; Kausar, T.; Haque, S.; Singh, R.; Ramteke, P.W.; Mishra, P.K.; Tuohy, M.; Leitgeb, M. Technological advances for improving fungal cellulase production from fruit wastes for bioenergy application: A review. Environ. Pollut. 2021, 287, 117370. [Google Scholar] [CrossRef]
- Panda, S.K.; Mishra, S.S.; Kayitesi, E.; Ray, R.C. Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: Biotechnology and scopes. Environ. Res. 2016, 146, 161–172. [Google Scholar] [CrossRef]
- Saeed, S.; Baig, U.U.R.; Tayyab, M.; Altaf, I.; Irfan, M.; Raza, S.Q.; Nadeem, F.; Mehmood, T. Valorization of banana peels waste into biovanillin and optimization of process parameters using submerged fermentation. Biocatal. Agric. Biotechnol. 2021, 36, 102154. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities—A review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef]
- Oliver-Simancas, R.; Muñoz, R.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Alañón, M.E. Mango by-products as a natural source of valuable odor-active compounds. J. Sci. Food Agric. 2020, 100, 4688–4695. [Google Scholar] [CrossRef]
- Steingass, C.B.; Carle, R.; Schmarr, H.-G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: I. Characterization of pineapple aroma compounds by comprehensive two-dimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2591–2608. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Porat, R. The flavor of pomegranate fruit: A review. J. Sci. Food Agric. 2014, 94, 21–27. [Google Scholar] [CrossRef]
- Garcia, C.V.; Quek, S.-Y.; Stevenson, R.J.; Winz, R.A. Kiwifruit flavour: A review. Trends Food Sci. Technol. 2012, 24, 82–91. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, H. Determination of organic acids in kiwifruit by reversed-phase HPLC method. Food Res. Dev. 2013, 34, 85–87. [Google Scholar]
- Dubourdieu, D.; Tominaga, T.; Masneuf, I.; des Gachons, C.P.; Murat, M.L. The role of yeasts in grape flavor development during fermentation: The example of Sauvignon blanc. Am. J. Enol. Vitic. 2006, 57, 81–88. [Google Scholar]
- Dimou, C.; Karantonis, H.C.; Skalkos, D.; Koutelidakis, A.E. Valorization of fruits by-products to unconventional sources of additives, oil, biomolecules and innovative functional foods. Curr. Pharm. Biotechnol. 2019, 20, 776–786. [Google Scholar] [CrossRef]
- Milovanovic, S.; Lukic, I.; Stamenic, M.; Kamiński, P.; Florkowski, G.; Tyśkiewicz, K.; Konkol, M. The effect of equipment design and process scale-up on supercritical CO2 extraction: Case study for Silybum marianum seeds. J. Supercrit. Fluids 2022, 188, 105676. [Google Scholar] [CrossRef]
- Abd-Talib, N.; Mohd-Setapar, S.H.; Khamis, A.K. The benefits and limitations of methods development in solid phase extraction: Mini review. J. Teknol. 2014, 69. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Abbas, K.; Mohamed, A.; Abdulamir, A.; Abas, H. A review on supercritical fluid extraction as new analytical method. Am. J. Biochem. Biotechnol. 2008, 4, 345–353. [Google Scholar]
- Barba, F.J.; Puértolas, E.; Brnčić, M.; Panchev, I.N.; Dimitrov, D.A.; Athès-Dutour, V.; Moussa, M.; Souchon, I. Emerging extraction. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2015; pp. 249–272. [Google Scholar]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Sirisompong, W.; Jirapakkul, W.; Klinkesorn, U. Response surface optimization and characteristics of rambutan (Nephelium lappaceum L.) kernel fat by hexane extraction. LWT-Food Sci. Technol. 2011, 44, 1946–1951. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Denny, E. Distillation of the lavender type oils: Theory and practice. In Lavender; CRC Press: Boca Raton, FL, USA, 2002; pp. 114–130. [Google Scholar]
- Calvi, L.; Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Giorgi, A. Quality traits of medical Cannabis sativa L. inflorescences and derived products based on comprehensive mass-spectrometry analytical investigation. In Recent Advances in Cannabinoid Research; IntechOpen: London, UK, 2018; pp. 55–78. [Google Scholar]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M. Public health problems of organic solvents. Toxicol. Lett. 1992, 64, 191–201. [Google Scholar] [CrossRef]
- Welton, T. Solvents and sustainable chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150502. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.; Duarte, A. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Naviglio, D. Naviglio’s principle and presentation of an innovative solid–liquid extraction technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Vyas, P.; Haque, I.; Kumar, M.; Mukhopadhyay, K. Photocontrol of differential gene expression and alterations in foliar anthocyanin accumulation: A comparative study using red and green forma Ocimum tenuiflorum. Acta Physiol. Plant. 2014, 36, 2091–2102. [Google Scholar] [CrossRef]
- Zulkifli, K.S.; Abdullah, N.; Abdullah, A.; Aziman, N.; Kamarudin, W. Bioactive phenolic compounds and antioxidant activity of selected fruit peels. In Proceedings of the International Conference on Environment, Chemistry and Biology, Hong Kong, China, 24 November 2012; pp. 66–70. [Google Scholar]
- Das, S.; Mondal, A.; Balasubramanian, S. Recent advances in modeling green solvents. Curr. Opin. Green Sustain. Chem. 2017, 5, 37–43. [Google Scholar] [CrossRef]
- Gutiérrez-Arnillas, E.; Álvarez, M.S.; Deive, F.J.; Rodríguez, A.; Sanromán, M.Á. New horizons in the enzymatic production of biodiesel using neoteric solvents. Renew. Energy 2016, 98, 92–100. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green solvents for the extraction of high added-value compounds from agri-food waste. Food Eng. Rev. 2020, 12, 83–100. [Google Scholar] [CrossRef]
- Pighin, E.; Diez, V.K.; Di Cosimo, J.I. Kinetic study of the ethyl lactate synthesis from triose sugars on Sn/Al2O3 catalysts. Catal. Today 2017, 289, 29–37. [Google Scholar] [CrossRef]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; Russo, M.; Rastrelli, L. Pressurized hot water extraction of bioactive compounds from artichoke by-products. Electrophoresis 2018, 39, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- El-Malah, M.H.; Hassanein, M.M.M.; Areif, M.H.; Al-Amrousi, E. Utilization of Egyptian tomato waste as a potential source of natural antioxidants using solvents, microwave and ultrasound extraction methods. Am. J. Food Technol. 2015, 10, 14–25. [Google Scholar]
- Silva, Y.; Ferreira, T.A.; Celli, G.B.; Brooks, M.S. Optimization of lycopene extraction from tomato processing waste using an eco-friendly ethyl lactate–ethyl acetate solvent: A green valorization approach. Waste Biomass Valorization 2019, 10, 2851–2861. [Google Scholar] [CrossRef]
- Strati, I.; Oreopoulou, V. Recovery and isomerization of carotenoids from tomato processing by-products. Waste Biomass Valorization 2016, 7, 843–850. [Google Scholar] [CrossRef]
- Matthäus, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Belwal, T.; Jiang, L.; Hu, J.; Limwachiranon, J.; Li, L.; Ren, G.; Zhang, X.; Luo, Z. Valorization of lotus byproduct (Receptaculum Nelumbinis) under green extraction condition. Food Bioprod. Processing 2019, 115, 110–117. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, G.; Wang, X.; Hu, X.; Chen, L. The study on optimization of Soxhlet extraction process for ursolic acid from Cynomorium. Shipin Yanjiu Yu Kaifa 2013, 34, 85–88. [Google Scholar]
- Szentmihályi, K.; Vinkler, P.; Lakatos, B.; Illés, V.; Then, M. Rose hip (Rosa canina L.) oil obtained from waste hip seeds by different extraction methods. Bioresour. Technol. 2002, 82, 195–201. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech 2016, 6, 3. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K. Mixed fermentation for natural product drug discovery. Appl. Microbiol. Biotechnol. 2009, 83, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Use of agro-industrial wastes in solid-state fermentation processes. In Industiral Waste; IntechOpen: London, UK, 2012; Volume 274. [Google Scholar]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef]
- Pérez-Guerra, N.; Torrado-Agrasar, A.; López-Macias, C.; Pastrana, L. Main characteristics and applications of solid substrate fermentation. Electron. J. Environ. Agric. Food Chem. 2003, 2. [Google Scholar]
- Knob, A.; Fortkamp, D.; Prolo, T.; Izidoro, S.C.; Almeida, J.M. Agro-residues as alternative for xylanase production by filamentous fungi. BioResources 2014, 9, 5738–5773. [Google Scholar]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Oloyede, O.O.; James, S.; Ocheme, O.B.; Chinma, C.E.; Akpa, V.E. Effects of fermentation time on the functional and pasting properties of defatted Moringa oleifera seed flour. Food Sci. Nutr. 2016, 4, 89–95. [Google Scholar] [CrossRef]
- Scherer, D. Pulsed Electric Field (PEF)—Assisted Protein Recovery from Microalgae Biomass for Food and Feed Applications. Ph.D. Thesis, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 2019. [Google Scholar]
- Manzoor, M.F.; Ahmad, N.; Aadil, R.M.; Rahaman, A.; Ahmed, Z.; Rehman, A.; Siddeeg, A.; Zeng, X.A.; Manzoor, A. Impact of pulsed electric field on rheological, structural, and physicochemical properties of almond milk. J. Food Process Eng. 2019, 42, e13299. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Zeng, X.-A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Zeng, X.A.; Ahmad, N.; Ahmed, Z.; Rehman, A.; Aadil, R.M.; Roobab, U.; Siddique, R.; Rahaman, A. Effect of pulsed electric field and thermal treatments on the bioactive compounds, enzymes, microbial, and physical stability of almond milk during storage. J. Food Process. Preserv. 2020, 44, e14541. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmed, Z.; Ahmad, N.; Aadil, R.M.; Rahaman, A.; Roobab, U.; Rehman, A.; Siddique, R.; Zeng, X.A.; Siddeeg, A. Novel processing techniques and spinach juice: Quality and safety improvements. J. Food Sci. 2020, 85, 1018–1026. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Angulo, J.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of carotenoids from fresh biomass of Rhodotorula glutinis. Innov. Food Sci. Emerg. Technol. 2018, 47, 421–427. [Google Scholar] [CrossRef]
- Dhua, S.; Kumar, K.; Sharanagat, V.S.; Nema, P.K. Bioactive compounds and its optimization from food waste: Review on novel extraction techniques. Nutr. Food Sci. 2022. ahead of print. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innov. Food Sci. Emerg. Technol. 2015, 29, 2–8. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A comparative analysis of the ‘green’techniques applied for polyphenols extraction from bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Hussain, A.; Sameen, A.; Sahar, A.; Khan, S.; Siddique, R.; Aadil, R.M.; Xu, B. Novel extraction, rapid assessment and bioavailability improvement of quercetin: A review. Ultrason. Sonochem. 2021, 78, 105686. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Mehmood, A.; Usman, M.; Zeng, X.A. Effect of dielectric barrier discharge plasma, ultra-sonication, and thermal processing on the rheological and functional properties of sugarcane juice. J. Food Sci. 2020, 85, 3823–3832. [Google Scholar] [CrossRef]
- Da Silva, R.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- De Melo, M.; Silvestre, A.; Silva, C. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Blicharski, T.; Oniszczuk, A. Extraction methods for the isolation of isoflavonoids from plant material. Open Chem. 2017, 15, 34–45. [Google Scholar] [CrossRef]
- Leiper, K.A.; Miedl, M. Brewhouse technology. In Handbook of Brewing; CRC Press: Boca Raton, FL, USA, 2006; pp. 398–461. [Google Scholar]
- Shi, J.; Xue, S.J.; Ma, Y.; Jiang, Y.; Ye, X.; Yu, D. Green separation technologies in food processing: Supercritical-CO2 fluid and subcritical water extraction. In Green Technologies in Food Production and Processing; Springer: Berlin/Heidelberg, Germany, 2012; pp. 273–294. [Google Scholar]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical water extraction of bioactive compounds from plants and algae: Applications in pharmaceutical and food ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- Cardenas-Toro, F.P.; Alcazar-Alay, S.C.; Forster-Carneiro, T.; Meireles, M.A.A. Obtaining oligo-and monosaccharides from agroindustrial and agricultural residues using hydrothermal treatments. Food Public Health 2014, 4, 123–139. [Google Scholar] [CrossRef]
- Ho, C.H.; Cacace, J.E.; Mazza, G. Extraction of lignans, proteins and carbohydrates from flaxseed meal with pressurized low polarity water. LWT-Food Sci. Technol. 2007, 40, 1637–1647. [Google Scholar] [CrossRef]
- Tunchaiyaphum, S.; Eshtiaghi, M.; Yoswathana, N. Extraction of bioactive compounds from mango peels using green technology. Int. J. Chem. Eng. Appl. 2013, 4, 194. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Gogus, F.; Lewis, A.C. Subcritical water extraction of essential oils from Thymbra spicata. Food Chem. 2003, 82, 381–386. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
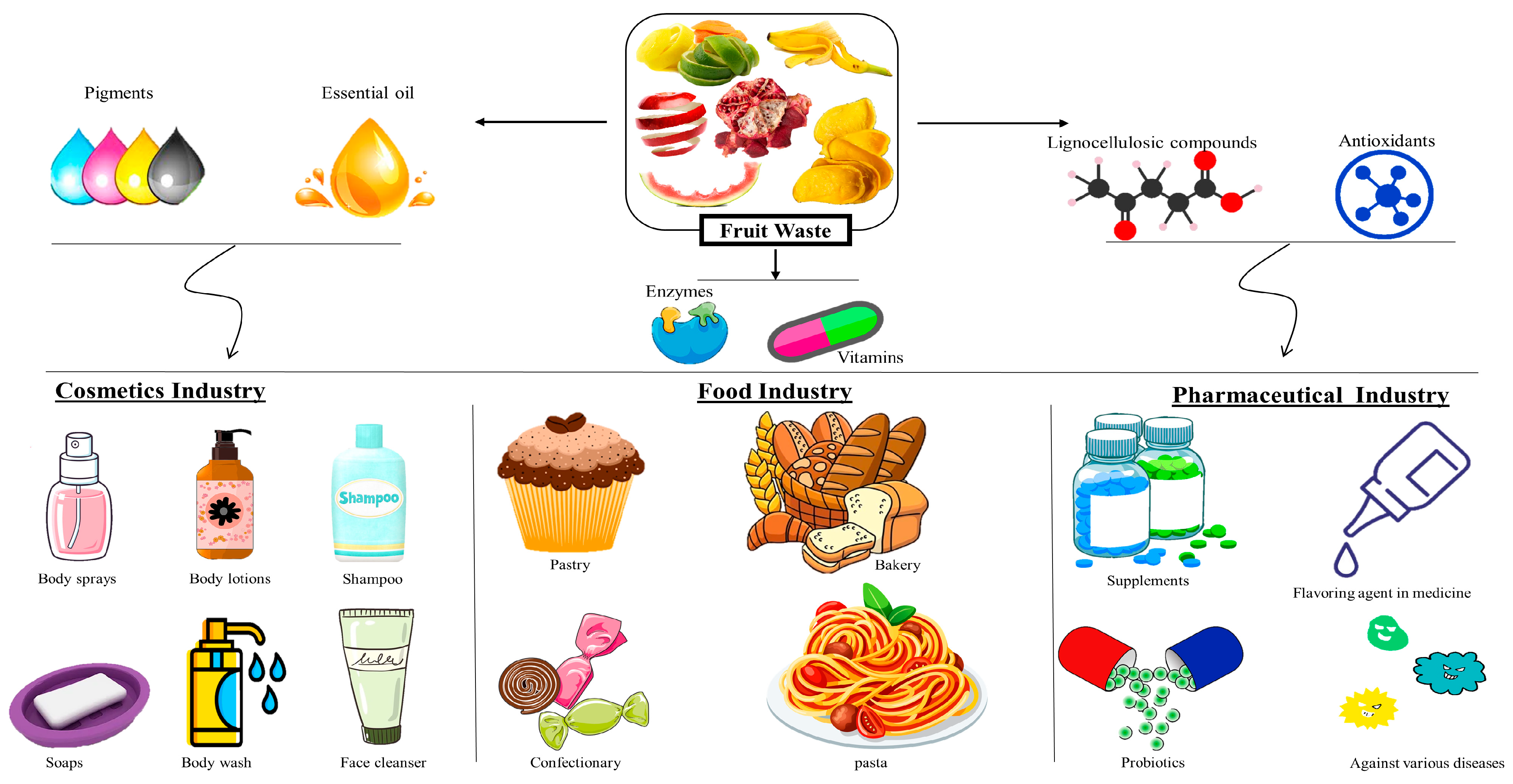

| Fruits | Type of Waste | Percentage of Losses (%) | References |
|---|---|---|---|
| Mango | Seed, peel | 25.51 | [20] |
| Apple | Seeds, peel | 28 | [21] |
| Banana | Peel | 26.5 | [22] |
| Guava | Peel, seeds | 20–40 (developing countries) | [23] |
| 10–15 (developed countries) | |||
| Papaya | Peel, seeds | 57 | [24] |
| Pineapple | Peel | 32.12 | [25] |
| Grapes | Stem, seeds, skin | 53 | [26] |
| Orange | Peel, seeds | 29 | [27] |
| Berries | Seeds, skin | 20 | [28] |
| Peach | Seed, peel | 18–31 | [29] |
| Apricot | Skin, seed | 60–65 | [30] |
| Fruits | Waste | Total Dietary Fiber Percentage (%) | Insoluble Dietary Fiber Percentage (%) | Soluble Dietary Fiber Percentage (%) | Reference |
|---|---|---|---|---|---|
| Mango | Seed, peel | 51.2 | 32 | 19 | [52,53] |
| Apple | Seeds, peel | 61.9 | 36.5 | 14.6 | [54] |
| Banana | Peel | 44.03 | 0.73 | 0.13 | [55,56] |
| Guava | Peel, seeds | 48.55–49.42 | 95 of total dietary fiber | 4.00–4.52 | [57] |
| Papaya | Peel, seeds | 44.66 | - | 36.99 | [58] |
| Pineapple | Peel | 46–48 | 44–47 | 0.78–0.80 | [59] |
| Grapes | Stem, seeds, peel | 25.8 | 17.4 | 8.4 | [60] |
| Orange | Peel, seeds | 0.58 | 0.53 | 0.05 | [61] |
| Peach | Seed, peel | 36 | 20–24 | 11–12 | [62] |
| Apricot | Skin, seed | 4.01 | n.a. | 1.07 | [63] |
| Watermelon | Peel, seed | 17.28 | n.a. | n.a. | [64] |
| Fruits | Waste | Phenolic Compounds | Structure | Reference | |||
|---|---|---|---|---|---|---|---|
| Mango | Seeds, peel | 1: Ellagic acid 2: Quercetin 3: Galic acid 4: Mangiferin | 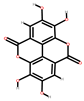 | 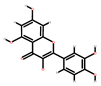 | 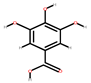 |  | [81] |
| Ellagic acid | Quercetin | Gallic acid | Mangiferin | ||||
| Apple | Seeds, peel | 1: Quercetin 2: Epicatechin 3: Phloridzin 4: Phloretin 5: Procyanidin B2 | 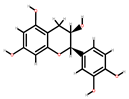 |  |  |  | [82] |
| Epicatechin | Phloridzin | Phloretin | Procyanidin B2 | ||||
| Banana | Peel | 1: Hydroxycinnamic acids (HCA) 2: Flavonols 3: Flavan-3-ols 4: Catecholamines |  |  |  | 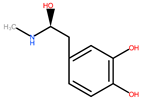 | [83] |
| HCA | Flavonols | Flavan-3-ols | Catecholamines | ||||
| Guava | Peel, seeds | 1: Galic acid 2: Galangin 3: Catechin 4: Homogentistic acid (HA) 5: Caffeic acid | 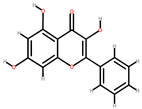 |  |  | 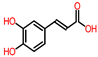 | [84] |
| Galangin | Catechin | HA | Caffeic acid | ||||
| Papaya | Peel, seeds | 1: Salicylic acid 2: Gentisyl alcohol 3: Chrysin 4: Protocatechuic acid (PA) |  | 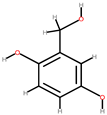 | 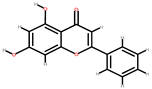 | 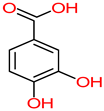 | [85] |
| Salicylic acid | Gentisyl alcohol | Chrysin | PA | ||||
| Pineapple | Peel, stem, crown | 1: p-Coumaric acid (p-CA) 2: Catechin 3: Epicatechin 4: Cinnamic acid 5: Vanillin 6: Syringic acid |  |  |  | 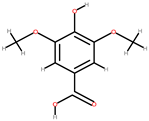 | [86] |
| p-CA | Cinnamic acid | Vanillin | Syringic acid | ||||
| Grapes | Stem, seeds, peel | 1: Hydroxybenzoic acid 2: hydroxycinnamic acids (HCA) 3: Anthocyanins 4: Proanthocyanidins 5: Catechins 6: Flavonols |  |  | 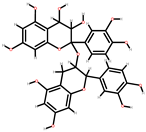 | [87] | |
| Hydroxybenzoic | Anthocyanins | Proanthocyanidins | |||||
| Orange | Peel, seeds | 1: Quercetin 2: Gallic acid: 3: Ferulic acid 4: Naringin 4: Hesperitin 5: Citric acid | 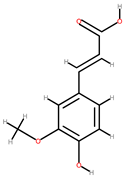 |  | 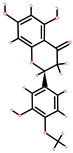 |  | [88] |
| Ferulic acid | Naringin | Hesperitin | Citric acid | ||||
| Peach | Seeds, peel | 1: Chlorogenic acid 2: Neochlorogenic acid 3: p-Coumaric acid 4: Gallic acid 5: Flavonols 6: Flavan-3-ols 7: Anthocyanidins | 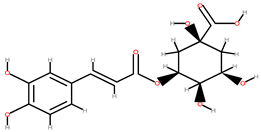 |  | [89] | ||
| Chlorogenic acid | Neochlorogenic acid | ||||||
| Apricot | Skin, seeds | 1: Gallic acid 2: Chlorogenic acid 3: Caffeic acid 4: Quercetin-3-galactoside (Q-3-galactoside) 5: Quercetin-3-glucoside (Q-3-glucoside) 6: Quercetin-3-rutinoside (Q-3-rutinoside) 7: Kaempferol-3-rutinoside (K-3-rutinoside) | 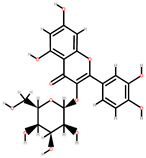 | 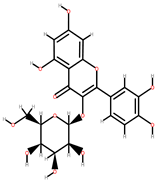 |  | [90] | |
| Q-3-galactoside | Q-3-glucoside | K-3-rutinoside | |||||
| Watermelon | Peel, seeds | 1: Gallic acid 2: Synapic acid 3: Myricetin 4: p-anisic acid |  | 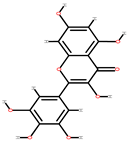 |  | [91] | |
| Sinapic acid | Myricetin | p-anisic acid | |||||
| Kiwi | Peel | 1: Benzoic acid 2: Chlorogenic acid 3: Gallic acid 4: Vanillic acid 5: Delphinidin 6: Cyanidin | 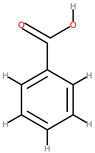 |  | [92] | ||
| Benzoic acid | Delphinidin | ||||||
| Berries | Peel, seeds | 1: Caffeic acid 2: Quercetin 3: Proanthocyanidins 4: Gallic acid 5: Secoisolariciresinol | 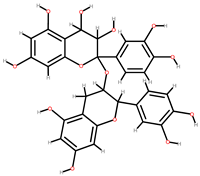 |  | [93] | ||
| Proanthocyanidins | Secoisolariciresinol | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Riaz, S.; Sameen, A.; Naumovski, N.; Iqbal, M.W.; Rehman, A.; Mehany, T.; Zeng, X.-A.; Manzoor, M.F. The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review. Processes 2022, 10, 2014. https://doi.org/10.3390/pr10102014
Ali A, Riaz S, Sameen A, Naumovski N, Iqbal MW, Rehman A, Mehany T, Zeng X-A, Manzoor MF. The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review. Processes. 2022; 10(10):2014. https://doi.org/10.3390/pr10102014
Chicago/Turabian StyleAli, Anwar, Sakhawat Riaz, Aysha Sameen, Nenad Naumovski, Muhammad Waheed Iqbal, Abdur Rehman, Taha Mehany, Xin-An Zeng, and Muhammad Faisal Manzoor. 2022. "The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review" Processes 10, no. 10: 2014. https://doi.org/10.3390/pr10102014
APA StyleAli, A., Riaz, S., Sameen, A., Naumovski, N., Iqbal, M. W., Rehman, A., Mehany, T., Zeng, X.-A., & Manzoor, M. F. (2022). The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review. Processes, 10(10), 2014. https://doi.org/10.3390/pr10102014













