Comparison of Four Density-Based Semi-Empirical Models for the Solubility of Azo Disperse Dyes in Supercritical Carbon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
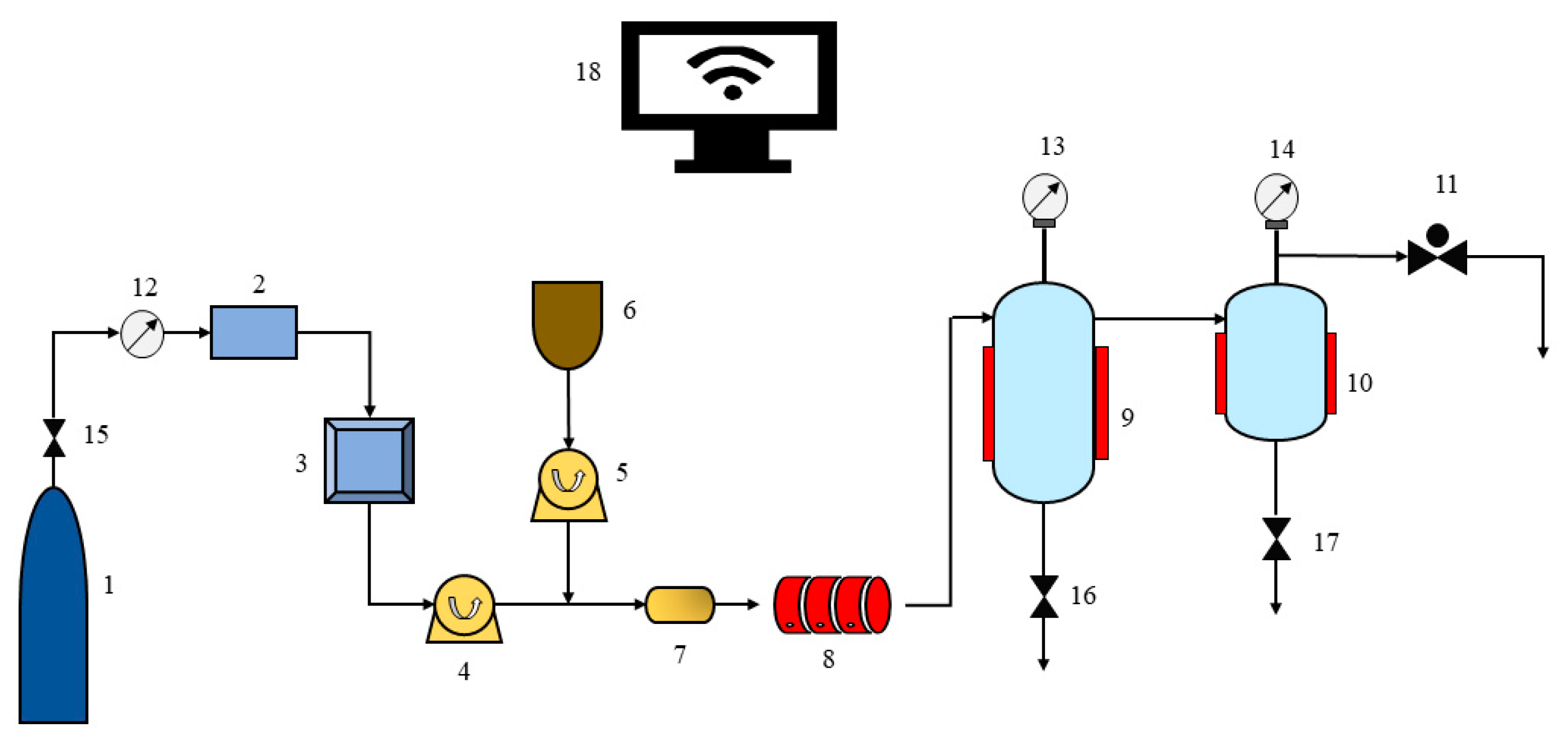
2.2. Apparatus and Procedures
2.3. Measurement of Solubility
3. Results and Discussion
3.1. Experimental Solubility Data in sc-CO2
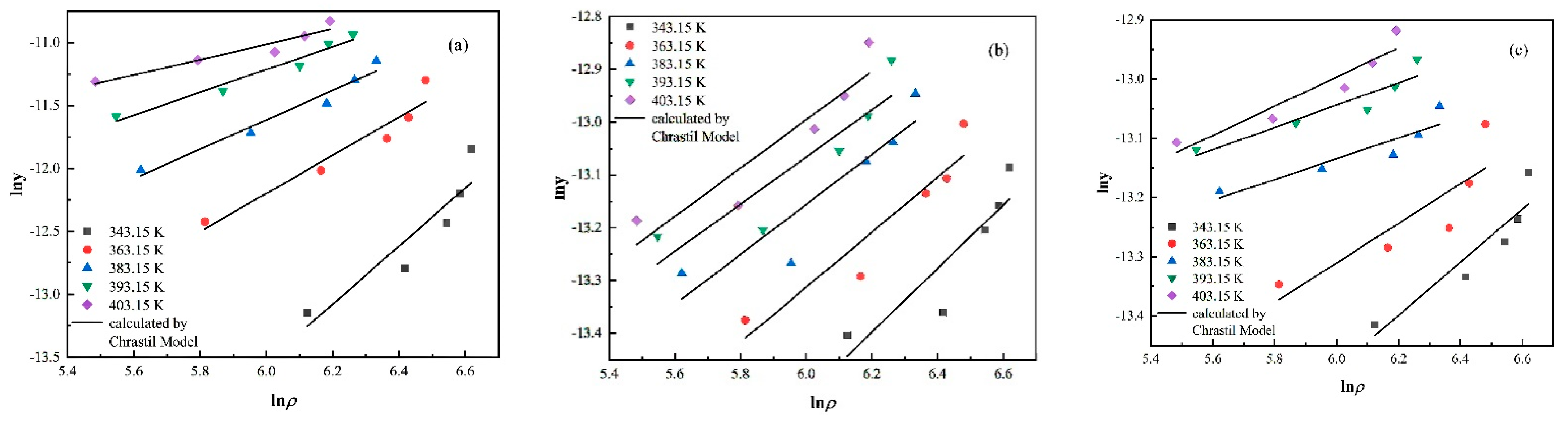
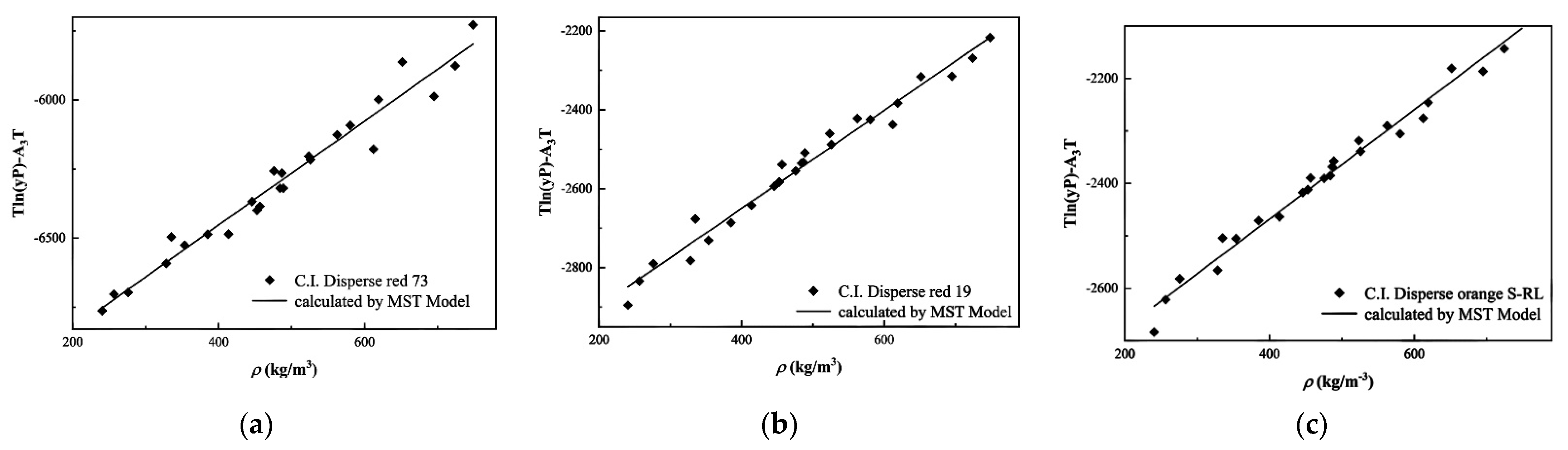
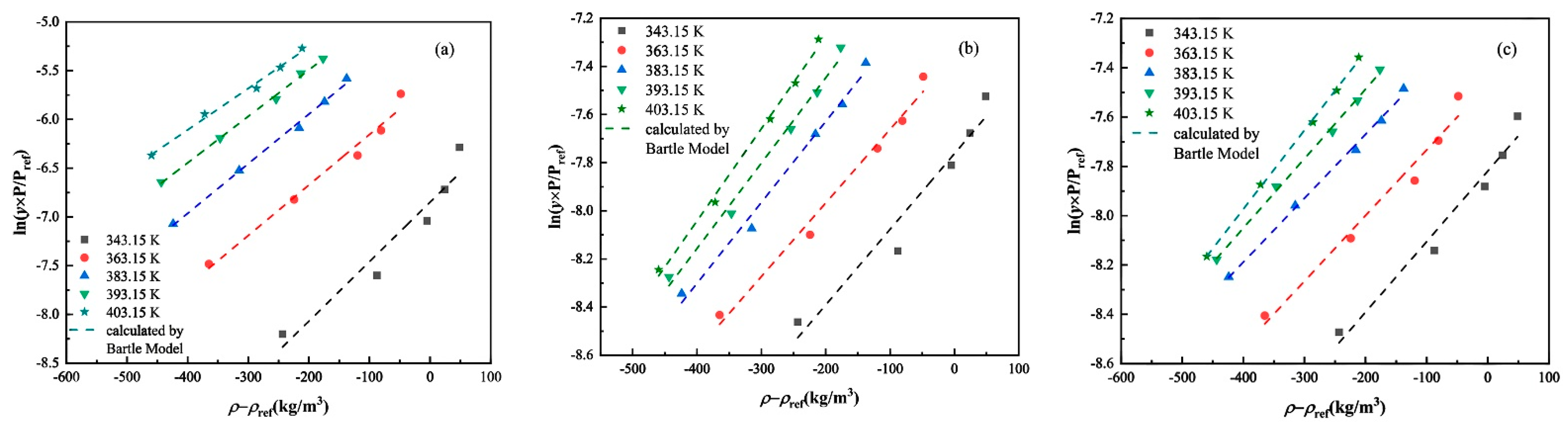
3.2. Experimental Solubility Data Correlation
3.3. Comparison Solubility with Literature Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abou Elmaaty, T.; Abd El-Aziz, E. Supercritical carbon dioxide as a green media in textile dyeing: A review. Text. Res. J. 2018, 88, 1184–1212. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical fluid dyeing of synthetic and natural textiles—A review. Color. Technol. 2013, 129, 2–17. [Google Scholar] [CrossRef]
- Banchero, M. Recent advances in supercritical fluid dyeing. Color. Technol. 2020, 136, 317–335. [Google Scholar] [CrossRef]
- Zha, X.; Han, S.; Wang, W.; Jiao, Z. Experimental measurement and correlation of solubility of ethosuximide in supercritical carbon dioxide. J. Chem. Thermodyn. 2019, 131, 104–110. [Google Scholar] [CrossRef]
- Sodeifian, G.; Razmimanesh, F.; Sajadian, S.A.; Hazaveie, S.M. Experimental data and thermodynamic modeling of solubility of Sorafenib tosylate, as an anti-cancer drug, in supercritical carbon dioxide: Evaluation of Wong-Sandler mixing rule. J. Chem. Thermodyn. 2020, 142, 105998. [Google Scholar] [CrossRef]
- Sawada, K.; Oshima, M.; Sugimoto, M.; Urakawa, H.; Ueda, M. Complexation of ionic substances with surfactants for solubilisation in supercritical carbon dioxide. Dyes Pigm. 2008, 76, 1–6. [Google Scholar] [CrossRef]
- Sawada, K.; Takagi, T.; Ueda, M. Solubilization of ionic dyes in supercritical carbon dioxide: A basic study for dyeing fiber in non-aqueous media. Dyes Pigm. 2004, 60, 129–135. [Google Scholar] [CrossRef]
- Montero, G.A.; Smith, C.B.; Hendrix, W.A.; Butcher, D.L. Supercritical fluid technology in textile processing: An overview. Ind. Eng. Chem. Res. 2000, 39, 4806–4812. [Google Scholar] [CrossRef]
- Bach, E.; Cleve, E.; Schollmeyer, E. Past, present and future of supercritical fluid dyeing technology—An overview. Color. Technol. 2002, 32, 88–102. [Google Scholar] [CrossRef]
- Fasihi, J.; Yamini, Y.; Nourmohammadian, F.; Bahramifar, N. Investigations on the solubilities of some disperse azo dyes in supercritical carbon dioxide. Dyes Pigm. 2004, 63, 161–168. [Google Scholar] [CrossRef]
- Long, J.J.; Ran, R.L.; Jiang, W.D.; Ding, Z.F. Solubility of a reactive disperse dye in supercritical carbon dioxide. Color. Technol. 2012, 128, 127–132. [Google Scholar] [CrossRef]
- Gao, D.; Yang, D.F.; Cui, H.S.; Huang, T.T.; Lin, J.X. Synthesis and measurement of solubilities of reactive disperse dyes for dyeing cotton fabrics in supercritical carbon dioxide. Ind. Eng. Chem. Res. 2014, 53, 13862–13870. [Google Scholar] [CrossRef]
- Hu, J.; Yang, F.; Yan, J.; Zheng, L.; Xiong, X.; Mou, J.; Li, H.; Tamura, K. Measurement and correlation of 1, 4-diamino-2-methoxyanthraquinone and 1- amino-2-methoxy-4-hydroxyanthraquinone in supercritical CO2. J. Chin. Inst. Eng. 2020, 44, 64–71. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Mazzer, H.R.; Machado, G.D.; Andreaus, J.; Cabral, V.F.; Zabaloy, M.S.; Cardozo-Filho, L. High-pressure phase behaviour of the system (CO2+C.I. Disperse Orange 30 dye). J. Chem. Thermodyn. 2012, 48, 284–290. [Google Scholar] [CrossRef]
- Draper, S.L.; Montero, G.A.; Smith, B.; Beck, K. Solubility relationships for disperse dyes in supercritical carbon dioxide. Dyes Pigm. 2000, 45, 177–183. [Google Scholar] [CrossRef]
- Chudgar, R.J.; Oakes, J. Dyes, azo. Kirk-Othmer Encycl. Chem. Technol. 2000, 9, 349. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Cadoni, E.; Maricca, D.; Piras, A. Dyeing polyester fibres with disperse dyes in supercritical CO2. Dyes Pigm. 2000, 45, 75–79. [Google Scholar] [CrossRef]
- Penthala, R.; Heo, G.; Kim, H.; Lee, I.Y.; Ko, E.H.; Son, Y.A. Synthesis of azo and anthraquinone dyes and dyeing of nylon-6, 6 in supercritical carbon dioxide. J. CO2 Util. 2020, 38, 49–58. [Google Scholar] [CrossRef]
- Joung, S.N.; Yoo, K.P. Solubility of disperse anthraquinone and azo dyes in supercritical carbon dioxide at 313.15 to 393.15 K and from 10 to 25 MPa. J. Chem. Eng. Data. 1998, 43, 9–12. [Google Scholar] [CrossRef]
- Gao, D.; Yang, D.F.; Cui, H.S.; Huang, T.T.; Lin, J.X. Supercritical carbon dioxide dyeing for PET and cotton fabric with synthesized dyes by a modified apparatus. ACS Sustain. Chem. Eng. 2015, 3, 668–674. [Google Scholar] [CrossRef]
- Ardestani, N.S.; Amani, M.; Moharrery, L. Determination of Anthraquinone Violet 3RN solubility in supercritical carbon dioxide with/without co-solvent: Experimental data and modeling (empirical and thermodynamic models). Chem. Eng. Res. Des. 2020, 159, 529–542. [Google Scholar] [CrossRef]
- Zacconi, F.C.; Nuñez, O.N.; Cabrera, A.L.; Valenzuela, L.M.; del Valle, J.M.; Juan, C. Synthesis and solubility measurement in supercritical carbon dioxide of two solid derivatives of 2-methylnaphthalene-1,4-dione (menadione): 2-(Benzylamino)-3-methylnaphthalene-1,4-dione and 3-(phenethylamino)-2-methylnaphthalene-1,4-dione. J. Chem. Thermodyn. 2016, 103, 325–332. [Google Scholar] [CrossRef]
- Zabihi, S.; Jamshidian, S.; Borousan, F.; Hezave, A.Z.; Pishnamazi, M.; Marjani, A.; Shirazian, S. Measuring salsalate solubility in supercritical carbon dioxide: Experimental and thermodynamic modelling. J. Chem. Thermodyn. 2021, 152, 106271. [Google Scholar] [CrossRef]
- Kong, X.J.; Huang, T.T.; Cui, H.S.; Yang, D.F.; Lin, J.X. Multicomponent system of trichromatic disperse dye solubility in supercritical carbon dioxide. J. CO2 Util. 2019, 33, 1–11. [Google Scholar] [CrossRef]
- Yamini, Y.; Moradi, M.; Hojjati, M.; Nourmohammadian, F.; Saleh, A. Solubilities of some disperse yellow dyes in supercritical CO2. J. Chem. Eng. Data 2010, 55, 3896–3900. [Google Scholar] [CrossRef]
- Josef, C. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Mendez-Santiago, J.; Teja, A.S. The solubility of solids in supercritical fluids. Fluid Phase Equilib. 1999, 158, 501–510. [Google Scholar] [CrossRef]
- Kumar, K.S.; Johnston, K.P. Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids 1988, 1, 15–22. [Google Scholar] [CrossRef]
- Bartle, K.D.; Clifford, A.A.; Jafar, S.A.; Shilstone, G.F. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 1991, 20, 713–756. [Google Scholar] [CrossRef]
- Black, S.; Dang, L.; Liu, C.; Wei, H. On the measurement of solubility. Org. Process Res. Dev. 2013, 17, 486–492. [Google Scholar] [CrossRef]
- NIST. Available online: http://webbook.nist.gov/chemistry/fluid/ (accessed on 12 February 2022).
- Span, R.; Wanger, W. Equations of state for technical applications. I. simultaneously optimized functional forms for nonpolar and polar fluids. Int. J. Thermophys. 2003, 24, 1–39. [Google Scholar] [CrossRef]
- Banchero, M.; Ferri, A.; Manna, L.; Sicardi, S. Solubility of disperse dyes in supercritical carbon dioxide and ethanol. Fluid Phase Equilib. 2006, 243, 107–114. [Google Scholar] [CrossRef]
- Zhan, S.; Li, S.; Zhao, Q.; Wang, W.; Wang, J. Measurement and correlation of curcumin solubility in supercritical carbon dioxide. J. Chem. Eng. Data 2017, 62, 1257–1263. [Google Scholar] [CrossRef]
- Alwi, R.S.; Tamura, K. Measurement and correlation of derivatized anthraquinone solubility in supercritical carbon dioxide. J. Chem. Eng. Data 2015, 60, 3046–3052. [Google Scholar] [CrossRef]
- Wang, H.L.; Sang, J.R.; Guo, L.T.; Zhu, J.; Jin, J.S. Solubility of polyacrylamide in supercritical carbon dioxide. J. Chem. Eng. Data 2016, 62, 341–347. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, M.; Lu, X.; Lin, C. Measurement and correlation of solubilities of C.I. Disperse Red 73, C.I. Disperse Blue 183 and their mixture in supercritical carbon dioxide. Chin. J. Chem. Eng. 2010, 18, 648–653. [Google Scholar] [CrossRef]
- Dong, P.; Xu, M.; Lu, X.; Lin, C. Measurement and correlation of solubilities of C.I. Disperse Red 73, C.I. Disperse Yellow 119 and their mixture in supercritical carbon dioxide. Fluid Phase Equilib. 2010, 297, 46–51. [Google Scholar] [CrossRef]


| Dye Name | Formula | Molecular Structure | MW | Melting Point/K | Mass Fraction Purity | Source |
|---|---|---|---|---|---|---|
| C.I. disperse red 73(2-[4-(2-Cyanoethylethylamino)phenyl]diazenyl-5-nitrobenzonitrile) CAS number 16889-10-4 | C18H16N6O2 |  | 348.36 | 402.15 ± 0.05 | >0.995 | Hebei Province Zize Chemical Co., Ltd. (Handan, China) |
| C.I. disperse red 19(2,2′-[[4-[(4-Nitrophenyl)azo]phenyl]imino]bis-ethano) CAS number 2734-52-3 | C16H18N4O4 | 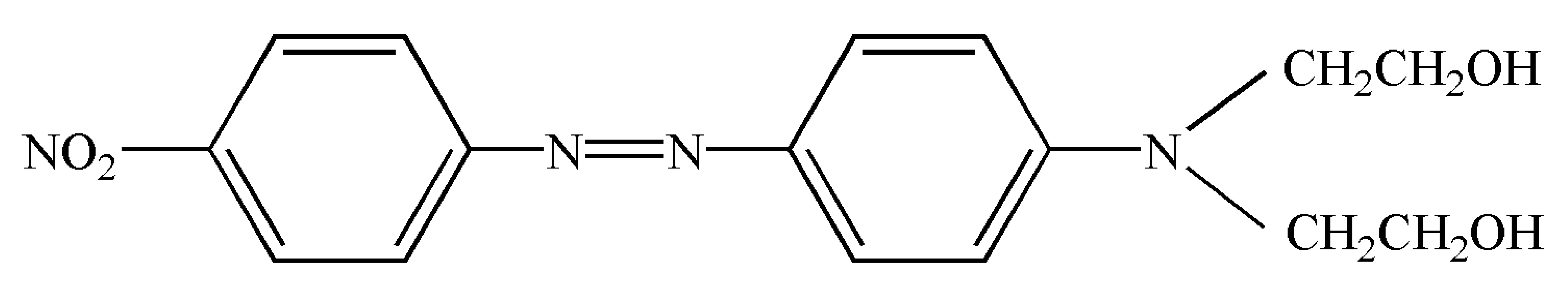 | 330.34 | 412.15 ± 0.06 | >0.995 | Hebei Province Zize Chemical Co., Ltd.(Handan, China) |
| C.I. disperse orange S-RL (3,3′-[[4-[(4-nitrophenyl)azo]phenyl]imino]bispropiononitrile) CAS number 4234-72-4 | C18H16N6O2 | 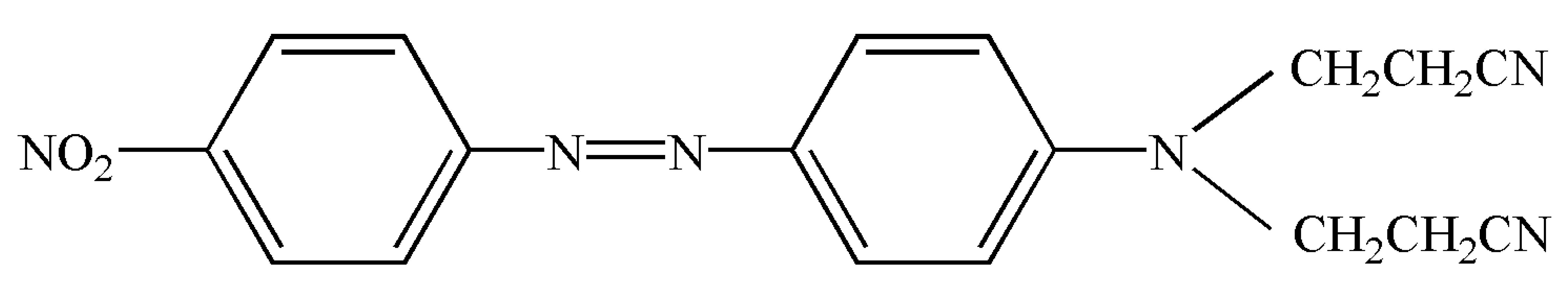 | 348.36 | 398.15 ± 0.03 | >0.995 | Hebei Province Zize Chemical Co., Ltd.(Handan, China) |
| Carbon dioxide CAS number 124-38-9 | CO2 | ⸺ | 44 | >0.999 | Dalian Zhonghao Guangming Chemical Research and Design Institute Co., Ltd. (Dalian, China) |
| T/K | P/MPa | ρ/kg∙m−3 | 106∙y/mol∙mol−1 | ||
|---|---|---|---|---|---|
| DR 73 | DR 19 | DO S-RL | |||
| 343.15 | 14 | 456.62 3 | 1.96 1 ± 0.28 2 | 1.51 ± 0.15 | 1.49 ± 0.44 |
| 18 | 612.24 | 2.78 ± 0.17 | 1.58 ± 0.14 | 1.62 ± 0.47 | |
| 22 | 695.1 | 3.98 ± 0.39 | 1.84 ± 0.17 | 1.72 ± 0.11 | |
| 24 | 724.23 | 5.03 ± 0.31 | 1.93 ± 0.24 | 1.79 ± 0.52 | |
| 26 | 748.61 | 7.16 ± 0.39 | 2.08 ± 0.29 | 1.93 ± 0.42 | |
| 363.15 | 14 | 335.08 | 4.02 ± 0.24 | 1.55 ± 0.20 | 1.60 ± 0.37 |
| 18 | 475.67 | 6.05 ± 0.20 | 1.69 ± 0.16 | 1.70 ± 0.67 | |
| 22 | 580.38 | 7.79 ± 0.15 | 1.98 ± 0.13 | 1.76 ± 0.56 | |
| 24 | 619.21 | 9.23 ± 0.18 | 2.03 ± 0.14 | 1.90 ± 0.40 | |
| 26 | 651.66 | 12.39 ± 0.49 | 2.25 ± 0.21 | 2.10 ± 0.50 | |
| 383.15 | 14 | 276.12 | 6.06 ± 0.42 | 1.70 ± 0.10 | 1.87 ± 0.53 |
| 18 | 384.99 | 8.16 ± 0.34 | 1.73 ± 0.22 | 1.94 ± 0.47 | |
| 22 | 484.04 | 10.31 ± 0.29 | 2.10 ± 0.19 | 1.99 ± 0.08 | |
| 24 | 525.8 | 12.38 ± 0.49 | 2.18 ± 0.21 | 2.06 ± 0.33 | |
| 26 | 562.32 | 14.48 ± 0.20 | 2.39 ± 0.37 | 2.16 ± 0.56 | |
| 393.15 | 14 | 256.41 | 9.31 ± 0.39 | 1.82 ± 0.16 | 2.01 ± 0.35 |
| 18 | 353.55 | 11.36 ± 0.40 | 1.84 ± 0.30 | 2.10 ± 0.63 | |
| 22 | 445.86 | 13.87 ± 0.45 | 2.14 ± 0.21 | 2.15 ± 0.38 | |
| 24 | 486.7 | 16.57 ± 0.60 | 2.29 ± 0.20 | 2.23 ± 0.71 | |
| 26 | 523.39 | 17.81 ± 0.28 | 2.54 ± 0.13 | 2.34 ± 0.56 | |
| 403.15 | 14 | 240.4 | 12.24 ± 0.20 | 1.88 ± 0.13 | 2.03 ± 0.40 |
| 18 | 328.23 | 14.55 ± 0.43 | 1.93 ± 0.09 | 2.11 ± 0.31 | |
| 22 | 413.72 | 15.48 ± 0.54 | 2.23 ± 0.17 | 2.23 ± 0.48 | |
| 24 | 452.85 | 17.59 ± 0.22 | 2.38 ± 0.24 | 2.32 ± 0.86 | |
| 26 | 488.81 | 19.78 ± 0.49 | 2.63 ± 0.19 | 2.45 ± 0.74 | |
| Model | Parameters | Dyestuff Name | ||
|---|---|---|---|---|
| DR 73 | DR 19 | DO S-RL | ||
| Chrastil | a1 | 1.18 | 0.49 | 0.26 |
| b1 | −4661.72 | −1087.52 | −967.42 | |
| c1 | −6.45 | −13.23 | −12.15 | |
| AARD (%) | 12.29 | 4.34 | 3.24 | |
| MST | A1 | −7203.86 | −3148.06 | −2885.14 |
| A2 | 1.88 | 1.24 | 1.04 | |
| A3 | 8.10 | −3.37 | −3.81 | |
| AARD (%) | 10.72 | 5.49 | 4.17 | |
| K-J | A | 0.24 | −10.50 | −10.68 |
| B | −4994.93 | −1203.16 | −1043.50 | |
| C | 0.00295 | 0.00119 | 0.000651 | |
| AARD (%) | 10.37 | 3.49 | 2.49 | |
| Bartle | a0 | 11.55 | −0.43 | −0.98 |
| a1 | −6299.55 | −2507.77 | −2348.12 | |
| a2 | 0.0051 | 0.00333 | 0.0028 | |
| AARD (%) | 10.51 | 4.42 | 3.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Du, S.; Du, H.; Zhang, H.; Jiao, A.; Li, H.; Du, B.; Gao, D.; Wang, K. Comparison of Four Density-Based Semi-Empirical Models for the Solubility of Azo Disperse Dyes in Supercritical Carbon Dioxide. Processes 2022, 10, 1960. https://doi.org/10.3390/pr10101960
Yan J, Du S, Du H, Zhang H, Jiao A, Li H, Du B, Gao D, Wang K. Comparison of Four Density-Based Semi-Empirical Models for the Solubility of Azo Disperse Dyes in Supercritical Carbon Dioxide. Processes. 2022; 10(10):1960. https://doi.org/10.3390/pr10101960
Chicago/Turabian StyleYan, Jun, Shuang Du, Hui Du, Huan Zhang, Andong Jiao, Hong Li, Bing Du, Dawei Gao, and Kaihua Wang. 2022. "Comparison of Four Density-Based Semi-Empirical Models for the Solubility of Azo Disperse Dyes in Supercritical Carbon Dioxide" Processes 10, no. 10: 1960. https://doi.org/10.3390/pr10101960
APA StyleYan, J., Du, S., Du, H., Zhang, H., Jiao, A., Li, H., Du, B., Gao, D., & Wang, K. (2022). Comparison of Four Density-Based Semi-Empirical Models for the Solubility of Azo Disperse Dyes in Supercritical Carbon Dioxide. Processes, 10(10), 1960. https://doi.org/10.3390/pr10101960





