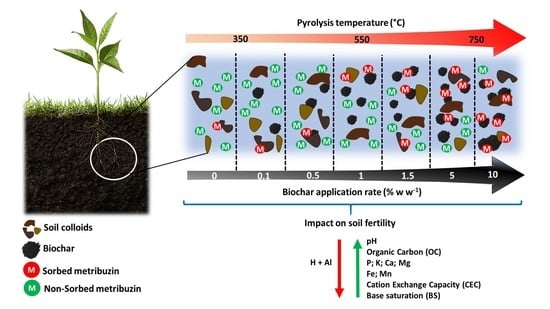
Pyrolysis Temperature and Application Rate of Sugarcane Straw Biochar Influence Sorption and Desorption of Metribuzin and Soil Chemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sugarcane Straw Biochar
2.2. Soil Collection and Analysis
2.3. Sorption–Desorption Studies
2.4. Freundlich Model for Sorption–Desorption and Apparent Coefficient
3. Results and Discussion
3.1. Biochar Characterization
3.2. Characterization of Biochar-Amended and Unamended Soils
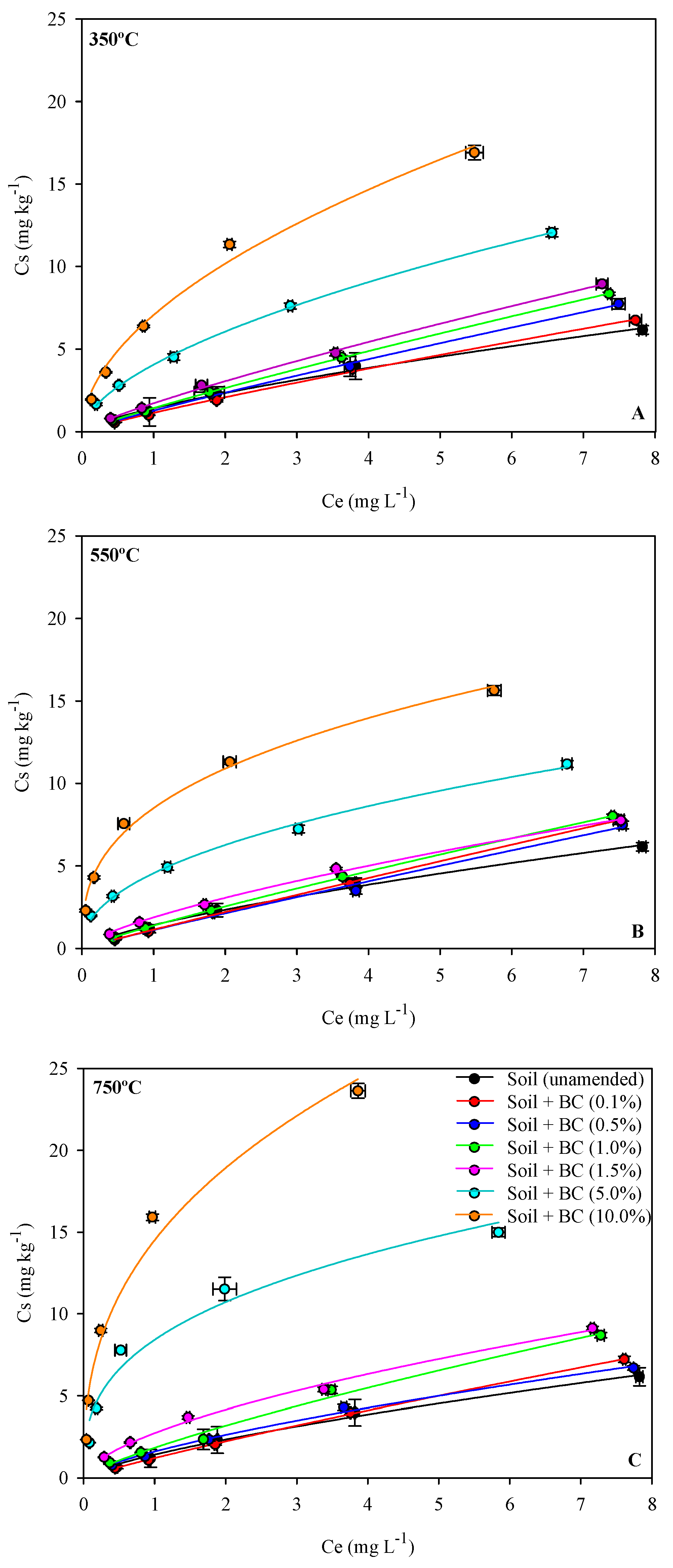
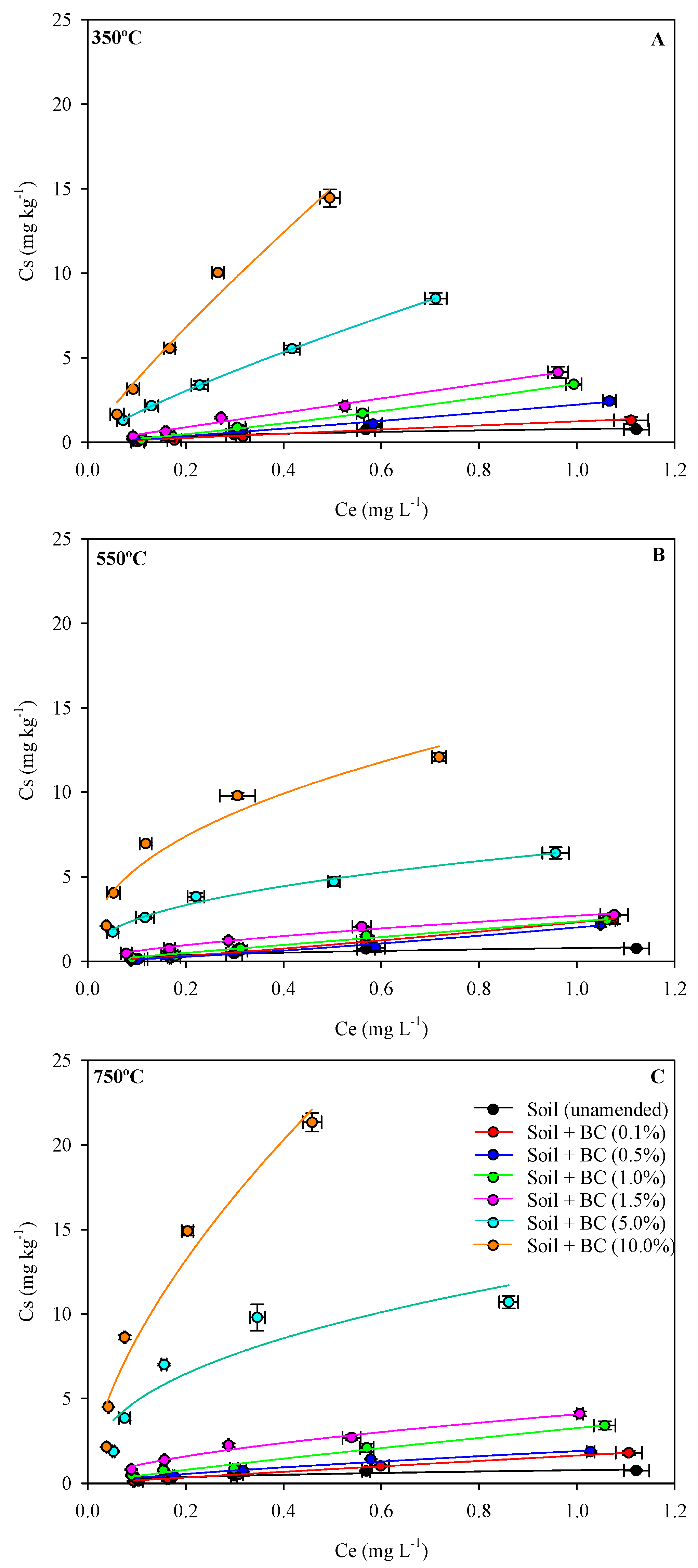
3.3. Sorption–Desorption Metribuzin in Biochar-Amended and Unamended Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction, In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 1–13. [Google Scholar]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar- amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Mosley, L.M.; Willson, P.; Hamilton, B.; Butler, G.; Seaman, R. The capacity of biochar made from common reeds to neutralise pH and remove dissolved metals in acid drainage. Environ. Sci. Pollut. Res. 2015, 22, 15113–15122. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 1–9. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F.; Besharati, H.; Khalaj, M.A. The effects of biochar on soil nutrients status, microbial activity and carbon sequestration potential in two calcareous soils. Biochar 2021, 3, 105–116. [Google Scholar] [CrossRef]
- Karimi, A.; Moezzi, A.; Chorom, M.; Enayatizamir, N. Application of biochar changed the status of nutrients and biological activity in a calcareous soil. J. Soil Sci. Plant Nutr. 2020, 20, 450–459. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A review of non-soil biochar applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanagef, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Yu, X.Y.; Mu, C.L.; Gu, C.; Liu, C.; Liu, X.J. Impact of woodchip biochar amendment on the sorption and dissipation of pesticide acetamiprid in agricultural soils. Chemosphere 2011, 85, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.; Cox, L.; Spokas, K.U.R.T.; Hermosín, M.C.; Cornejo, J.; Koskinen, W.C. Influence of biochar amendments on the sorption-desorption of aminocyclopyrachlor, bentazone and pyraclostrobin pesticides to an agricultural soil. Sci. Total Environ. 2014, 470, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, C.; Zhang, P.; Cao, M.; Xu, G.; Wu, H.; Zhang, J.; Li, C.; Rong, Q. Effects of biochar amendment on the sorption and degradation of atrazine in different soils. Soil Sediment Contam. 2018, 27, 643–657. [Google Scholar] [CrossRef]
- Szmigielski, A.M.; Hangs, R.D.; Schoenau, J.J. Bioavailability of metsulfuron and sulfentrazone herbicides in soil as affected by amendment with two contrasting willow biochars. Bull. Environ. Contam. Toxicol. 2018, 100, 298–302. [Google Scholar] [CrossRef]
- Mendes, K.F.; Júnior, A.F.D.; Takeshita, V.; Régo, A.P.J.; Tornisielo, V.L. Effect of biochar amendments on the sorption and desorption herbicides in agricultural soil. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: London, UK, 2019; pp. 1–25. [Google Scholar]
- Jensen, L.C.; Becerra, J.R.; Escudey, M. Impact of physical/chemical properties of volcanic ash-derived soils on mechanisms involved during sorption of ionisable and non-ionisable herbicides. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: London, UK, 2018; pp. 1–23. [Google Scholar]
- Mielke, K.C.; Mendes, K.F.; Guimarães, T. Biochars effects on sorption and desorption of herbicides in soil. In Interactions of Biochar and Herbicides in the Environment; Mendes, K.F., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 131–157. [Google Scholar]
- Trebst, A.; Wietoska, H. Mode of action and structure-acitivity-relationships of the aminotriazinone herbicide Metribuzin. Inhibition of photosynthetic electron transport in chloroplasts by Metribuzin. Z. fur Nat. Sect. C Biosci. 1975, 30, 499–504. [Google Scholar]
- Saritha, J.D.; Ramprakash, T.; Rao, P.C.; Madhavi, M. Persistence of metribuzin in tomato growing soils and tomato fruits. Nat. Environ. Pollut. Technol. 2017, 16, 505. [Google Scholar]
- Guimarães, A.C.D.; Mendes, K.F.; Campion, T.F.; Christoffoleti, P.J.; Tornisielo, V.L. Leaching of herbicides commonly applied to sugarcane in five agricultural soils. Planta Daninha. 2019, 37, e019181505. [Google Scholar] [CrossRef]
- PPDB—Pesticide Properties Database. Footprint: Creating Tools for Pesticide Risk Assessment and Management in Europe. Developed by the Agriculture & Environment Research Unit (AERU), University of Hertfordshire, Funded by UK National Sources and the EU-funded FOOTPRINT Project (FP6-SSP-022704). Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/469.htm (accessed on 10 March 2022).
- Dores, E.F.G.C.; Navickiene, S.; Cunha, M.L.; Carbo, L.; Ribeiro, M.L.; De-Lamonica-Freire, E.M. Multiresidue determination of herbicides in environmental waters from Primavera do Leste Region (Middle West of Brazil) by SPE-GC-NPD. J. Braz. Chem. Soc. 2006, 17, 866–873. [Google Scholar] [CrossRef]
- Kjær, J.; Olsen, P.; Henriksen, T.; Ullum, M. Leaching of metribuzin metabolites and the associated contamination of a sandy Danish aquifer. Environ. Sci. Technol. 2005, 39, 8374–8381. [Google Scholar] [CrossRef]
- López-Piñeiro, A.; Peña, D.; Albarrán, A.; Becerra, D.; Sánchez-Llerena, J. Sorption, leaching and persistence of metribuzin in Mediterranean soils amended with olive mill waste of different degrees of organic matter maturity. J. Environ. Manag. 2013, 122, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, E.; Parlavecchia, M.; Perri, G.; Gattullo, R. Comparative assessment of metribuzin sorption efficiency of biochar, hydrochar and vermicompost. J. Environ. Sci. Health Part B 2019, 54, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.F.; de Sousa, R.N.; Takeshita, V.; Alonso, F.G.; Régo, A.P.J.; Tornisielo, V.L. Cow bone char as a sorbent to increase sorption and decrease mobility of hexazinone, metribuzin, and quinclorac in soil. Geoderma 2019, 343, 40–49. [Google Scholar] [CrossRef]
- Sigua, G.C.; Stone, K.C.; Hunt, P.G.; Cantrell, K.B.; Novak, J.M. Increasing biomass of winter wheat using sorghum biochars. Agron. Sustain. Dev. 2015, 35, 739–748. [Google Scholar] [CrossRef]
- Jafri, N.; Wong, W.Y.; Doshi, V.; Yoon, L.W.; Cheah, K.H. A review on production and characterization of biochars for application in direct carbon fuel cells. Process Saf. Environ. Prot. 2018, 118, 152–166. [Google Scholar] [CrossRef]
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef]
- Soares, M.B.; dos Santos, F.H.; Alleoni, L.R.F. Iron-modified biochar from sugarcane straw to remove arsenic and lead from contaminated water. Water Air Soil Pollut. 2021, 232, 1–13. [Google Scholar] [CrossRef]
- Soares, M.B.; Milori, D.M.B.P.; Alleoni, L.R.F. How does the biochar of sugarcane straw pyrolysis temperature change arsenic and lead availabilities and the activity of the microorganisms in a contaminated sediment? J. Soils Sediments 2021, 21, 3185–3200. [Google Scholar] [CrossRef]
- USEPA. Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils and Oils; EPA: Washington, DC, USA, 2007. [Google Scholar]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis. 2012, 94, 138–145. [Google Scholar] [CrossRef]
- OECD—Organisation for Economic Co-Operation and Development. Adsorption—Desorption Using a Batch Equilibrium Method; OECD: Paris, France, 2000; 44p. [Google Scholar]
- Mendes, K.F.; Sousa, R.N.; Soares, M.B.; Viana, D.G.; Souza, A.J. Sorption and desorption studies of herbicides in the soil by batch equilibrium and stirred flow methods. In Radioisotopes in Weed Research; Mendes, K.F., Ed.; CRC Press: Boca Raton, FL, USA, 2021; Volume 1, pp. 17–61. [Google Scholar]
- Melo, L.C.; Coscione, A.R.; Abreu, C.A.; Puga, A.P.; Camargo, O.A. Influence of pyrolysis temperature on cadmium and zinc sorption capacity of sugar cane straw–derived biochar. BioResources 2013, 8, 4992–5004. [Google Scholar] [CrossRef]
- Riaz, M.; Khan, M.; Ali, S.; Khan, M.D.; Ahmad, R.; Khan, M.J.; Rizwan, M. Sugarcane waste straw biochar and its effects on calcareous soil and agronomic traits of okra. Arab. J. Geosci. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 1–18. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and quantification of biochar alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Novotny, E.H.; Maia, C.M.B.F.; Carvalho, M.T.M.; Madari, B.E. Biochar—Pyrogenic carbon for agricultural use—A critical review. Rev. Bras. Cienc. Solo. 2015, 39, 321–344. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Farid, I.M.; Siam, H.S.; Abbas, M.H.; Mohamed, I.; Mahmoud, S.A.; Tolba, M.; Abbas, H.H.; Yang, X.; Antoniadis, V.; Rinklebe, J.; et al. Co-composted biochar derived from rice straw and sugarcane bagasse improved soil properties, carbon balance, and zucchini growth in a sandy soil: A trial for enhancing the health of low fertile arid soils. Chemosphere 2022, 292, e133389. [Google Scholar] [CrossRef]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of pyrolysis temperature on chemical and physical properties of biochar from sewage sludge. Arch. Agron. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; Melo, I.C.N.D.; Melo, L.C.; Magriotis, Z.M.; Sanchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, S.M.; Wu, R.R.; Zhang, L.; Wang, P.; Xiao, R.B. Magnetic biochars have lower adsorption but higher separation effectiveness for Cd2+ from aqueous solution compared to nonmagnetic biochars. Environ. Pollut. 2021, 275, 116485. [Google Scholar] [CrossRef]
- Lopes, R.P.; Astruc, D. Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar] [CrossRef]
- Trivedi, M.; Branton, A.; Trivedi, D.; Nayak, G.; Singh, R.; Jana, S. Characterization of physical, spectral and thermal properties of biofield treated 1, 2, 4-Triazole. Curr. Org. Chem. 2015, 3, 1000128. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. MnO2-decorated biochar composites of coconut shell and rice husk: An efficient lithium ions adsorption-desorption performance in aqueous media. Chemosphere 2020, 260, 127500. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Bhattacharya, J. Influence of temperature and duration of pyrolysis on the property heterogeneity of rice straw biochar and optimization of pyrolysis conditions for its application in soils. J. Clean. Prod. 2019, 215, 1123–1139. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sust. Energ. Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, B.; Wang, L.; Feng, Z.; Fan, H.; Sun, T. Hierarchically structured two-dimensional magnetic microporous biochar derived from hazelnut shell toward effective removal of p-arsanilic acid. Appl. Surf. Sci. 2021, 540, 148372. [Google Scholar] [CrossRef]
- Silva, R.C.F.; Ardisson, J.D.; Cotta, A.A.C.; Araujo, M.H.; Carvalho, A.P.T. Use of iron mining tailings from dams for carbon nanotubes synthesis in fluidized bed for 17α-ethinylestradiol removal. Environ. Pollut. 2020, 260, 114099. [Google Scholar] [CrossRef]
- Huang, H.; Guo, T.; Wang, K.; Li, Y.; Zhang, G. Efficient activation of persulfate by a magnetic recyclable rape straw biochar catalyst for the degradation of tetracycline hydrochloride in water. Sci. Total Environ. 2021, 758, 143957. [Google Scholar] [CrossRef]
- Sousa, R.N.; Soares, M.B.; Santos, F.H.; Leite, C.N.; Mendes, K.F. Interaction mechanisms between biochar and herbicides. In Interactions of Biochar and Herbicides in the Environment; Mendes, K.F., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 80–105. [Google Scholar]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Naeem, M.A.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Yang, X.; Ryu, C.; Rizwan, M.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]
- Oliveira, F.S.; Takeshita, V.; Mendes, K.F.; Tornisielo, V.L.; Alonso, F.G.; Junqueira, L.V.; Neto, M.B.; Lins, H.A.; Silva, D.V. Addition of raw feedstocks and biochars to the soil on the sorption–desorption and biodegradation of 14C-saflufenacil. Int. J. Environ. Sci. Technol. 2022, 1, 1–18. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus sorption and availability from biochars and soil/Biochar mixtures. Clean—Soil Air Water 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Fernandes, J.D.; Chaves, L.H.G.; Dantas, E.R.B.; Tito, G.A.; Guerra, H.O.C. Phosphorus availability in soil incubated with biochar: Adsorption study. Rev. Caatinga. 2022, 35, 206–215. [Google Scholar] [CrossRef]
- Hong, C.; Lu, S. Does biochar affect the availability and chemical fractionation of phosphate in soils? Environ. Sci. Pollut. Res. 2018, 25, 8725–8734. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Tahir, M.; Amjad, M.; Murtaza, B.; Yan, A.; Akhtar, S.S. Effect of wheat and rice straw biochar produced at different temperatures on maize growth and nutrient dynamics of a calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 2048–2061. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Envrionmental Management: Science, Technology and Implementation; Lehmann, J., Stephen, J., Eds.; Routledge: New York, NY, USA, 2015; pp. 137–161. [Google Scholar]
- Kongthod, T.; Thanachit, S.; Anusontpornperm, S.; Wiriyakitnateekul, W. Effects of biochars and other organic soil amendments on plant nutrient availability in an ustoxic quartzipsamment. Pedosphere 2015, 25, 790–798. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood biochar influences calcareous soil physicochemical and microbiological status. J. Environ. Manag. 2014, 43, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrolysis. 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Da Silva Mendes, J.; Fernandes, J.D.; Chaves, L.H.G.; Guerra, H.O.C.; Tito, G.A.; de Brito Chaves, I. Chemical and physical changes of soil amended with biochar. Water Air Soil Pollut. 2021, 232, 1–13. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Rigi, M.R.; Farahbakhsh, M.; Rezaei, K. Adsorption and desorption behavior of herbicide metribuzin in different soils of Iran. J. Agric. Sci. Technol. 2015, 17, 777–787. [Google Scholar]
- Peña, D.; López-Piñeiro, A.; Albarrán, Á.; Rato-Nunes, J.M.; Sánchez-Llerena, J.; Becerra, D.; Ramírez, M. De-oiled two-phase olive mill waste may reduce water contamination by metribuzin. Sci. Total Environ. 2016, 541, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Saritha, J.D.; Prakash, T.R.; Madhavi, M.; Rao, P.C. Adsorption of metribuzin in tomato growing soils. Int. J. Chem. Stud. 2017, 5, 740–746. [Google Scholar]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of Biochar. In Biochar for Environmental Management, Science and Technology; Lehmann, J.L., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 13–32. [Google Scholar]
- White, P.M., Jr.; Potter, T.L.; Lima, I.M. Sugarcane and pinewood biochar effects on activity and aerobic soil dissipation of metribuzin and pendimethalin. Ind. Crops Prod. 2015, 74, 737–744. [Google Scholar] [CrossRef]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding activation effects on low-temperature biochar for optimization of herbicide sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Xiao, F.; Pignatello, J.J. π+–π Interactions between (Hetero) aromatic Amine cations and the graphitic surfaces of pyrogenic carbonaceous materials. Environ. Sci. Technol. 2015, 49, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Cara, I.G.; Filip, M.; Bulgariu, L.; Raus, L.; Topa, D.; Jitareanu, G. Environmental remediation of metribuzin herbicide by mesoporous carbon-rich from wheat straw. Appl. Sci. 2021, 11, 4935. [Google Scholar] [CrossRef]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y.; Li, Y.; Huang, L.; Mar, N.N.; Huang, Q.; Liu, Z. Biochar characteristics produced from rice husks and their sorption properties for the acetanilide herbicide metolachlor. Environ. Sci. Pollut. Res. 2017, 24, 4552–4561. [Google Scholar] [CrossRef]
- Liu, K.; He, Y.; Xu, S.; Hu, L.; Luo, K.; Liu, X.; Liu, M.; Zhou, X.; Bai, L. Mechanism of the effect of pH and biochar on the phytotoxicity of the weak acid herbicides imazethapyr and 2,4-D in soil to rice (Oryza sativa) and estimation by chemical methods. Ecotoxicol. Environ. Saf. 2018, 161, 602–609. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Almendros, G.; Peña, A. Raw or incubated olive-mill wastes and its biotransformed products as agricultural soil amendments e effect on sorption e desorption of triazine herbicides. J. Agric. Food Chem. 2007, 55, 836e843. [Google Scholar] [CrossRef]
- Majumdar, K.; Singh, N. Effect of soil amendments on sorption and mobility of metribuzin in soils. Chemosphere 2007, 66, 630e637. [Google Scholar] [CrossRef]
- Mendes, K.F.; Furtado, I.F.; Sousa, R.N.D.; Lima, A.D.C.; Mielke, K.C.; Brochado, M.G.D.S. Cow bonechar decreases indaziflam pre-emergence herbicidal activity in tropical soil. J. Environ. Sci. Health Part B 2021, 56, 532–539. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B. Black carbon (biochar) in water/soil environments: Molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef]
- Barriuso, E.; Laird, D.A.; Koskinen, W.C.; Dowdy, R.H. Atrazine desorption from smectites. Soil Sci. Soc. Am. J. 1994, 58, 1632–1638. [Google Scholar] [CrossRef]
- Singh, N.; Raunaq; Singh, S.B. Effect of fly ash on sorption behavior of metribuzin in agricultural soils. J. Environ. Sci. Health Part B 2012, 47, 89–98. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Sorption and desorption behaviors of diuron in soils amended with charcoal. J. Agric. Food Chem. 2006, 54, 8545–8550. [Google Scholar] [CrossRef]
- Deng, H.; Feng, D.; He, J.X.; Li, F.Z.; Yu, H.M.; Ge, C.J. Influence of biochar amendments to soil on the mobility of atrazine using sorption-desorption and soil thin-layer chromatography. Ecol. Eng. 2017, 99, 381–390. [Google Scholar] [CrossRef]
- Liu, Y. Is the free energy change of adsorption correctly calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Carter, M.C.; Kilduff, J.E.; Weber, W.J. Site energy distribution analysis of preloaded adsorbents. Environ. Sci. Technol. 1995, 29, 1773–1780. [Google Scholar] [CrossRef]
- Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
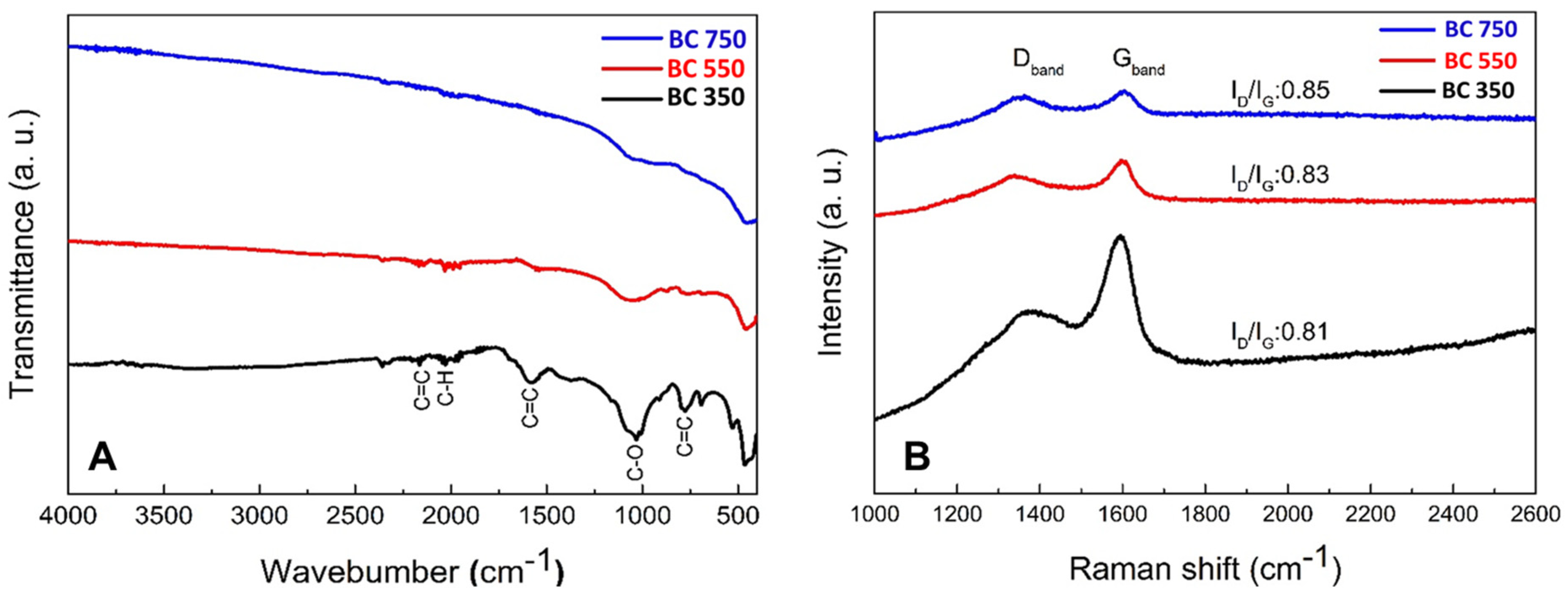
| T °C | pH | C | N | C/N | Ash | SSA |
|---|---|---|---|---|---|---|
| H2O | % | m2 g−1 | ||||
| 350 | 8.6 | 48.7 | 0.832 | 58.51 | 5.0 | 17 |
| 550 | 9.3 | 49.1 | 0.647 | 75.83 | 10.3 | 129 |
| 750 | 9.8 | 59.0 | 0.403 | 146.36 | 11.6 | 223 |
| Pyrolysis Temperature | Application Rate | Chemical Attributes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | OC | P | K | Ca | Mg | H + Al | Zn | Fe | Mn | Cu | B | CEC | BS | ||
| (°C) | (%) w w−1 | H2O | % | mg kg−1 | mmolc kg−1 | mg kg−1 | mmolc kg−1 | % | |||||||
| - | unamended | 5.5 | 1.2 | 1.3 | 77.0 | 15.9 | 5.4 | 33.0 | 3.0 | 129.8 | 91.0 | 3.9 | 0.1 | 23.3 | 41.0 |
| 350 | 0.1 | 5.5 | 1.2 | 1.5 | 97.0 | 16.0 | 5.7 | 33.3 | 2.9 | 129.6 | 99.1 | 3.8 | 0.1 | 24.2 | 40.0 |
| 0.5 | 5.5 | 1.2 | 2.0 | 111.0 | 17.9 | 6.5 | 33.0 | 3.1 | 123.6 | 127.0 | 3.6 | 0.1 | 27.8 | 46.0 | |
| 1 | 5.8 | 1.2 | 3.3 | 125.0 | 17.5 | 6.8 | 26.4 | 2.8 | 148.1 | 130.0 | 3.7 | 0.1 | 29.3 | 52.0 | |
| 1.5 | 5.9 | 1.2 | 6.3 | 139.0 | 17.1 | 7.2 | 23.1 | 2.9 | 154.7 | 144.0 | 4.1 | 0.1 | 29.4 | 56.0 | |
| 5 | 6.8 | 1.2 | 10.0 | 240.0 | 17.7 | 8.3 | 13.3 | 2.9 | 234.4 | 155.0 | 3.8 | 0.1 | 36.7 | 73.0 | |
| 10 | 7.2 | 1.2 | 30.0 | 290.0 | 17.4 | 9.6 | 6.6 | 2.8 | 245.5 | 212.0 | 3.6 | 0.1 | 37.1 | 85.0 | |
| 550 | 0.1 | 5.4 | 1.2 | 2.2 | 99.0 | 16.5 | 5.7 | 29.4 | 2.8 | 128.5 | 94.5 | 3.6 | 0.1 | 24.7 | 48.0 |
| 0.5 | 5.6 | 1.2 | 2.7 | 132.0 | 16.2 | 5.8 | 29.7 | 3.1 | 157.4 | 97.9 | 4.1 | 0.1 | 24.8 | 45.0 | |
| 1 | 5.8 | 1.2 | 4.4 | 158.0 | 17.3 | 6.1 | 29.7 | 3.0 | 228.5 | 91.2 | 4.0 | 0.1 | 26.6 | 47.0 | |
| 1.5 | 5.9 | 1.2 | 8.7 | 161.0 | 17.8 | 5.8 | 19.8 | 2.8 | 266.5 | 157.0 | 3.4 | 0.1 | 25.2 | 56.0 | |
| 5 | 7.0 | 1.2 | 15.0 | 250.0 | 17.7 | 7.6 | 9.9 | 2.7 | 273.5 | 183.0 | 3.1 | 0.1 | 33.2 | 77.0 | |
| 10 | 7.3 | 1.3 | 33.0 | 340.0 | 18.1 | 8.4 | 3.3 | 2.9 | 297.5 | 202.0 | 3.6 | 0.1 | 38.5 | 90.0 | |
| 750 | 0.1 | 5.4 | 1.2 | 2.9 | 108.0 | 16.8 | 5.6 | 33.0 | 2.7 | 135.0 | 96.6 | 3.6 | 0.1 | 25.2 | 43.0 |
| 0.5 | 5.5 | 1.2 | 3.7 | 144.0 | 17.4 | 6.8 | 29.7 | 3.0 | 148.8 | 135.0 | 3.9 | 0.1 | 27.6 | 48.0 | |
| 1 | 5.8 | 1.2 | 7.8 | 178.0 | 17.8 | 7.0 | 29.7 | 2.8 | 147.6 | 122.0 | 4.0 | 0.1 | 29.4 | 49.0 | |
| 1.5 | 6.2 | 1.2 | 12.0 | 240.0 | 18.1 | 7.1 | 13.2 | 2.5 | 238.5 | 123.0 | 3.8 | 0.1 | 30.6 | 70.0 | |
| 5 | 7.2 | 1.3 | 55.0 | 500.0 | 19.7 | 9.8 | 3.3 | 2.9 | 267.5 | 177.0 | 3.7 | 0.1 | 39.3 | 92.0 | |
| 10 | 7.6 | 1.4 | 65.0 | 550.0 | 20.0 | 11.1 | 0.0 | 2.9 | 294.5 | 178.0 | 3.8 | 0.1 | 40.6 | 100.0 | |
| Physical Attributes (g kg−1) | |||||||||||||||
| Sand | Silt | Clay | Texture class | ||||||||||||
| Soil | unamended | 500 | 120 | 380 | sandy clay | ||||||||||
| Pyrolysis Temperature | Application Rate | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kf | Kfoc | Kd-app | Koc | ∆G | |||||
| (°C) | (%) w w−1 | (mg(1−1/n) L1/n kg−1) | 1/n | R2 | L kg−1 | Sorbed (%) | kJ mol−1 | ||
| - | unamended | 1.42 ± 0.26 a | 114.5 | 0.721 ± 0.04 | 0.99 | 1.66 ± 0.71 | 133 | 23.17 ± 2.80 | −868.77 |
| 350 | 0.1 | 1.13 ± 0.05 | 96 | 0.875 ± 0.05 | 0.99 | 0.96 ± 0.07 | 82 | 18.9 ± 0.28 | −302.80 |
| 0.5 | 1.27 ± 0.14 | 116 | 0.894 ± 0.03 | 0.99 | 1.39 ± 0.14 | 127 | 22.8 ± 0.76 | −592.18 | |
| 1 | 1.33 ± 0.05 | 122 | 0.885 ± 0.03 | 0.99 | 1.37 ± 0.04 | 117 | 23.8 ± 0.48 | −706.55 | |
| 1.5 | 1.71 ± 0.10 | 137 | 0.830 ± 0.01 | 0.99 | 1.60 ± 0.10 | 129 | 28.0 ± 0.61 | −1329.20 | |
| 5 | 4.05 ± 0.15 | 311 | 0.579 ± 0.02 | 0.99 | 3.04 ± 0.34 | 233 | 45.2 ± 1.56 | −3465.42 | |
| 10 | 7.06 ± 0.16 | 504 | 0.527 ± 0.01 | 0.99 | 6.99 ± 0.11 | 499 | 63.8 ± 0.98 | −4842.27 | |
| 550 | 0.1 | 1.21 ± 0.08 | 105 | 0.807 ± 0.02 | 0.99 | 1.22 ± 0.15 | 104 | 20.4 ± 0.62 | −472.27 |
| 0.5 | 1.38 ± 0.10 | 117 | 0.877 ± 0.03 | 0.99 | 1.23 ± 0.05 | 105 | 21.9 ± 0.72 | −797.98 | |
| 1 | 1.13 ± 0.08 | 91 | 0.854 ± 0.01 | 0.99 | 1.33 ± 0.23 | 107 | 22.0 ± 0.82 | −302.80 | |
| 1.5 | 1.87 ± 0.04 | 159 | 0.708 ± 0.02 | 0.99 | 1.58 ± 0.16 | 134 | 26.4 ± 0.39 | −1550.80 | |
| 5 | 4.55 ± 0.15 | 417 | 0.460 ± 0.02 | 0.99 | 4.77 ± 0.32 | 437 | 49.2 ± 1.38 | −3753.83 | |
| 10 | 8.50 ± 0.10 | 726 | 0.358 ± 0.02 | 0.99 | 14.0 ± 0.14 | 1196 | 75.5 ± 0.74 | −5302.16 | |
| 750 | 0.1 | 1.54 ± 0.07 | 107 | 0.881 ± 0.01 | 0.99 | 1.03 ± 0.09 | 83 | 23.6 ± 0.56 | −532.95 |
| 0.5 | 1.60 ± 0.09 | 136 | 0.704 ± 0.03 | 0.99 | 1.35 ± 0.18 | 115 | 23.4 ± 0.75 | −1164.46 | |
| 1 | 1.84 ± 0.21 | 157 | 0.788 ± 0.06 | 0.99 | 1.09 ± 1.0 | 93 | 23.5 ± 1.90 | −1510.73 | |
| 1.5 | 2.73 ± 0.08 | 220 | 0.606 ± 0.03 | 0.99 | 2.58 ± 0.17 | 208 | 39.9 ± 0.79 | −2488.22 | |
| 5 | 8.41 ± 0.22 | 718 | 0.349 ± 0.04 | 0.98 | 14.2 ± 0.18 | 1210 | 77.8 ± 1.31 | −5275.79 | |
| 10 | 14.51 ± 0.15 | 1239 | 0.383 ± 0.06 | 0.98 | 45.0 ± 0.21 | 3846 | 89.4 ± 0.70 | −6627.10 | |
| Biochar | Freundlich | |||||||||
| Pyrolysis Temperature | Application Rate | Kf | Kfoc | Kd-app | Koc | ∆G | ||||
| (°C) | (%) w w−1 | (mg(1−1/n) L1/n kg−1) | 1/n | H | R2 | L kg−1 | Desorbed (%) | kJ mol−1 | ||
| - | unamended | 0.78 ± 0.09 a | 62 | 0.468 ± 0.13 | 0.65 | 0.91 | 1.49 ± 0.09 | 117 | 15.8 ± 0.54 | −615.58 |
| 350 | 0.1 | 1.23 ± 0.08 | 105 | 0.974 ± 0.30 | 1.11 | 0.96 | 1.07 ± 0.04 | 91 | 15.5 ± 0.25 | −512.89 |
| 0.5 | 2.22 ± 0.18 | 203 | 1.006 ± 0.10 | 1.24 | 0.99 | 2.27 ± 0.10 | 208 | 15.7 ± 0.45 | −1975.88 | |
| 1 | 3.45 ± 0.06 | 294 | 1.029 ± 0.04 | 1.39 | 0.99 | 2.86 ± 0.10 | 244 | 15.1 ± 0.72 | −3068.16 | |
| 1.5 | 3.27 ± 0.12 | 344 | 0.976 ± 0.08 | 1.18 | 0.99 | 5.33 ± 0.06 | 429 | 13.5 ± 0.15 | −2935.40 | |
| 5 | 6.51 ± 0.18 | 597 | 0.418 ± 0.03 | 0.91 | 0.99 | 14.76 ± 0.07 | 1135 | 11.0 ± 0.32 | −5985.60 | |
| 10 | 14.64 ± 0.11 | 1251 | 0.425 ± 0.07 | 1.19 | 0.96 | 33.10 ± 0.07 | 2364 | 5.8 ± 0.19 | −8218.33 | |
| 550 | 0.1 | 2.30 ± 0.09 | 196 | 1.023 ± 0.06 | 1.52 | 0.99 | 2.03 ± 0.07 | 173 | 15.2 ± 0.13 | −2062.59 |
| 0.5 | 2.00 ± 0.08 | 170 | 1.059 ± 0.20 | 1.44 | 0.97 | 2.21 ± 0.03 | 188 | 15.1 ± 0.30 | −1717.32 | |
| 1 | 2.35 ± 0.09 | 189 | 0.970 ± 0.08 | 1.02 | 0.99 | 2.40 ± 0.14 | 193 | 15.5 ± 0.23 | −2116.87 | |
| 1.5 | 2.69 ± 0.05 | 229 | 0.645 ± 0.05 | 0.91 | 0.99 | 4.23 ± 0.10 | 361 | 14.3 ± 0.18 | −2451.65 | |
| 5 | 11.28 ± 0.17 | 961 | 0.818 ± 0.03 | 0.91 | 0.99 | 17.26 ± 0.22 | 1583 | 11.4 ± 0.44 | −4641.33 | |
| 10 | 27.58 ± 0.18 | 1970 | 0.815 ± 0.07 | 1.19 | 0.96 | 58.86 ± 0.09 | 5030 | 8.3 ± 0.32 | −6649.20 | |
| 750 | 0.1 | 1.64 ± 0.06 | 132 | 0.960 ± 0.02 | 1.09 | 0.99 | 1.72 ± 0.09 | 138 | 15.1 ± 0.37 | −1225.61 |
| 0.5 | 1.90 ± 0.18 | 162 | 0.766 ± 0.09 | 1.09 | 0.98 | 2.40 ± 0.07 | 205 | 15.7 ± 0.26 | −1590.23 | |
| 1 | 3.26 ± 0.12 | 281 | 0.877 ± 0.09 | 1.11 | 0.99 | 7.02 ± 0.03 | 600 | 14.9 ± 0.33 | −2927.80 | |
| 1.5 | 4.08 ± 0.09 | 329 | 0.583 ± 0.05 | 0.96 | 0.99 | 7.85 ± 0.11 | 566 | 14.3 ± 0.18 | −3483.70 | |
| 5 | 12.44 ± 0.27 | 1063 | 0.468 ± 0.10 | 1.34 | 0.91 | 44.9 ± 0.06 | 3837 | 11.8 ± 0.31 | −6245.75 | |
| 10 | 35.91 ± 0.19 | 3069 | 0.623 ± 0.09 | 1.62 | 0.97 | 114.5 ± 0.13 | 9786 | 3.7 ± 0.17 | −8872.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielke, K.C.; Laube, A.F.S.; Guimarães, T.; Brochado, M.G.d.S.; Medeiros, B.A.d.P.; Mendes, K.F. Pyrolysis Temperature and Application Rate of Sugarcane Straw Biochar Influence Sorption and Desorption of Metribuzin and Soil Chemical Properties. Processes 2022, 10, 1924. https://doi.org/10.3390/pr10101924
Mielke KC, Laube AFS, Guimarães T, Brochado MGdS, Medeiros BAdP, Mendes KF. Pyrolysis Temperature and Application Rate of Sugarcane Straw Biochar Influence Sorption and Desorption of Metribuzin and Soil Chemical Properties. Processes. 2022; 10(10):1924. https://doi.org/10.3390/pr10101924
Chicago/Turabian StyleMielke, Kamila C., Ana Flávia S. Laube, Tiago Guimarães, Maura Gabriela da S. Brochado, Bruna Aparecida de P. Medeiros, and Kassio F. Mendes. 2022. "Pyrolysis Temperature and Application Rate of Sugarcane Straw Biochar Influence Sorption and Desorption of Metribuzin and Soil Chemical Properties" Processes 10, no. 10: 1924. https://doi.org/10.3390/pr10101924
APA StyleMielke, K. C., Laube, A. F. S., Guimarães, T., Brochado, M. G. d. S., Medeiros, B. A. d. P., & Mendes, K. F. (2022). Pyrolysis Temperature and Application Rate of Sugarcane Straw Biochar Influence Sorption and Desorption of Metribuzin and Soil Chemical Properties. Processes, 10(10), 1924. https://doi.org/10.3390/pr10101924