Thermal Behavior of Estonian Graptolite–Argillite from Different Deposits
Abstract
:1. Introduction
2. Materials and Methods
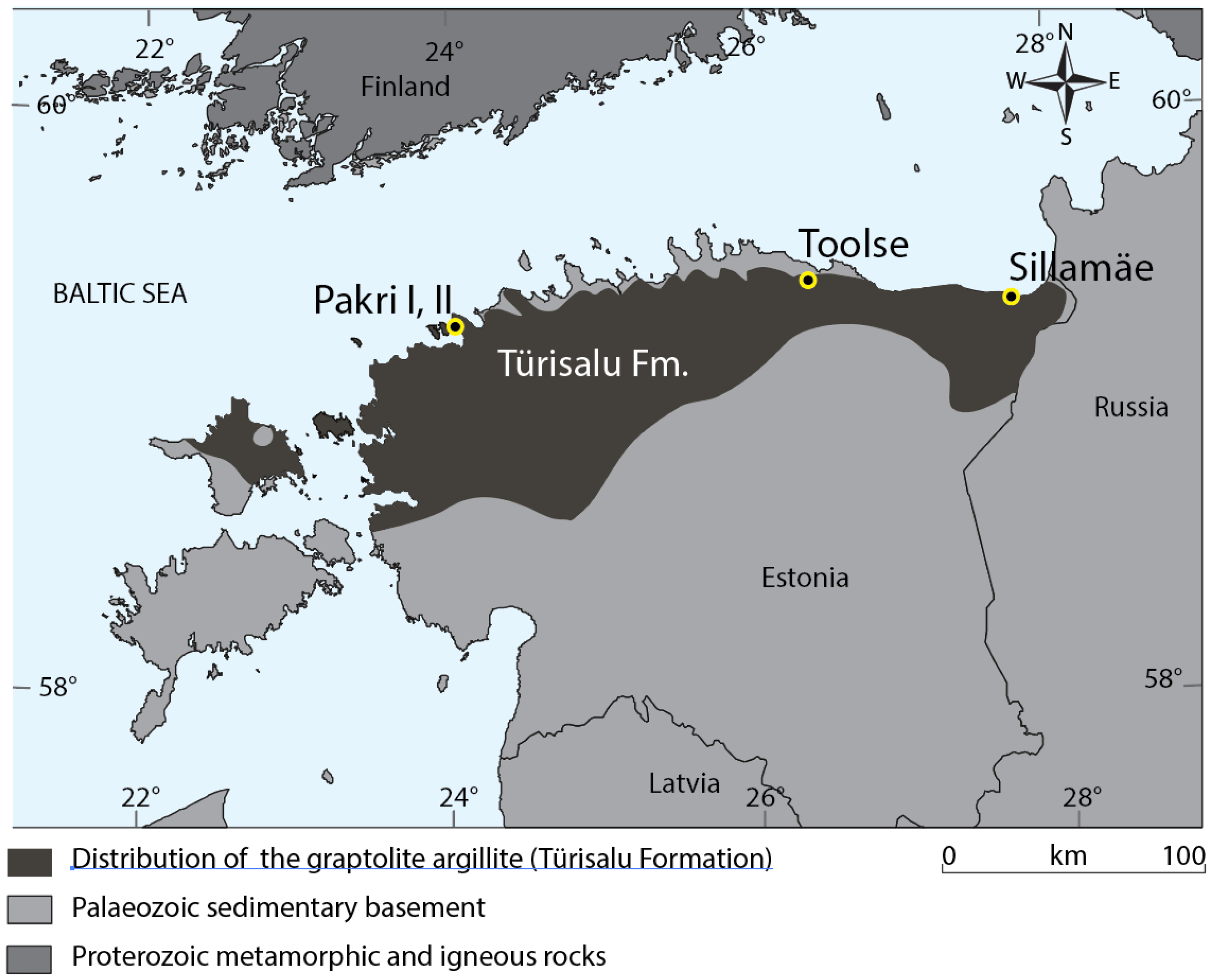
2.1. Materials
2.2. Methods
2.2.1. Material Characterization
2.2.2. Thermal and Kinetic Analysis
3. Results and Discussion
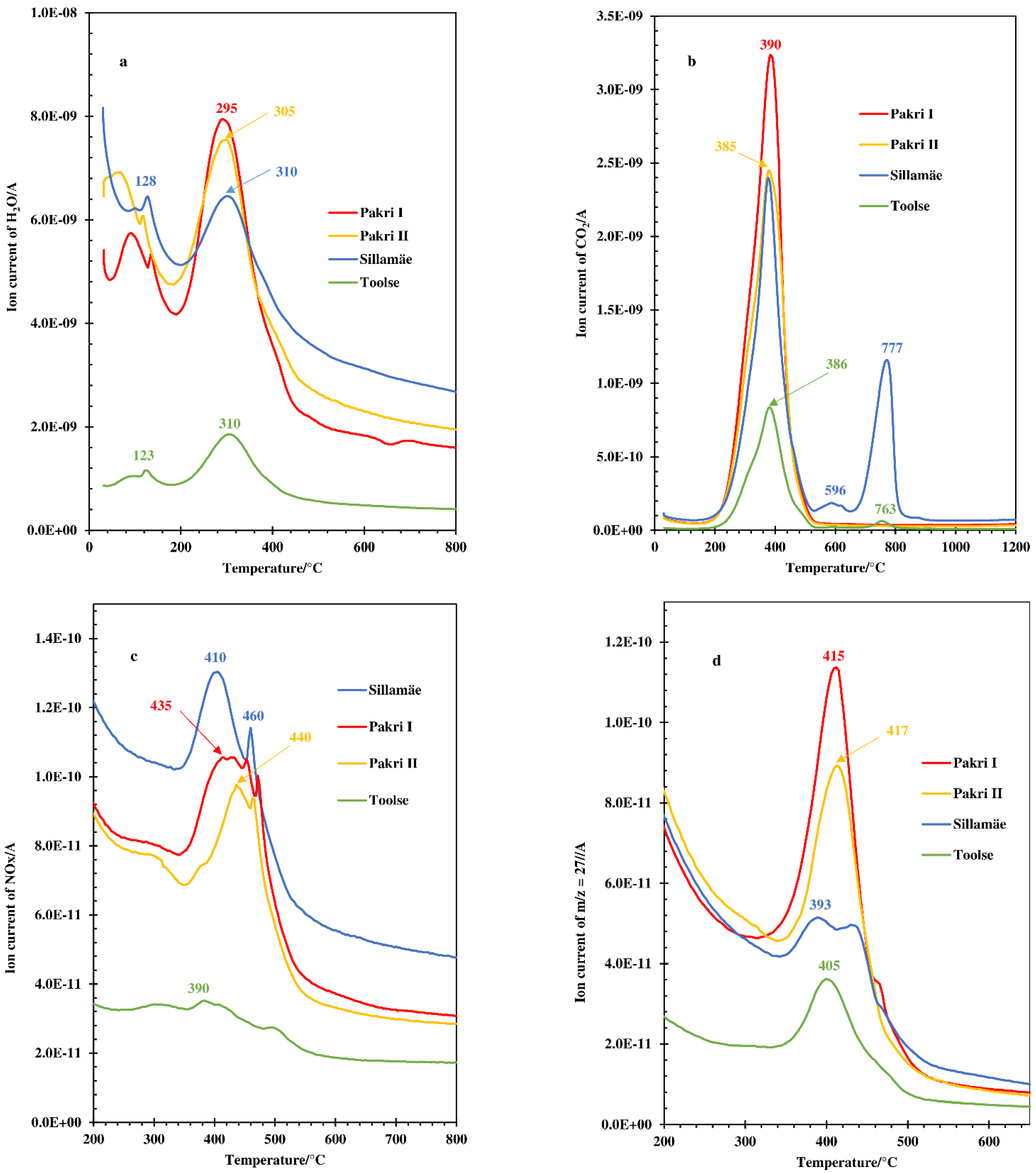
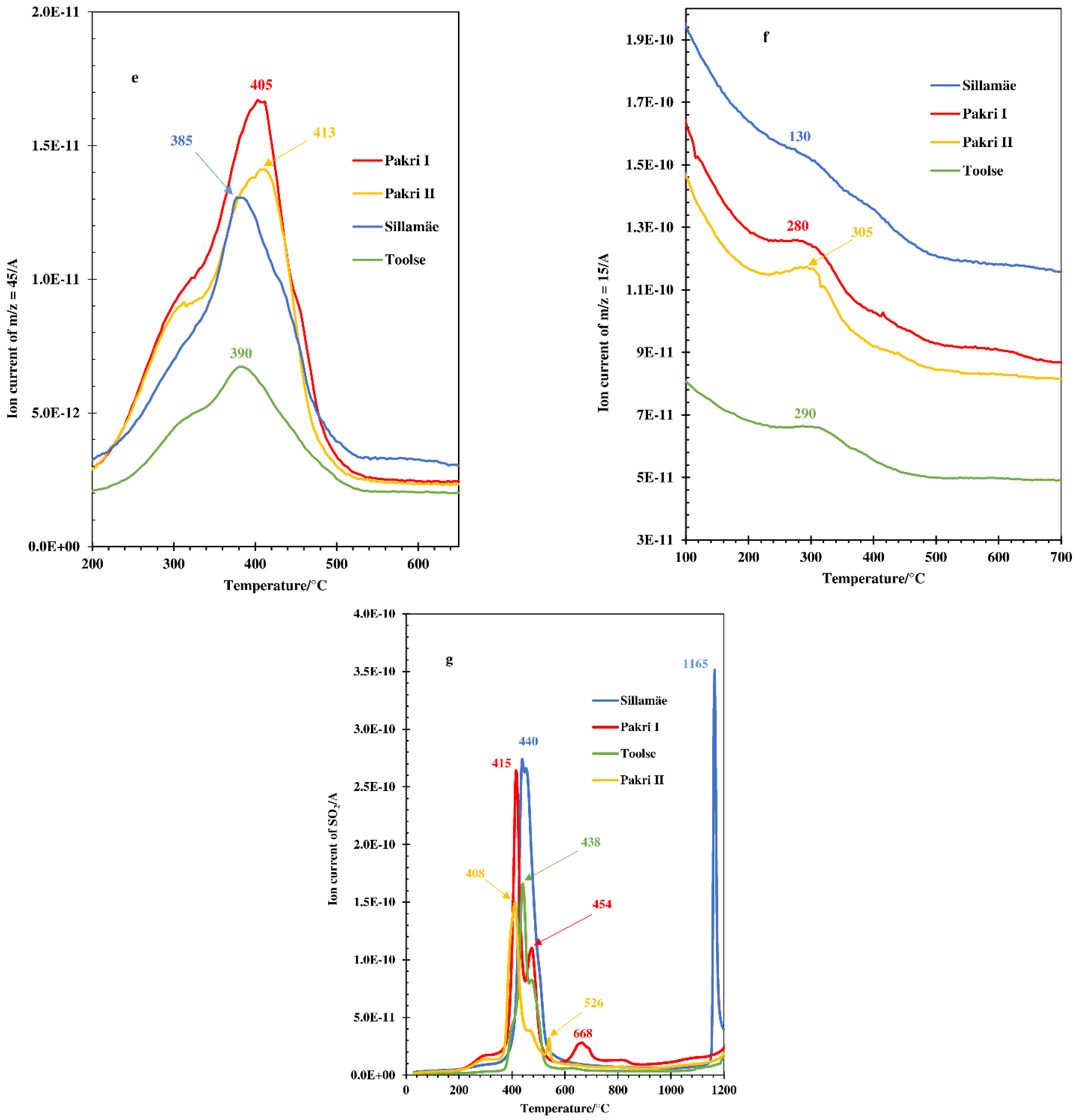
3.1. Thermal and MS Analysis
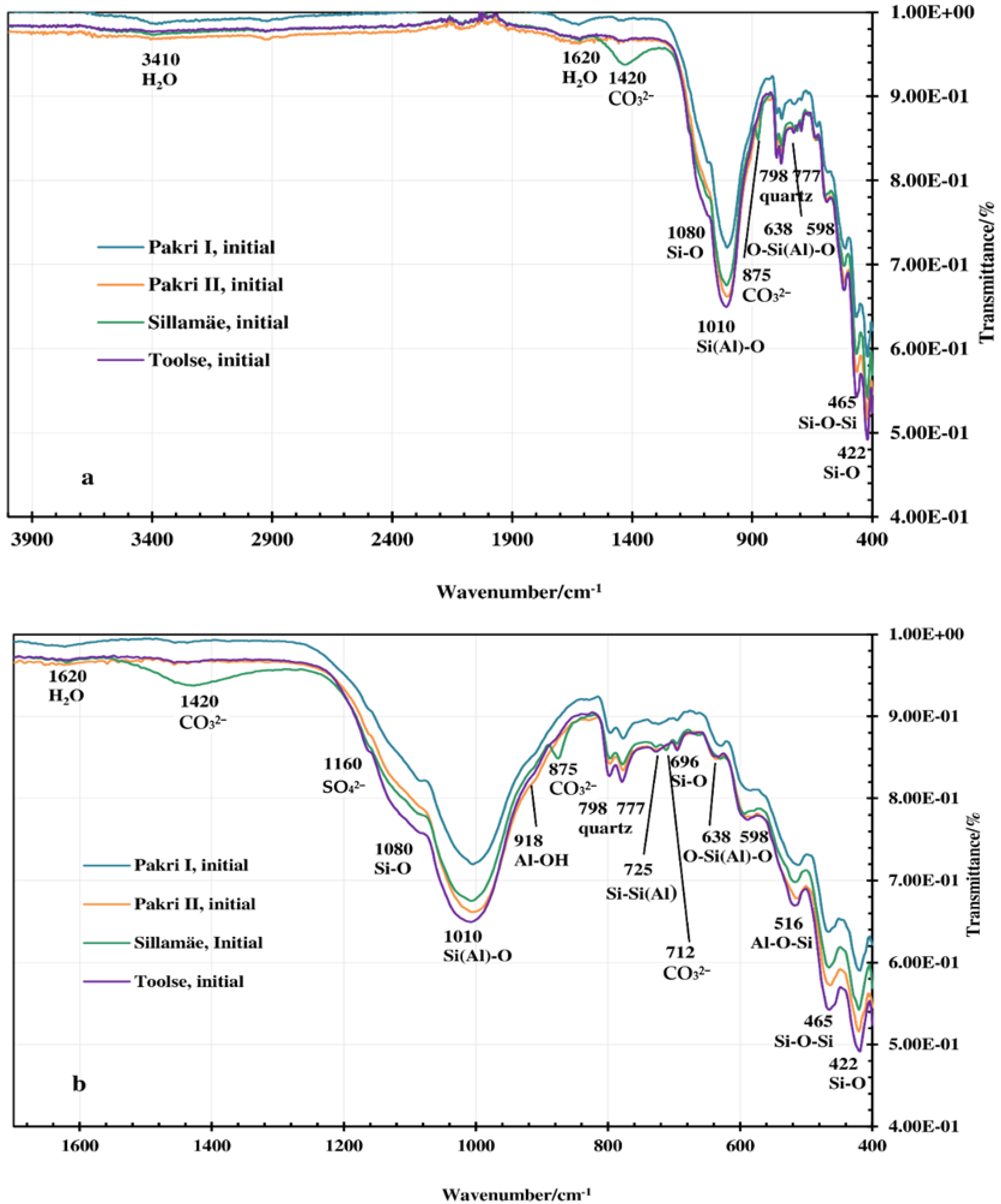
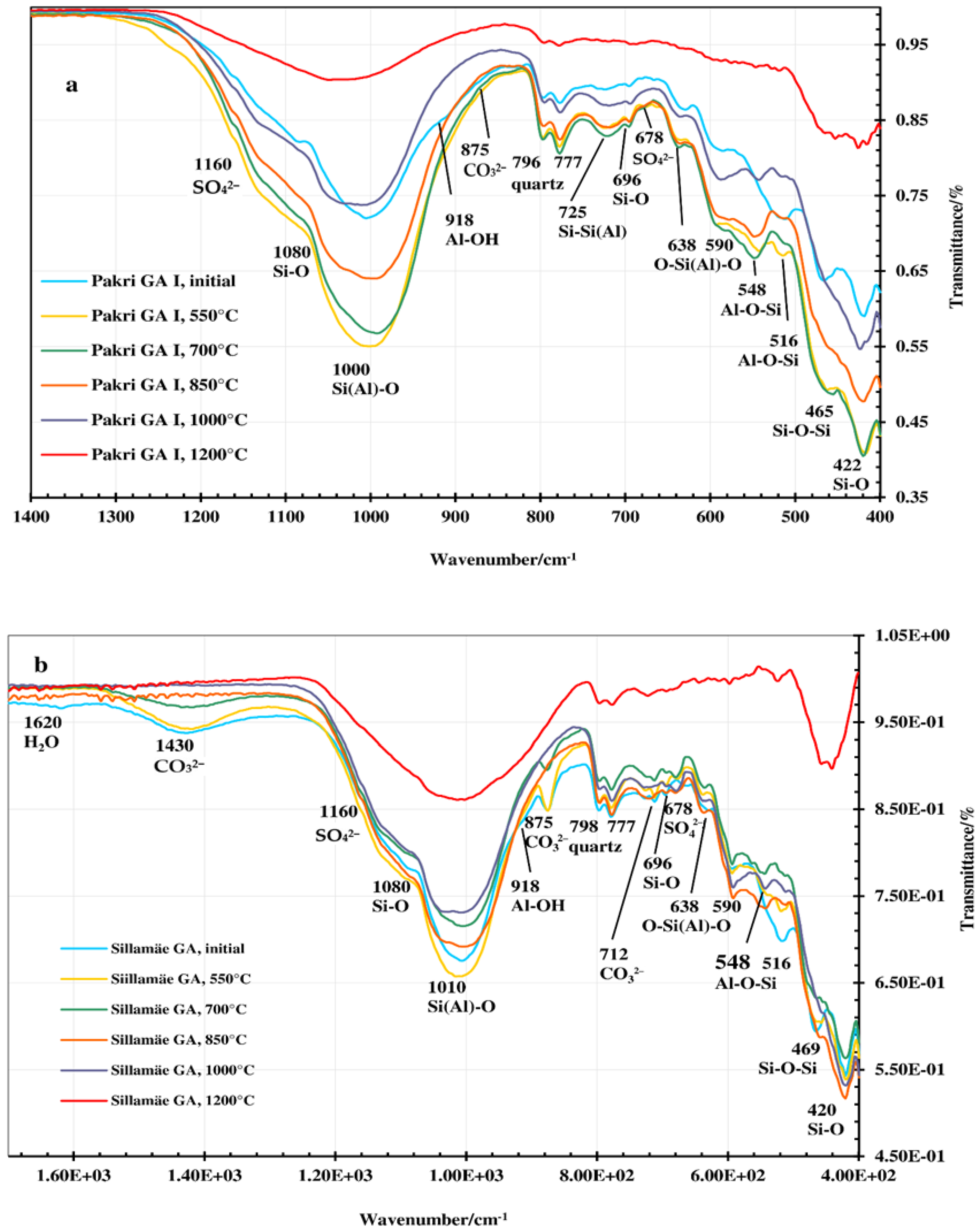
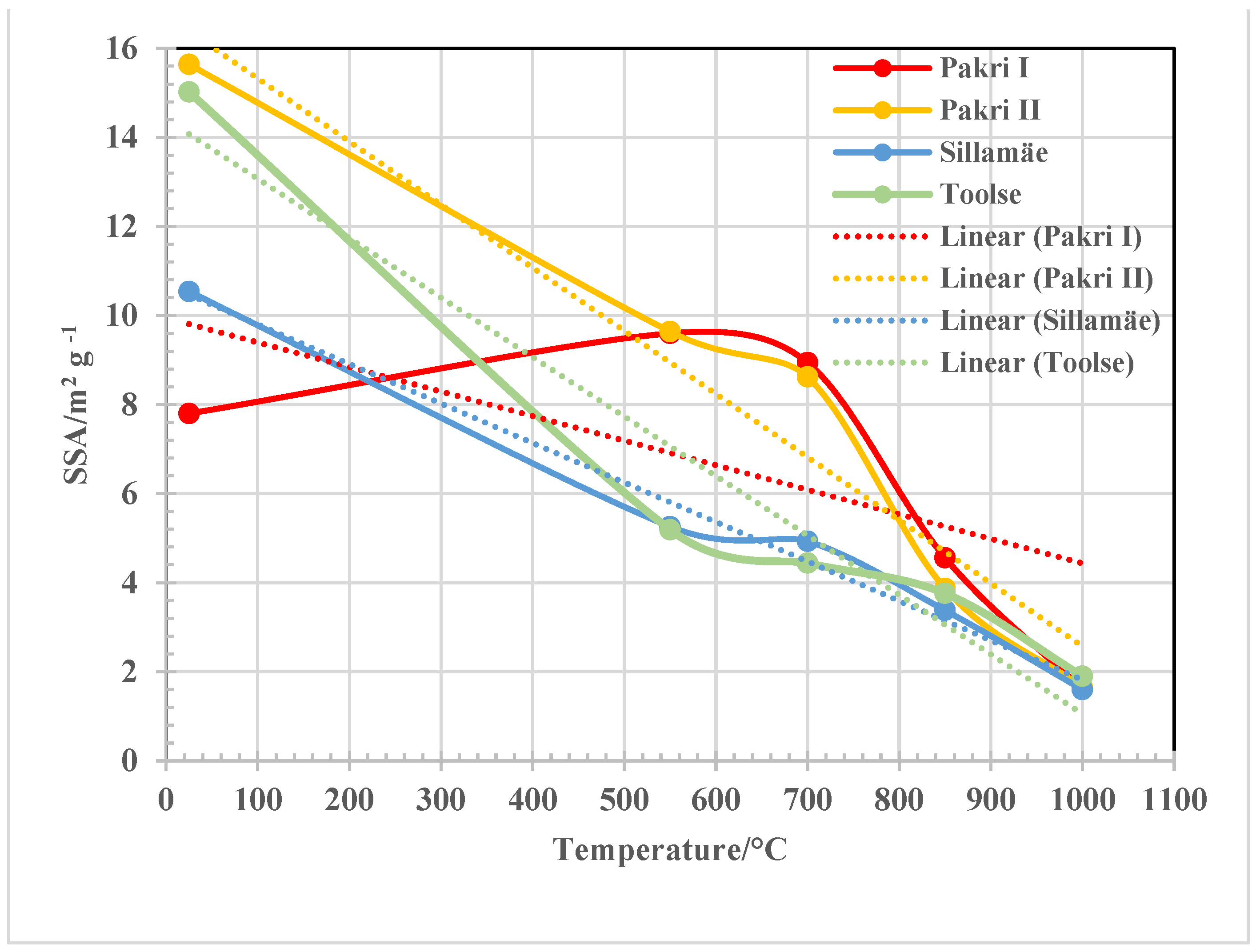
3.2. FT-IR, XRD and Morphology Analysis
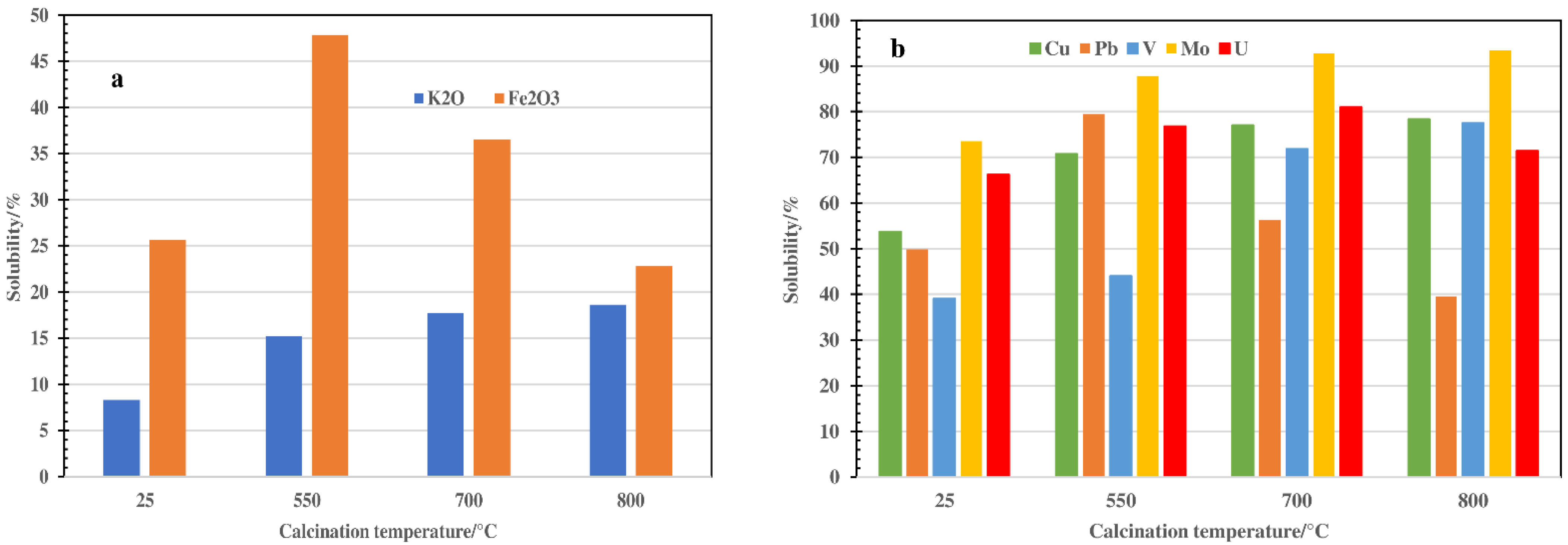
3.3. Solubility Test in Sulpuric Acid
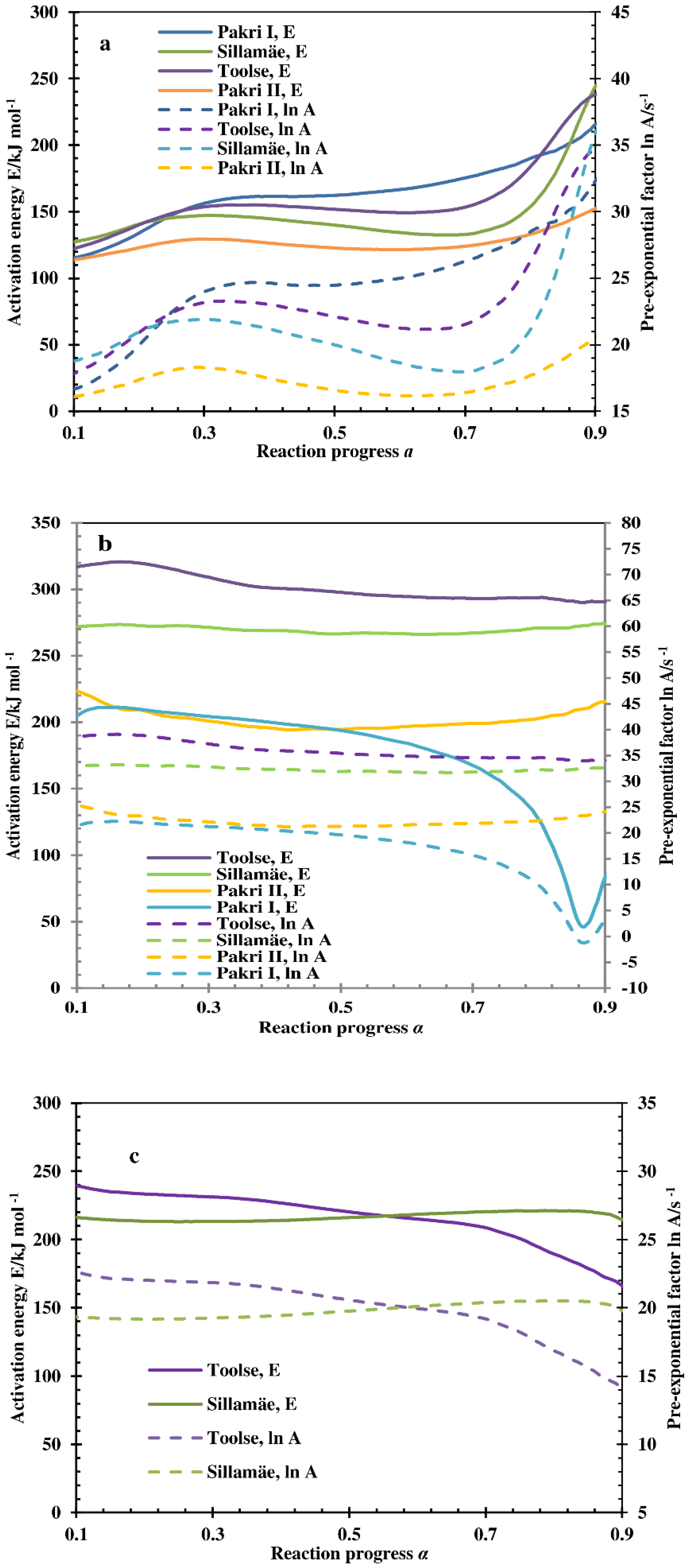
3.4. Kinetic Calculations
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DTA | Differential thermal analysis |
| DTG | Differential TG |
| EGA | Evolved gas analysis |
| FTIR | Fourier transform infrared |
| GA | Graptolite–argillite |
| MP-AES | Microwave plasma atomic emission spectroscopy |
| MS | Mass spectroscopy |
| SEM | Scanning electron spectroscopy |
| BET SSA | Brunauer–Emmett–Teller specific surface area |
| TG | Thermogravimetry |
| XRD | X-ray diffraction |
References
- Brumsack, H.-J. The trace metal content of recent organic carbon-rich sediments: Implications for Cretaceous black shale formation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 344–361. [Google Scholar] [CrossRef]
- Johnson, S.C.; Large, R.R.; Coveney, R.M.; Kelley, K.D.; Slack, J.F.; Steadman, J.A.; Gregory, D.D.; Sack, P.J.; Meffre, S. Secular distribution of highly metalliferous black shales corresponds with peaks in past atmosphere oxigenation. Min. Deposita. 2017, 52, 791–798. [Google Scholar] [CrossRef]
- Lavergren, U.; Åström, M.E.; Bergback, B.; Holmström, H. Mobility of trace elements in black shale assessed by leaching tests and sequential chemical reaction. Geochem. Explor. Environ. Analysis. 2009, 9, 71–79. [Google Scholar] [CrossRef]
- Canfield, D.E. A new model for Proterozoic ocean chemistry. Nature 1998, 396, 450–453. [Google Scholar] [CrossRef]
- Meyer, K.; Kump, L. Oceanic Euxinia in Earth History: Causes and Consequences. Annu. Rev. Earth Planet. Sci. 2008, 36, 251–288. [Google Scholar] [CrossRef] [Green Version]
- Lyons, T.W.; Anbar, A.D.; Severmann, S.; Scott, C.; Gill, B.C. Tracking Euxinia in the Ancient Ocean: A Multiproxy Perspective and Proterozoic Case Study. Annu. Rev. Earth Planet. Sci. 2009, 37, 507–534. [Google Scholar] [CrossRef]
- Blumenberg, M.; Wiese, F. Imbalanced nutrients as triggers for black shale formation in a shallow shelf setting during the OAE 2 (Wunstorf, Germany). Biogeosciences 2012, 9, 4139–4153. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, K.; Tajima, S.; Tajika, E. Conditions required for oceanic anoxia/euxinia: Constraints from a one-dimensional ocean biochemical cycle model. Earth Planet. Sci. Lett. 2011, 304, 270–279. [Google Scholar] [CrossRef]
- Turgeon, S.C.; Creaser, R.A. Cretaceous oceanic anoxic event 2 triggered by a massive magmatic episode. Nature 2008, 454, 323–326. [Google Scholar] [CrossRef]
- Jenkyns, H.C. Geochemistry of oceanic anoxic events. Geochem. Geophys. Geosyst. 2010, 11, Q03004. [Google Scholar] [CrossRef]
- Voigt, S.; Gale, A.S.; Voigt, T. Sea-level change, carbon cycling and paleoclimate during the Late Cenomanian of northwest Europe; an integrated paleoenvironmental analysis. Creteaceous Res. 2006, 27, 836–858. [Google Scholar] [CrossRef]
- Weissert, H.; Erba, E. Volcanism, CO2 and palaeoclimate: A Late Jurassic—Early Cretaceous carbon and oxygen isotope record. J. Geol. Soc. 2004, 161, 695–702. [Google Scholar] [CrossRef]
- Mattioli, E.; Pittet, B.; Palliani, R.B.; Röhl, H.J.; Schmid-Röhl, A.; Morettini, E. Phytoplankton evidence for the timing and correlation of palaeoceanographical changes during the early Toarcian oceanic anoxic event (Early Jurassic). J. Geol. Soc. 2004, 161, 685–693. [Google Scholar] [CrossRef]
- Nederbragt, A.J.; Thurow, J.; Vonhof, H.; Brumsack, H.-J. Modelling oceanic carbon and phosphorus fluxes: Implications for the cause of the late Cenomanian oceanic Anoxic Event (OAE2). J. Geol. Soc. 2004, 161, 721–728. [Google Scholar] [CrossRef]
- Algeo, T.J.; Ingall, E. Sedimentary Corg:P ratios, paleocean ventilation, and Phanerozoic atmospheric pO2. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 256, 130–155. [Google Scholar] [CrossRef]
- Mort, H.P.; Adatte, T.; Keller, G.; Bartels, D.; Föllmi, K.B.; Steinmann, P.; Berner, Z.; Chellai, E.H. Organic carbon deposition and phosphorus accumulation during Oceanic Anoxic Event 2 in Tarfaya, Morocco. Creteaceous Res. 2008, 29, 1008–1023. [Google Scholar] [CrossRef] [Green Version]
- Hade, S.; Soesoo, A. Estonian graptolite argillites revisited: A future resource? Oil Shale. 2014, 31, 4–18. [Google Scholar] [CrossRef] [Green Version]
- Voolma, M.; Soesoo, A.; Hade, S.; Hints, R.; Kallaste, T. Geochemical heterogeneity of Estonian graptolite argillite. Oil Shale. 2013, 30, 377–401. [Google Scholar] [CrossRef] [Green Version]
- Pagés, A.; Barnes, S.; Schmid, S.; Coveney, R.M.J.; Schwark, L.; Liu, W.; Grice, K.; Fan, H.; Wen, H. Geochemical investigation of the lower Cambrian mineralised black shales of South China and the late Devonian nick deposit, Canada. Ore Geol. Rev. 2018, 94, 396–413. [Google Scholar] [CrossRef]
- Dai, S.; Zheng, X.; Wang, X.; Finkelman, R.B.; Jiang, Y.; Ren, D.; Yan, X.; Zhou, Y. Stone coal in China: A review. Int. Geol. Review. 2018, 60, 736–753. [Google Scholar] [CrossRef]
- Marynowski, L.; Zatoń, M.; Rakociński, M.; Filipiak, P.; Kurkiewicz, S.; Pearce, T.J. Deciphering the upper Famennian Hangenberg Black Shale depositional environments based on multi-proxy record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 346–347, 66–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Zhang, Y.; Song, S.; Bao, S. In -situ investigation on mineral phase transition during roasting of vanadium-bearing stone coal. Adv. Powder Techn. 2017, 28, 1103–1107. [Google Scholar] [CrossRef]
- Li, W.; Ma, C.; Gong, W.; Zhu, X. Clean production technology for effective recovery of vanadium from shale: Interaction between activators and vanadium-loaded minerals. J. Clean. Prod. 2021, 315, 128170. [Google Scholar] [CrossRef]
- Lippmaa, E.; Maremäe, E.; Pihlak, A.T.; Aguraiuja, R. Estonian graptolitic argillites—ancient ores or future fuels? Oil Shale. 2009, 26, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Palvadre, R. Possibilities of utilization organic-poor metalliferous black shales (argillite). Oil Shale. 2020, 37, 242–267. [Google Scholar] [CrossRef]
- Hints, R.; Hade, S.; Soesoo, A.; Voolma, M. Depositional framework of the East Baltic Tremadocian black shale revisited. GFF 2014, 136, 464–482. [Google Scholar] [CrossRef]
- Hints, R.; Pajusaar, S.; Urtson, K.; Liiv, M.; Kallaste, T. Metal enrichment in lithologically complex black shales: A case study from the Tremadocian of NE Estonia. Est. J. Earth Sci. 2021, 70, 36–50. [Google Scholar] [CrossRef]
- Lippmaa, E.; Maremäe, E.; Pihlak, A.T. Resources, production and processing of Baltoscandian multimetal black shales. Oil Shale. 2011, 28, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Tribovillard, N.; Algeo, J.T.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Soesoo, A.; Vind, J.; Hade, S. Uranium and Thorium resources of Estonia. Minerals 2020, 10, 798. [Google Scholar] [CrossRef]
- Vind, J.; Bauert, H. Geochemical Characterization of the Tremadocian Black Shale in North-Western Estonia; Research Report No. EGF9330; Geological Survey of Estonia: Rakvere, Estonia, 2020; Volume 91. [Google Scholar]
- Schmidt, C.M.; Heide, K. Thermal analysis of hydrocarbons in Paleozoic black shales. J. Therm. Anal. Calorim. 2001, 64, 1297–1302. [Google Scholar] [CrossRef]
- Labus, M. Thermal methods implementation in analysis of fine-grained rocks containing organic matter. J. Therm. Anal. Calorim. 2017, 129, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Labus, M.; Lempart, M. Studies of Polish Paleozoic shale rocks using FTIR and TG/DSC methods. J. Petrol. Sci. Eng. 2018, 161, 311–318. [Google Scholar] [CrossRef]
- Taylor, J.C. Computer programs for standardless quantitative analysis of minerals using the full powder diffraction profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Ward, C.R.; Taylor, J.C.; Cohen, D.R. Quantitative mineralogy of sandstones by X-ray diffractrometry and normative analysis. J. Sed. Geo. 1999, 69, 1050–1062. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to phenolic plastic. J. Polym. Sci. 1965, 6, 183–195. [Google Scholar] [CrossRef]
- AKTS Softwear and Setaram Instruments: A Global Solution for Kinetic Analysis and Determination of the Thermal Stability of Materials; AKTS AG: Sider, Switzerland, 2006; p. 88.
- Gaines, G.L.; Vedder, W. Dehydroxylation of Muscovite. Nature 1964, 4918, 495. [Google Scholar] [CrossRef]
- Kodama, H.; Brydon, J.E. Dehydroxylation of Microcrystalline Muscovite. Trans. Faraday Soc. 1968, 64, 3112–3119. [Google Scholar] [CrossRef]
- Lempart, M.; Derkowski, A.; Luberda-Durnaś, K.; Skiba, M.; Błachowski, A. Dehydrogenation and dihydroxylation as drivers of the thermal decomposition of Fe-chlorites. Am. Mineral. 2018, 103, 1837–1850. [Google Scholar] [CrossRef]
- Frost, R.L.; Weier, M.L.; Martens, W. Thermal decomposition of jarosite of potassium, sodium and lead. J. Therm. Anal. Calorim. 2005, 82, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Paulik, F.; Paulik, J.; Arnold, M. Kinetics and mechanism of decomposition of pyrite under conventional and quasi-isothermal –quasi-isobaric thermoanalytical conditions. J. Therm. Anal. 1982, 25, 313–325. [Google Scholar] [CrossRef]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Diko, M.; Ekosse, G.; Ogola, J. Fourier Transform Infrared Spectroscopy and thermal analysis of kaolinite clays from South Africa and Cameroon. Acta Geodyn. Geomater. 2016, 13, 149–158. [Google Scholar]
- Zhirong, L.; Uddin, M.A.; Zhanxue, S. FT-IR and XRD analysis of Natural Na-bentonite and Cu (II)-loaded Na-bentonite. Spectrochim. Acta Part A 2011, 79, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Eisazadeh, A.; Kassim, K.A.; Nur, H. Solid-state NMR and FTIR studies of lime stabilized montmorillonitic and lateritic clays. Appl. Clay Sci. 2012, 67–68, 5–10. [Google Scholar] [CrossRef]
- Cizer, Ö.; Rodrigues-Navarro, C.; Ruiz-Agudo, E.; Elsen, J.; Van Gemert, D.; Van Balen, K. Phase and morphology evolution of carbonate precipitated by carbonation of hydrated lime. J. Mat. Sci. 2012, 47, 6151–6165. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.M.; Pejcic, B.; Esteban, L.; Piane, C.D.; Raven, M.; Mizaikoff, B. Infrared Attenuated Total Reflectance Spectroscopy: An Innovative Strategy for Analyzing Mineral components in Energy Relevant Systems. Sci. Rep. 2014, 4, 6764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Davarcioğlu, B.; Ҫiftҁi, E. Investigation of Central Anatolian Clays by FTIR Spectroscopy (Arapli-Yesilhisar-Kayseri, Turkey). Int. J. Nat. Engin. Sci. 2009, 3, 167–174. [Google Scholar]
- Theodosoglou, E.; Koroneos, A.; Soldatos, T.; Zorba, T.; Paraskevopoulos, K.M. Comparative Fourier transform infrared and X-ray powder diffraction analysis of naturally occurred K-feldspars. Bull. Geol. Soc. Greece Jan. 2010, XLIII, 2752–2761. [Google Scholar] [CrossRef] [Green Version]
- Mozgawa, W.; Król, M.; Dyczek, J.; Deja, J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta Part A 2000, 56, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Matteson, A.; Herron, M.M. End-member feldspar concentrations determined by FTIR spectral analysis. J. Sediment. Petrol. 1993, 63, 1144–1148. [Google Scholar]
- Alver, B.E.; Dikmen, G.; Alver, Ö. Investigation of the influence of heat treatment on the structural properties of illite-rich clay mineral using FT-IR, 29Si MAS NMR, TG and DTA methods. Anadolu. Univ. J. Sci. Technol. A Appl. Sci. Engin. 2016, 17, 823–829. [Google Scholar]
- Smith, D.H.; Seshadri, K.S. Infrared spectra of Mg2Ca(SO4)3, MgSO4, hexagonal CaSO4 and orthorhombic CaSO4. Spectrochim. Acta Part A 1999, 55, 795–805. [Google Scholar] [CrossRef]
- Bishop, J.L.; Murad, E. The visible and infrared properties of jarosite and alunite. Am. Mineral. 2005, 90, 1100–1107. [Google Scholar] [CrossRef]
- Barlow, S.G.; Manning, D.A.C. Influence of time and temperature on reactions and transformations of muscovite mica. Br. Ceram. Trans. 1999, 98, 122–126. [Google Scholar] [CrossRef]



| Compound/Formula | Pakri I | Pakri II | Sillamäe | Toolse |
|---|---|---|---|---|
| Quartz, SiO2 | 24.2 | 24.7 | 23.8 | 35.5 |
| Orthoclase, KAlSi3O8 | 54.7 | 57.3 | 38.0 | 41.8 |
| Muscovite, KAl2(AlSi3O10)(F,OH)2 | 7.2 | 7.8 | 3.8 | 9.1 |
| Jarosite, KFe33+(OH)6(SO4)2 | 4.5 | 1.0 | - | 0.4 |
| Chlorite, (Mg,Fe)3(SiAl)4O10(OH)2(Mg,Fe)3(OH)6 | 0.5 | 1.7 | 0.4 | - |
| Pyrite, FeS2 | 8.4 | 5.7 | 7.2 | 9.9 |
| Anatase, TiO2 | 0.5 | 0.7 | 0.3 | 0.4 |
| Hematite, Fe2O3 | - | 1.1 | - | 1.1 |
| Calcite, CaCO3 | - | - | 19.1 | - |
| Dolomite, CaMg(CO3)2 | - | - | 6.9 | 1.8 |
| Sphalerite, (Zn,Fe)S | - | - | 0.5 | - |
| Item | Sample/Content | |||
|---|---|---|---|---|
| Pakri I | Pakri II | Sillamäe | Toolse | |
| SiO2, % | 45.3 | 45.2 | 41.2 | 54.0 |
| Al2O3, % | 11.4 | 11.3 | 9.1 | 9.6 |
| K2O, % | 6.8 | 8.4 | 5.5 | 5.9 |
| Fe2O3, % | 7.9 | 5.4 | 8.1 | 9.4 |
| CaO, % | 0.3 | 0.2 | 11.8 | 1.6 |
| MgO, % | 0.8 | 1.0 | 1.4 | 1.8 |
| SO3total, % | 12.7 | 8.1 | 10.3 | 13.2 |
| CTC, % | 12.97 | 14.42 | 9.51 | 9.59 |
| CTIC, % | 0.40 | 0.24 | 2.23 | 0.32 |
| Corg *, % | 12.57 | 14.18 | 7.28 | 9.27 |
| V, ppm | 890 | 958 | 868 | 916 |
| Mo, ppm | 336 | 137 | 956 | 414 |
| U, ppm | 90 | 120 | 254 | 146 |
| Cu, ppm | 110 | 97 | 89 | 87 |
| Pb, ppm | 135 | 104 | 194 | 158 |
| BET SSA, m2 g−1 | 7.81 | 15.64 | 10.54 | 15.02 |
| Goss calorific value, MJ kg−1 | 5.27 | 6.13 | 3.25 | 4.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaljuvee, T.; Tõnsuaadu, K.; Einard, M.; Mikli, V.; Kivimäe, E.-K.; Kallaste, T.; Trikkel, A. Thermal Behavior of Estonian Graptolite–Argillite from Different Deposits. Processes 2022, 10, 1986. https://doi.org/10.3390/pr10101986
Kaljuvee T, Tõnsuaadu K, Einard M, Mikli V, Kivimäe E-K, Kallaste T, Trikkel A. Thermal Behavior of Estonian Graptolite–Argillite from Different Deposits. Processes. 2022; 10(10):1986. https://doi.org/10.3390/pr10101986
Chicago/Turabian StyleKaljuvee, Tiit, Kaia Tõnsuaadu, Marve Einard, Valdek Mikli, Eliise-Koidula Kivimäe, Toivo Kallaste, and Andres Trikkel. 2022. "Thermal Behavior of Estonian Graptolite–Argillite from Different Deposits" Processes 10, no. 10: 1986. https://doi.org/10.3390/pr10101986
APA StyleKaljuvee, T., Tõnsuaadu, K., Einard, M., Mikli, V., Kivimäe, E.-K., Kallaste, T., & Trikkel, A. (2022). Thermal Behavior of Estonian Graptolite–Argillite from Different Deposits. Processes, 10(10), 1986. https://doi.org/10.3390/pr10101986







