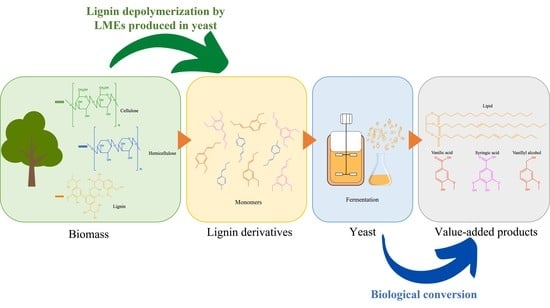
Valorization of Lignin and Its Derivatives Using Yeast
Abstract
:1. Introduction
1.1. Lignin Resources
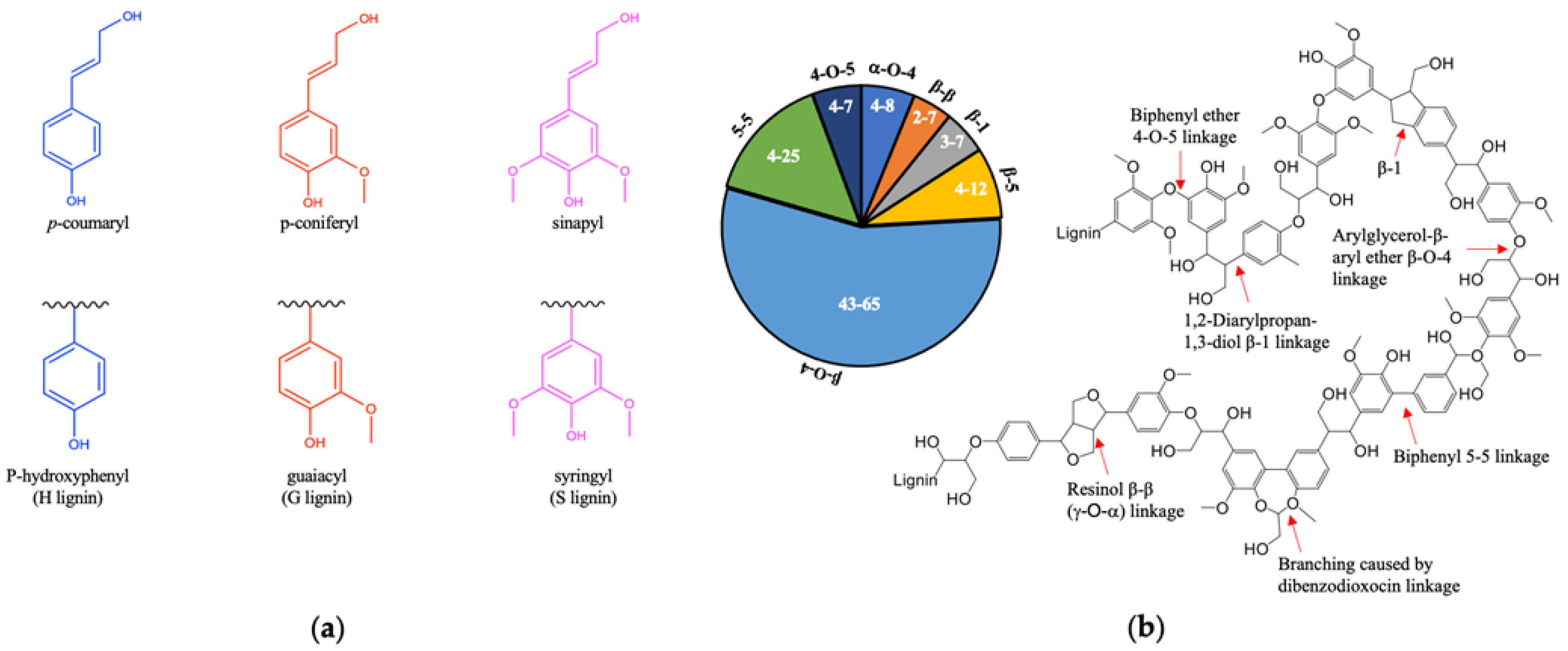
1.2. Structure of Lignin
2. Current Lignin Valorization by Microbes
3. The Use of Yeasts
3.1. Lipids
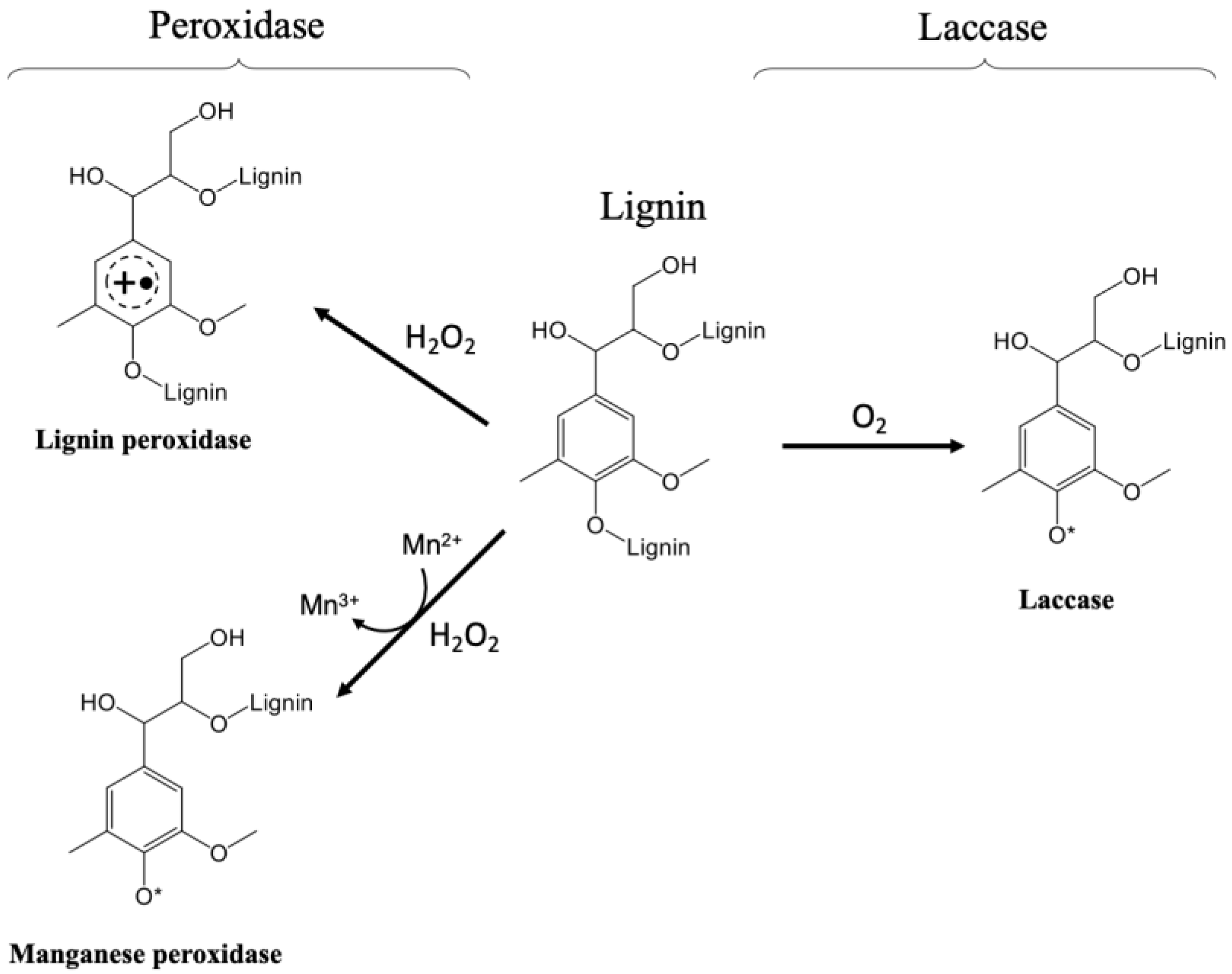
3.2. Enzyme for Lignin Degradation
3.2.1. Laccase Producing Yeasts
3.2.2. Peroxidases Producing Yeast
4. Other Biochemicals Produced by Yeasts
5. Future Perspectives and Feasibility of the Use of Yeasts for Lignin Valorization
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.-D.; Patel, A.K.; Puri, M. Global Status of Lignocellulosic Biorefinery: Challenges and Perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y. Conversion of Technical Lignins to Functional Materials with Retained Polymeric Properties. J. Wood Sci. 2015, 61, 230–250. [Google Scholar] [CrossRef] [Green Version]
- Sivagurunathan, P.; Raj, T.; Mohanta, C.S.; Semwal, S.; Satlewal, A.; Gupta, R.P.; Puri, S.K.; Ramakumar, S.S.V.; Kumar, R. 2G Waste Lignin to Fuel and High Value-Added Chemicals: Approaches, Challenges and Future Outlook for Sustainable Development. Chemosphere 2021, 268, 129326. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Agrawal, R.; Satlewal, A.; Kumar, R.; Gupta, R.P.; Ramakumar, S.S.V. Next Generation Applications of Lignin Derived Commodity Products, Their Life Cycle, Techno-Economics and Societal Analysis. Int. J. Biol. Macromol. 2022, 197, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Amore, A.; Ciesielski, P.N.; Lin, C.-Y.; Salvachúa, D.; Sànchez i Nogué, V. Development of Lignocellulosic Biorefinery Technologies: Recent Advances and Current Challenges. Aust. J. Chem. 2016, 69, 1201. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Cardona Alzate, C.A. The Potential Use of Lignin as a Platform Product in Biorefineries: A Review. Renew Sustain. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- Zhang, C. Lignocellulosic Ethanol: Technology and Economics. In Alcohol Fuels-Current Technologies and Future Prospect; Yun, Y., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Phan, D.-P.; Sarwar, A.; Tran, M.H.; Lee, O.K.; Lee, E.Y. Valorization of Industrial Lignin to Value-Added Chemicals by Chemical Depolymerization and Biological Conversion. Ind. Crops Prod. 2021, 161, 113219. [Google Scholar] [CrossRef]
- Chatel, G.; Rogers, R.D. Review: Oxidation of Lignin Using Ionic Liquids—An Innovative Strategy to Produce Renewable Chemicals. ACS Sustain. Chem. Eng. 2014, 2, 322–339. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial Enzymes Involved in Lignin Degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef]
- Schuetz, M.; Benske, A.; Smith, R.A.; Watanabe, Y.; Tobimatsu, Y.; Ralph, J.; Demura, T.; Ellis, B.; Samuels, A.L. Laccases Direct Lignification in the Discrete Secondary Cell Wall Domains of Protoxylem. Plant. Physiol. 2014, 166, 798–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [Green Version]
- Aftab, M.N.; Iqbal, I.; Riaz, F.; Karadag, A.; Tabatabaei, M. Different Pretreatment Methods of Lignocellulosic Biomass for Use in Biofuel Production. In Biomass for Bioenergy-Recent Trends and Future Challenges; Abomohra, A.E., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Thilakarathna, W.P.D.W.; He, Q.S.; Rupasinghe, H.P.V. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Xu, C.; Ferdosian, F. Conversion of Lignin into Bio-Based Chemicals and Materials; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-54957-5. [Google Scholar]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Grossman, A.; Vermerris, W. Lignin-Based Polymers and Nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Eklund, R.; Gustafsson, L.; Niklasson, C.; Lidén, G. Characterization and Fermentation of Dilute-Acid Hydrolyzates from Wood. Ind. Eng. Chem. Res. 1997, 36, 4659–4665. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Galgano, A. Biomass Screening for the Production of Furfural via Thermal Decomposition. Ind. Eng. Chem. Res. 2010, 49, 2658–2671. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Zhang, L.; Dai, G.; Zhao, Y.; Wang, X.; Ru, B. Structural Characterization and Pyrolysis Behavior of Cellulose and Hemicellulose Isolated from Softwood Pinus armandii Franch. Energy Fuels 2016, 30, 5721–5728. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Saka, S. Comparative Study on Chemical Composition of Various Biomass Species. RSC Adv. 2013, 3, 3946. [Google Scholar] [CrossRef] [Green Version]
- Demirbaş, A. Thermochemical Conversion of Biomass to Liquid Products in the Aqueous Medium. Energy Sources 2005, 27, 1235–1243. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic Agriculture Wastes as Biomass Feedstocks for Second-Generation Bioethanol Production: Concepts and Recent Developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the Valorization of Lignocellulosic Materials by Biotechnology: An Overview. Bioresources 2013, 8, 3157–3176. [Google Scholar] [CrossRef] [Green Version]
- Cerino-Córdova, F.J.; Dávila-Guzmán, N.E.; García León, A.M.; Salazar-Rabago, J.J.; Soto-Regalado, E. Revalorization of Coffee Waste. In Coffee-Production and Research; IntechOpen: London, UK, 2020. [Google Scholar]
- Kai, D.; Chow, L.P.; Loh, X.J. Lignin and Its Properties. In Functional Materials from Lignin: Methods and Advances; World Scientific Publishing: Singapore, 2018; pp. 1–28. [Google Scholar]
- Zhang, C.; Wang, F. Catalytic Lignin Depolymerization to Aromatic Chemicals. Acc. Chem. Res. 2020, 53, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-Degrading Enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Stephanopoulos, G. Co-culture Engineering for Microbial Biosynthesis of 3-amino-benzoic Acid in Escherichia coli. Biotechnol. J. 2016, 11, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and Analysis of the Molecular Weight of Lignin for Biorefining Studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Lin, S.Y.; Dence, C.W. (Eds.) Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 978-3-642-74067-1. [Google Scholar]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parthasarathi, R.; Romero, R.A.; Redondo, A.; Gnanakaran, S. Theoretical Study of the Remarkably Diverse Linkages in Lignin. J. Phys. Chem. Lett. 2011, 2, 2660–2666. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Cheng, H.; Lei, M.; Liang, J.; Tong, H. Theoretical Study of Bond Dissociation Energies for Lignin Model Compounds. J. Fuel Chem. Technol. 2015, 43, 429–436. [Google Scholar] [CrossRef]
- Weng, C.; Peng, X.; Han, Y. Depolymerization and Conversion of Lignin to Value-Added Bioproducts by Microbial and Enzymatic Catalysis. Biotechnol. Biofuels 2021, 14, 84. [Google Scholar] [CrossRef]
- Kosa, M.; Ragauskas, A.J. Bioconversion of Lignin Model Compounds with Oleaginous Rhodococci. Appl. Microbiol. Biotechnol. 2012, 93, 891–900. [Google Scholar] [CrossRef]
- Sainsbury, P.D.; Hardiman, E.M.; Ahmad, M.; Otani, H.; Seghezzi, N.; Eltis, L.D.; Bugg, T.D.H. Breaking Down Lignin to High-Value Chemicals: The Conversion of Lignocellulose to Vanillin in a Gene Deletion Mutant of Rhodococcus Jostii RHA1. ACS Chem. Biol. 2013, 8, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Kuhl, M.; Kohlstedt, M.; Starck, S.; Wittmann, C. Metabolic Engineering of Corynebacterium glutamicum for the Production of Cis, Cis-Muconic Acid from Lignin. Microb. Cell Fact. 2018, 17, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Stahl, H. Microbial Utilization and Biopolyester Synthesis of Bagasse Hydrolysates. Bioresour. Technol. 2008, 99, 8042–8048. [Google Scholar] [CrossRef]
- Wu, W.; Dutta, T.; Varman, A.M.; Eudes, A.; Manalansan, B.; Loqué, D.; Singh, S. Lignin Valorization: Two Hybrid Biochemical Routes for the Conversion of Polymeric Lignin into Value-Added Chemicals. Sci. Rep. 2017, 7, 8420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshmy, R.; Athiyaman Balakumaran, P.; Divakar, K.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sirohi, R.; Binod, P.; Kumar Awasthi, M.; Sindhu, R. Microbial Valorization of Lignin: Prospects and Challenges. Bioresour. Technol. 2022, 344, 126240. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sridharan, S.; Sowmya, V.; Yuvaraj, D.; Praveenkumar, R. Microbial Oil–A Plausible Alternate Resource for Food and Fuel Application. Bioresour. Technol. 2017, 233, 423–432. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Peltier, G. Third-Generation Biofuels: Current and Future Research on Microalgal Lipid Biotechnology. OCL 2013, 20, D606. [Google Scholar] [CrossRef]
- Anthony, W.E.; Carr, R.R.; DeLorenzo, D.M.; Campbell, T.P.; Shang, Z.; Foston, M.; Moon, T.S.; Dantas, G. Development of Rhodococcus Opacus as a Chassis for Lignin Valorization and Bioproduction of High-Value Compounds. Biotechnol. Biofuels 2019, 12, 192. [Google Scholar] [CrossRef] [Green Version]
- Shields-Menard, S.A.; AmirSadeghi, M.; Green, M.; Womack, E.; Sparks, D.L.; Blake, J.; Edelmann, M.; Ding, X.; Sukhbaatar, B.; Hernandez, R.; et al. The Effects of Model Aromatic Lignin Compounds on Growth and Lipid Accumulation of Rhodococcus Rhodochrous. Int. Biodeterior. Biodegrad. 2017, 121, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Botstein, D.; Chervitz, S.A.; Cherry, M. Yeast as a Model Organism. Science 1997, 277, 1259–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huccetogullari, D.; Luo, Z.W.; Lee, S.Y. Metabolic Engineering of Microorganisms for Production of Aromatic Compounds. Microb. Cell Fact. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Q.; Zhang, H.; Bao, J. Inhibitor Degradation and Lipid Accumulation Potentials of Oleaginous Yeast Trichosporon cutaneum Using Lignocellulose Feedstock. Bioresour. Technol. 2016, 218, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Šantek, M.I.; Lisičar, J.; Mušak, L.; Špoljarić, I.V.; Beluhan, S.; Šantek, B. Lipid Production by Yeast Trichosporon oleaginosus on the Enzymatic Hydrolysate of Alkaline Pretreated Corn Cobs for Biodiesel Production. Energy Fuels 2018, 32, 12501–12513. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Zong, M.H.; Wu, H. Efficient Lipid Production with Trichosporon fermentans and Its Use for Biodiesel Preparation. Bioresour. Technol. 2008, 99, 7881–7885. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Zhang, X.; Hu, F.; Ryu, D.D.Y.; Bao, J. Screening of Oleaginous Yeast Strains Tolerant to Lignocellulose Degradation Compounds. Appl. Biochem. Biotechnol. 2009, 159, 591–604. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Gao, Q.; Bao, J. Converting Lignin Derived Phenolic Aldehydes into Microbial Lipid by Trichosporon cutaneum. J. Biotechnol. 2018, 281, 81–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, J. Tolerance of Trichosporon Cutaneum to Lignin Derived Phenolic Aldehydes Facilitate the Cell Growth and Cellulosic Lipid Accumulation. J. Biotechnol. 2022, 343, 32–37. [Google Scholar] [CrossRef]
- Yaguchi, A.; Robinson, A.; Mihealsick, E.; Blenner, M. Metabolism of Aromatics by Trichosporon oleaginosus While Remaining Oleaginous. Microb. Cell Fact. 2017, 16, 206. [Google Scholar] [CrossRef] [Green Version]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Dutta, T.; Mirsiaghi, M.; Sundstrom, E.R.; Rodriguez, A.; Baidoo, E.; Tanjore, D.; et al. Rhodosporidium toruloides: A New Platform Organism for Conversion of Lignocellulose into Terpene Biofuels and Bioproducts. Biotechnol. Biofuels 2017, 10, 241. [Google Scholar] [CrossRef] [Green Version]
- Juanssilfero, A.B.; Kahar, P.; Amza, R.L.; Miyamoto, N.; Otsuka, H.; Matsumoto, H.; Kihira, C.; Thontowi, A.; Yopi; Ogino, C.; et al. Selection of Oleaginous Yeasts Capable of High Lipid Accumulation during Challenges from Inhibitory Chemical Compounds. Biochem. Eng. J. 2018, 137, 182–191. [Google Scholar] [CrossRef]
- Sànchez i Nogué, V.; Black, B.A.; Kruger, J.S.; Singer, C.A.; Ramirez, K.J.; Reed, M.L.; Cleveland, N.S.; Singer, E.R.; Yi, X.; Yeap, R.Y.; et al. Integrated Diesel Production from Lignocellulosic Sugars via Oleaginous Yeast. Green Chem. 2018, 20, 4349–4365. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.-W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.-H.; Dong, C.-D. Lignin Valorisation via Enzymes: A Sustainable Approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science 1983, 221, 661–663. [Google Scholar] [CrossRef] [Green Version]
- Thurston, C.F. The Structure and Function of Fungal Laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Claus, H. Laccases and Their Occurrence in Prokaryotes. Arch. Microbiol. 2003, 179, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [Green Version]
- Dikshit, P.K.; Jun, H.-B.; Kim, B.S. Biological Conversion of Lignin and Its Derivatives to Fuels and Chemicals. Korean J. Chem. Eng. 2020, 37, 387–401. [Google Scholar] [CrossRef]
- Antošová, Z.; Sychrová, H. Yeast Hosts for the Production of Recombinant Laccases: A Review. Mol. Biotechnol. 2016, 58, 93–116. [Google Scholar] [CrossRef]
- Rodgers, C.J.; Blanford, C.F.; Giddens, S.R.; Skamnioti, P.; Armstrong, F.A.; Gurr, S.J. Designer Laccases: A Vogue for High-Potential Fungal Enzymes? Trends Biotechnol. 2010, 28, 63–72. [Google Scholar] [CrossRef]
- Vieira Gomes, A.; Souza Carmo, T.; Silva Carvalho, L.; Mendonça Bahia, F.; Parachin, N. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia Pastoris: A Highly Successful Expression System for Optimal Synthesis of Heterologous Proteins. J. Cell Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Plácido, J.; Capareda, S. Ligninolytic Enzymes: A Biotechnological Alternative for Bioethanol Production. Bioresour. Bioprocess. 2015, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Bourbonnais, R.; Paice, M.G. Oxidation of Non-Phenolic Substrates. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-M.; Kalyani, D.; Tiwari, M.K.; Kim, T.-S.; Dhiman, S.S.; Lee, J.-K.; Kim, I.-W. Enhanced Enzymatic Hydrolysis of Rice Straw by Removal of Phenolic Compounds Using a Novel Laccase from Yeast Yarrowia Lipolytica. Bioresour. Technol. 2012, 123, 636–645. [Google Scholar] [CrossRef]
- Kalyani, D.; Tiwari, M.K.; Li, J.; Kim, S.C.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. A Highly Efficient Recombinant Laccase from the Yeast Yarrowia lipolytica and Its Application in the Hydrolysis of Biomass. PLoS ONE 2015, 10, e0120156. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhao, L.; Li, Y.; Wang, F.; Li, S.; Shi, G.; Ding, Z. Comparative Transcriptomics and Transcriptional Regulation Analysis of Enhanced Laccase Production Induced by Co-Culture of Pleurotus eryngii Var. Ferulae with Rhodotorula mucilaginosa. Appl. Microbiol. Biotechnol. 2020, 104, 241–255. [Google Scholar] [CrossRef]
- Wakil, S.; Adebayo-Tayo, B.; Odeniyi, O.; Salawu, K.; Eyiolawi, S.; Onilude, A. Production, Characterization and Purification of Laccase by Yeasts Isolated from Ligninolytic Soil. J. Pure Appl. Microbiol. 2017, 11, 847–869. [Google Scholar] [CrossRef]
- Piscitelli, A.; Giardina, P.; Mazzoni, C.; Sannia, G. Recombinant Expression of Pleurotus ostreatus Laccases in Kluyveromyces lactis and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2005, 69, 428–439. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, Expression Regulation, and Applications in Pharmaceutical Biodegradation. Front. Microbiol. 2017, 8, 832. [Google Scholar] [CrossRef] [Green Version]
- Arana-Cuenca, A.; Téllez-Jurado, A.; Yagüe, S.; Fermiñan, E.; Carbajo, J.M.; Domínguez, A.; Gónzalez, T.; Villar, J.C.; González, A.E. Delignification of Pinus Radiata Kraft Pulp by Treatment with a Yeast Genetically Modified to Produce Laccases. For. Syst. 2010, 19, 234. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Wang, H.; Luo, Y.; Guo, L. An Efficient System for Pre-Delignification of Gramineous Biofuel Feedstock in Vitro: Application of a Laccase from Pycnoporus sanguineus H275. Process Biochem. 2010, 45, 1141–1147. [Google Scholar] [CrossRef]
- Bao, W.; Peng, R.; Zhang, Z.; Tian, Y.; Zhao, W.; Xue, Y.; Gao, J.; Yao, Q. Expression, Characterization and 2,4,6-Trichlorophenol Degradation of Laccase from Monilinia fructigena. Mol. Biol. Rep. 2012, 39, 3871–3877. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, Y.; Du, B.; Tong, C.; Liang, S.; Han, S.; Zheng, S.; Lin, Y. Overexpression of a Novel Thermostable and Chloride-Tolerant Laccase from Thermus thermophilus SG0.5JP17-16 in Pichia pastoris and Its Application in Synthetic Dye Decolorization. PLoS ONE 2015, 10, e0119833. [Google Scholar] [CrossRef]
- Popović, N.; Pržulj, D.; Mladenović, M.; Prodanović, O.; Ece, S.; Ilić Đurđić, K.; Ostafe, R.; Fischer, R.; Prodanović, R. Immobilization of Yeast Cell Walls with Surface Displayed Laccase from Streptomyces cyaneus within Dopamine-Alginate Beads for Dye Decolorization. Int. J. Biol. Macromol. 2021, 181, 1072–1080. [Google Scholar] [CrossRef]
- Ji, L.; Shen, Y.; Xu, L.; Peng, B.; Xiao, Y.; Bao, X. Enhanced Resistance of Saccharomyces cerevisiae to Vanillin by Expression of LacA from Trametes Sp. AH28-2. Bioresour. Technol. 2011, 102, 8105–8109. [Google Scholar] [CrossRef]
- Larsson, S.; Cassland, P.; Jönsson, L.J. Development of a Saccharomyces cerevisiae Strain with Enhanced Resistance to Phenolic Fermentation Inhibitors in Lignocellulose Hydrolysates by Heterologous Expression of Laccase. Appl. Environ. Microbiol. 2001, 67, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- Nishibori, N.; Masaki, K.; Tsuchioka, H.; Fujii, T.; Iefuji, H. Comparison of Laccase Production Levels in Pichia pastoris and Cryptococcus Sp. S-2. J. Biosci. Bioeng. 2013, 115, 394–399. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Higuchi, T. Microbial Degradation of Lignin: Role of Lignin Peroxidase, Manganese Peroxidase, and Laccase. Proc. Jpn. Acad. Ser. B 2004, 80, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Biko, O.D.V.; Viljoen-Bloom, M.; van Zyl, W.H. Microbial Lignin Peroxidases: Applications, Production Challenges and Future Perspectives. Enzym. Microb. Technol. 2020, 141, 109669. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Al-Tohamy, R.; Sun, J. Performance of Meyerozyma caribbica as a Novel Manganese Peroxidase-Producing Yeast Inhabiting Wood-Feeding Termite Gut Symbionts for Azo Dye Decolorization and Detoxification. Sci. Total Environ. 2022, 806, 150665. [Google Scholar] [CrossRef] [PubMed]
- Samir Ali, S.; Al-Tohamy, R.; Khalil, M.A.; Ho, S.-H.; Fu, Y.; Sun, J. Exploring the Potential of a Newly Constructed Manganese Peroxidase-Producing Yeast Consortium for Tolerating Lignin Degradation Inhibitors While Simultaneously Decolorizing and Detoxifying Textile Azo Dye Wastewater. Bioresour. Technol. 2022, 351, 126861. [Google Scholar] [CrossRef]
- González, M.; Brito, N.; Hernández-Bolaños, E.; González, C. New Tools for High-throughput Expression of Fungal Secretory Proteins in Saccharomyces cerevisiae and Pichia pastoris. Microb. Biotechnol. 2019, 12, 1139–1153. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wen, X. Expression of Lignin Peroxidase H2 from Phanerochaete chrysosporium by Multi-Copy Recombinant Pichia Strain. J. Environ. Sci. 2009, 21, 218–222. [Google Scholar] [CrossRef]
- Majeke, B.M.; García-Aparicio, M.; Biko, O.D.; Viljoen-Bloom, M.; van Zyl, W.H.; Görgens, J.F. Synergistic Codon Optimization and Bioreactor Cultivation toward Enhanced Secretion of Fungal Lignin Peroxidase in Pichia pastoris: Enzymatic Valorization of Technical (Industrial) Lignins. Enzym. Microb. Technol. 2020, 139, 109593. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, F.; Sun, Y.; Du, L. Heterologous Expression of Lignin Peroxidase of Phanerochaete chrysosporium in Pichia methanolica. Biotechnol. Lett. 2004, 26, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.; Hwang, S.Y.; Kim, K.H.; Kang, J.H.; Lee, E.K. Functionality Improvement of Fungal Lignin Peroxidase by DNA Shuffling for 2,4-Dichlorophenol Degradability and H2O2 Stability. J. Biotechnol. 2008, 133, 110–115. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into Lignin Degradation and Its Potential Industrial Applications. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2013; pp. 1–28. [Google Scholar]
- Gu, L.; Lajoie, C.; Kelly, C. Expression of a Phanerochaete Chrysosporium Manganese Peroxidase Gene in the Yeast Pichia pastoris. Biotechnol. Prog. 2003, 19, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Kongsaeree, P.; Schilke, K.; Lajoie, C.; Kelly, C. Effects of PH and Temperature on Recombinant Manganese Peroxidase Production and Stability. Appl. Biochem. Biotechnol. 2008, 146, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Kongsaeree, P.; Charron, R.; Lajoie, C.; Xu, H.; Scott, G.; Kelly, C. Production and Separation of Manganese Peroxidase from Heme Amended Yeast Cultures. Biotechnol. Bioeng. 2008, 99, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Scott, G.M.; Jiang, F.; Kelly, C. Recombinant Manganese Peroxidase (RMnP) from Pichia pastoris. Part 1: Kraft Pulp Delignification. Holzforschung 2010, 64, 145–151. [Google Scholar] [CrossRef]
- Xu, H.; Guo, M.-Y.; Gao, Y.-H.; Bai, X.-H.; Zhou, X.-W. Expression and Characteristics of Manganese Peroxidase from Ganoderma Lucidum in Pichia pastoris and Its Application in the Degradation of Four Dyes and Phenol. BMC Biotechnol. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, K.L.; Jansen, L.E.; Lajoie, C.A.; Penner, M.H.; Morse, L.; Kelly, C.J. Furfural and 5-Hydroxymethyl-Furfural Degradation Using Recombinant Manganese Peroxidase. Enzym. Microb. Technol. 2018, 108, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Geng, A. Expression of a Novel Manganese Peroxidase from Cerrena unicolor BBP6 in Pichia pastoris and Its Application in Dye Decolorization and PAH Degradation. Biochem. Eng. J. 2020, 153, 107402. [Google Scholar] [CrossRef]
- Garcia-Ruiz, E.; Gonzalez-Perez, D.; Ruiz-Dueñas, F.J.; Martínez, A.T.; Alcalde, M. Directed Evolution of a Temperature-, Peroxide- and Alkaline PH-Tolerant Versatile Peroxidase. Biochem. J. 2012, 441, 487–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colao, M.; Lupino, S.; Garzillo, A.; Buonocore, V.; Ruzzi, M. Heterologous Expression of Lcc1 Gene from Trametes trogii in Pichia Pastoris and Characterization of the Recombinant Enzyme. Microb. Cell Fact. 2006, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, D.; Colao, M.C.; Ruzzi, M.; Romagnoli, G.; Bianchi, M.M. Optimization of Recombinant Fungal Laccase Production with Strains of the Yeast Kluyveromyces Lactis from the Pyruvate Decarboxylase Promoter. FEMS Yeast Res. 2009, 9, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Lu, F.; Liu, M.; Li, T.; Pu, J.; Wang, N.; Liang, P.; Zhang, C. Purification of Recombinant Laccase from Trametes versicolor in Pichia methanolica and Its Use for the Decolorization of Anthraquinone Dye. Biotechnol. Lett. 2008, 30, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chi, Y.; Yi, H.; Shao, S. Decolorization of Alizarin Red and Other Synthetic Dyes by a Recombinant Laccase from Pichia pastoris. Biotechnol. Lett. 2014, 36, 39–45. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Liang, S.-C.; Zhao, L.-Y.; Li, D.-B.; Zhang, B.-B. Production and Synthetic Dyes Decolourization Capacity of a Recombinant Laccase from Pichia pastoris. J. Appl. Microbiol. 2009, 107, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zheng, F.; Long, L.; Wang, J.; Ding, S. Engineering the Expression and Characterization of Two Novel Laccase Isoenzymes from Coprinus comatus in Pichia pastoris by Fusing an Additional Ten Amino Acids Tag at N-Terminus. PLoS ONE 2014, 9, e93912. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhang, Z.; Tian, Y.; Zhao, W.; Zhu, B.; Xu, Z.; Peng, R.; Yao, Q. Purification and Characterization of a Novel Laccase from Coprinus Cinereus and Decolorization of Different Chemically Dyes. Mol. Biol. Rep. 2013, 40, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Hong, Y.Z.; Xiao, Y.Z.; Xu, Y.H.; Fang, W. High Production of Laccase B from Trametes Sp. in Pichia pastoris. World J. Microbiol. Biotechnol. 2007, 23, 741–745. [Google Scholar] [CrossRef]
- Li, J.; Hong, Y.; Xiao, Y. Cloning and Heterologous Expression of the Gene of Laccase C from Trametes Sp. 420 and Potential of Recombinant Laccase in Dye Decolorization. Wei Sheng Wu Xue Bao 2007, 47, 54–58. [Google Scholar] [PubMed]
- Hong, Y.; Zhou, H.; Tu, X.; Li, J.; Xiao, Y. Cloning of a Laccase Gene from a Novel Basidiomycete Trametes Sp. 420 and Its Heterologous Expression in Pichia pastoris. Curr. Microbiol. 2007, 54, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pei, J.; Zhao, L.; Xie, J.; Cao, F.; Wang, G. Overexpression and Characterization of Laccase from Trametes Versicolor in Pichia pastoris. Appl. Biochem. Microbiol. 2014, 50, 140–147. [Google Scholar] [CrossRef]
- Li, Q.; Ge, L.; Cai, J.; Pei, J.; Xie, J.; Zhao, L. Comparison of Two Laccases from Trametes versicolor for Application in the Decolorization of Dyes. J. Microbiol. Biotechnol. 2014, 24, 545–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilić Đurđić, K.; Ece, S.; Ostafe, R.; Vogel, S.; Balaž, A.M.; Schillberg, S.; Fischer, R.; Prodanović, R. Flow Cytometry-Based System for Screening of Lignin Peroxidase Mutants with Higher Oxidative Stability. J. Biosci. Bioeng. 2020, 129, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Ilić Đurđić, K.; Ece, S.; Ostafe, R.; Vogel, S.; Schillberg, S.; Fischer, R.; Prodanović, R. Improvement in Oxidative Stability of Versatile Peroxidase by Flow Cytometry-Based High-Throughput Screening System. Biochem. Eng. J. 2020, 157, 107555. [Google Scholar] [CrossRef]
- Deparis, Q.; Claes, A.; Foulquié-Moreno, M.R.; Thevelein, J.M. Engineering Tolerance to Industrially Relevant Stress Factors in Yeast Cell Factories. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.S.; Parales, R.E. The Beta-Ketoadipate Pathway and the Biology of Self-Identity. Annu Rev. Microbiol. 1996, 50, 553–590. [Google Scholar] [CrossRef] [PubMed]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Kim, I.S.; Choi, D.-K.; Jung, H.J. Neuroprotective Effects of Vanillyl Alcohol in Gastrodia Elata Blume Through Suppression of Oxidative Stress and Anti-Apoptotic Activity in Toxin-Induced Dopaminergic MN9D Cells. Molecules 2011, 16, 5349–5361. [Google Scholar] [CrossRef] [Green Version]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.-K. Improving Alkaline Pretreatment Method for Preparation of Whole Rice Waste Biomass Feedstock and Bioethanol Production. RSC Adv. 2015, 5, 97171–97179. [Google Scholar] [CrossRef]
| Lignocellulosic Biomass | Lignin | Hemicellulose | Cellulose | Reference |
|---|---|---|---|---|
| (%) | (%) | (%) | ||
| Hardwood | ||||
| Aspen | 19.5 | 21.7 | 52.7 | [19] |
| Beech | 20 | 33 | 45 | [20] |
| Cherry wood | 18 | 29 | 46 | [20] |
| Poplar | 20 | 24 | 49 | [20] |
| Willow | 29.3 | 16.7 | 41.7 | [19] |
| Softwood | ||||
| Fir | 30 | 22 | 45 | [20] |
| Pine armandii | 24.1 | 17.8 | 48.4 | [21] |
| Japanese cedar | 33.8 | 23.1 | 38.6 | [22] |
| Spruce | 27.6 | 29.4 | 43 | [23] |
| Others | ||||
| Barley straw | 14–19 | 27–38 | 31–45 | [24] |
| Bamboo | 20.81 | 19.49 | 39.8 | [22] |
| Corn cobs | 18.2 | 33.1 | 34.6 | [22] |
| Corn strover | 7–21 | 28 | 38–40 | [24] |
| Rice straw | 12–14 | 23–28 | 28–36 | [24] |
| Wheat straw | 20.2 | 34.4 | 37.5 | [22] |
| Banana waste | 14 | 14.8 | 13.2 | [25] |
| Nut shells | 30–40 | 25–30 | 25–30 | [25] |
| Coffee grounds | 19.8–26.5 | 5–10 | 59.2–62.9 | [26] |
| Newspaper | 18–30 | 25–40 | 40–55 | [25] |
| Enzymes | Native | Gene | Yeast | Reference |
|---|---|---|---|---|
| Laccase | Pleurotus ostreatus | POXA1b | Kluyveromyces lactis | [75] |
| POXC | Saccharomyces cerevisiae | |||
| Coriolopsis gallic | LCC1 | Kluyveromyces lactis | [77] | |
| Pycnoporus sanguineus H275 | LCC1 | Pichia pastoris | [78] | |
| Trametes trogii | LCC1 | Pichia pastoris | [105] | |
| Trametes trogii | LCC1 | Kluyveromyces lactis | [106] | |
| Trametes versicolor | LCC1 | Pichia methalonica | [107] | |
| Monilinia fructigena | LCC2 | Pichia pastoris | [79] | |
| Trametes versicolor | LCC2 | Saccharomyces cerevisiae | [83] | |
| Lenzites gibbosa | LAC | Pichia pastoris | [108] | |
| Pleurotus sanguineus | LAC | Pichia pastoris | [109] | |
| Streptomyces cyaneus | LAC | Saccharomyces cerevisiae | [81] | |
| Gaeumannomyces graminis | LAC2 | Cryptococcus sp. S-2 | [84] | |
| Coprinus comatus | LAC3 | Pichia pastoris | [110] | |
| Thermus thermophillus | LACTt | Pichia pastoris | [80] | |
| Coprinus cinereus | LCC5I | Pichia pastoris | [111] | |
| Trametes sp. AH28-2 | LACA | Saccharomyces cerevisiae | [82] | |
| Trametes sp. AH28-2 | LACB | Pichia pastoris | [112] | |
| Trametes sp. 420 | LACC | Pichia pastoris | [113] | |
| Trametes sp. 420 | LACD | Pichia pastoris | [114] | |
| Trametes versicolor | LCCA | Pichia pastoris | [115] | |
| Trametes versicolor | LCCB | Pichia pastoris | [116] | |
| Yarrowia lipolytica | YILAC | Pichia pastoris | [72] | |
| Lignin peroxidase | Phanerochaete chrysosporium BKM-F-1767 | LiPH2 | Pichia pastoris | [92] |
| Phanerochaete chrysosporium | LiPH2 | Saccharomyces cerevisiae | [95] | |
| Phanerochaete chrysosporium | LiPH8 | Pichia methalonica | [94] | |
| Phanerochaete chrysosporium | LiPH8 | Saccharomyces cerevisiae | [117] | |
| Manganese-dependent peroxidase | Phanerochaete chrysosporium | MnP1 | Pichia pastoris | [97] |
| Ganoderma lucidum | MnP1 | Pichia pastoris | [101] | |
| Phanerochaete chrysosporium | MnP1 | Saccharomyces cerevisiae | [102] | |
| Cerrena unicolor BB6P | MnP3 | Pichia pastoris | [103] | |
| Versatile peroxidase | Pleurotus eryngii | VPL2 | Saccharomyces cerevisiae | [104] |
| Pleurotus eryngii | wtVP | Saccharomyces cerevisiae | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, F.J.N.; Kahar, P.; Kondo, A.; Ogino, C. Valorization of Lignin and Its Derivatives Using Yeast. Processes 2022, 10, 2004. https://doi.org/10.3390/pr10102004
Putra FJN, Kahar P, Kondo A, Ogino C. Valorization of Lignin and Its Derivatives Using Yeast. Processes. 2022; 10(10):2004. https://doi.org/10.3390/pr10102004
Chicago/Turabian StylePutra, Filemon Jalu Nusantara, Prihardi Kahar, Akihiko Kondo, and Chiaki Ogino. 2022. "Valorization of Lignin and Its Derivatives Using Yeast" Processes 10, no. 10: 2004. https://doi.org/10.3390/pr10102004