Abstract
In this study, the mixture of NaOH and deep eutectic solvent (DES) ChCl:UA-TA was firstly used to pretreat waste tomato stalk (TS). The effects of pretreatment time, pretreatment temperature, NaOH dosage, and DES dose were investigated, and the synergistic effects of dilute NaOH and DES combination pretreatment were tested on the influence of enzymatic saccharification. It was found that the relationship between delignification and saccharification rate had a significant linear correction. When TS was pretreated with NaOH (7 wt%)–ChCl:UA-TA (8 wt%) in a solid-to-liquid ratio of 1:10 (wt:wt) at 75 °C for 60 min, the delignification reached 82.1%. The highest yield of reducing sugars from NaOH–ChCl:UA-TA-treated TS could reach 62.5% in an acetate buffer (50 mM, pH 4.8) system containing cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS) at 50 °C. In summary, effective enzymatic saccharification of TS was developed by a combination pretreatment with dilute NaOH and ChCl:UA-TA, which has potential application in the future.
1. Introduction
In recent years, the valorization of renewable and sustainable biomass into useful biobased chemicals and biofuels has attracted rapidly increasing attention due to the gradual depletion of petrochemical resources [,,]. Lignocellulosic biomass (LCB), which is composed of carbohydrates (hemicellulose and cellulose) and lignin [], is regarded as one of the most promising alternative energy resources owing to its abundance, low-cost, non-toxicity, and renewability []. However, these three main polymers (e.g., hemicellulose, cellulose, and lignin) in the cell wall are tightly complexed by hydrogen and covalent bonds []. To enhance the enzymatic saccharification of lignocellulosic biomass, it is necessary to utilize an efficient pretreatment strategy for the disruption of recalcitrant structure in lignocellulosic biomass [,].
Pretreatment is known as one of the most important units in the valorization of lignocellulosic materials [], which currently presents the most critical challenge and determines the capital cost of the biomass-to-product process []. Presently, various pretreatments (e.g., physical, physicochemical, biological methods or their combinations) have been developed and utilized to disrupt a series of lignocellulosic biomasses for subsequent conversion [,,,,,,]. Alkaline treatment can be used to modify the fibers based on the removal of lignin, pectin, wax, and other components, thereby enhancing the enzymatic hydrolysis of lignocellulosic biomass []. NaOH solution has been utilized for swelling and dissolving the cellulose components, which can destroy the neighboring crystalline regions []. Aqueous urea/NaOH and thiourea/NaOH solutions may dissolve cotton linter [,,]. Urea/NaOH can be utilized to soak biomass at low temperatures, achieving a high saccharification yield (80.2%) []. Probably, NaOH, urea (UA), and thiourea (TA) might capture free water molecules to confine the intermolecular interactions between hydrogen bonds and cellulose molecules, resulting in the enhancement of enzymatic saccharification of lignocellulose [,]. In addition, deep eutectic solvents (DESs) have attracted much attention as benign solvents to pretreat lignocellulosic biomass due to their biodegradability, low corrosion, and high stability [,,]. Considering the merit of NaOH and DES pretreatment, the combination by mixing NaOH and DES ChCl:UA-TA in a one-pot manner can be attempted to pretreat biomass.
According to the statistics, the annual production of tomato (Solanum Lycopersicum L.) is over 60 million tons (http://www.fao.org/), which accounts for ~30% of the production of total greenhouse vegetables. As a byproduct, waste tomato stalk (TS) is nearly 8.5 million tons of dry weight per year []. However, there is limited information about the efficient enzymatic saccharification of TS. In this work, dilute NaOH and DES ChCl:UA-TA were mixed as a pretreatment solvent to pretreat TS. The pretreatment parameters, including pretreatment time, pretreatment temperature, NaOH dosage, and DES dose, were investigated to influence the enzymatic saccharification of TS, and the synergistic effects of dilute NaOH and DES in a one-pot manner were evaluated. The relationship between delignification and saccharification rate was explored. To effectively utilize TS, significant enzymatic saccharification of TS was developed by a combination pretreatment with dilute NaOH and ChCl:UA-TA in a one-pot manner.
2. Materials and Methods
2.1. Materials
Tomato stalk (TS) was collected from a suburb in Changzhou, China. The dried TS powder (40 mesh) was stored at room temperature for future use. Cellulase (C805042, 10,000 U/g) and xylanase (X832362, 100,000 U/g) were obtained from Macklin (Shanghai, China). Choline chloride, urea, thiourea, tetracycline hydrochloride, sodium acetate, acetic acid and other chemicals were bought from Sinopharm Group Chemical Reagent Co., (Shanghai, China).
2.2. Synthesis of DES ChCl:UA-TA
Choline chloride (ChCl), urea (UA), and thiourea (TA) were mixed in the molar ratio of 1:1:1. This mixture was stirred continuously (200 rpm) in a stoppered three-neck flask Nanjing Kangluoda Experiment Technology Co.; Nanjing, China) (at 80 °C until a clear solvent was formed. Then, the generated DES ChCl:UA-TA was stored under low-temperature drying conditions.
2.3. Pretreatment of TS with Dilute NaOH and ChCl: UA-TA
Dry TS (solid-liquid ratio of 1:10, wt:wt) was added to aqueous solutions containing NaOH (0.5−9 wt%) or/and ChCl:UA-TA (0−16 wt%) in a 250 mL round bottom flask at −25−125 °C by using a magnetic stirring device. After being pretreated for 10−120 min, an equal volume of deionized water (DI water, as an antisolvent) was added to the pretreatment system under vigorous agitation. After 5 min, the regenerated TS solid was washed repeatedly with DI water to remove NaOH, ChCl:UA-TA, or other residuals. Subsequently, the solid TS was oven-dried at 60 °C and further used for enzymatic saccharification.
2.4. Enzymatic Hydrolysis of TS
Enzymatic hydrolysis of untreated or pretreated TS (50 g/L) was conducted in a 25 mL of acetate buffer (50 mM, pH 4.8) system containing cellulase (10.0 FPU/g TS), xylanase (30.0 CBU/g TS) and 40 μL of tetracycline hydrochloride (10.0 g/L).
2.5. Analytical Methods
The compositions of pretreated and unpretreated samples were determined according to National Renewable Energy Laboratory (NREL) standard analytical procedures []. Reduced sugars were determined by the DNS method []. Glucose and xylose were assayed with HPLC (Waters 600, Milford, MA, USA) []. The yield of reducing sugars was calculated as previously reported []. The total delignification and solid recovery of TS were calculated as previously reported [,].
3. Results and Discussion
3.1. Optimization of NaOH–ChCl:UA-TA Pretreatment
ChCl:UA-TA was confirmed by 1H NMR (Figure S1) and 13C NMR (Figure S2). 13C NMR (101 MHz, DMSO): δ 184.32 (TA), 160.23 (UA), 67.4 (ChCl, -CH2-N), 55.54 (ChCl, -CH2-O), 53.62 (ChCl, CH3-N). 1H NMR (400 MHz, DMSO): δ 7.22 (4H, TA), 5.52 (4H, UA), 3.83 (2H, ChCl, -CH2-N), 3.44 (2H, ChCl, -CH2-O), 3.41 (1H, ChCl, -OH), 3.15 (9H, ChCl, -CH3). ChCl:UA-TA was also assayed with FT-IR (Figure S3); the peaks near 3287 and 3170 cm−1 are ascribed to the stretching mode of -OH and -NH2. The peaks around 1661, 1604, and 1392 cm−1 are associated with TA and UA. The peak near 1079 cm−1 is related to the stretching mode of C-O. The peak around 951 cm−1 is ascribed to the C-N group of ChCl.
To efficiently saccharify biomass, it is of great interest to optimize the pretreatment conditions [,]. In this work, the effects of pretreatment temperature, pretreatment time, NaOH dosage, DES (ChCl:UA-TA) dose, and TS concentration were examined during the pretreatment.
Efficient removal of lignin in lignocellulosic biomass with NaOH can facilitate enzymatic saccharification []. Since NaOH might be used to remove lignin that can adsorb or inhibit cellulase [], thereby decreasing the concentration of enzyme required. Figure 1 depicts that different NaOH dosages could significantly affect enzymatic saccharification. In the presence of 7 wt%, the highest reducing sugar yield of 62.5% was obtained. The highest yield of reducing sugars reached 62.5% in a solid-to-liquid ratio of 1:10 (wt:wt). The concentration of DES might have a profound influence on the enzymatic saccharification of TS [,]. Various ChCl:UA-TA dosages (0−16 wt%) were examined on the enzymatic saccharification of TS. As illustrated in Figure 2, the yield of reducing sugars increased from 51.4% to 62.5% when the DES dosage was raised from 0 wt% to 8 wt%. Further increasing the DES dosage from 8 to 16 wt%, the yield of reducing sugar dropped from 62.5% to 54.5%. High concentration of DES ChCl:UA-TA (over 12 wt%) might promote hemicellulose removal, which led to a reduced reducing sugar yield. Hence, the optimum dose of DES was 8 wt%.
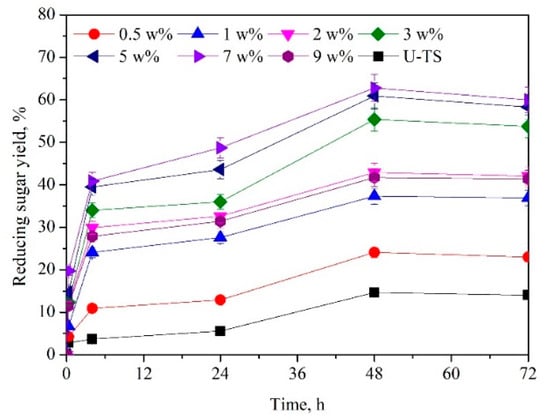
Figure 1.
Effects of NaOH concentration on the enzymatic saccharification of tomato stalk (TS). [Pretreatment condition: NaOH (0.5−9 wt%)–ChCl:UA-TA (8 wt%), 75 °C, 60 min; Enzymatic hydrolysis condition: cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS), 50 oC, pH 4.8]. [U-TS represents untreated TS].
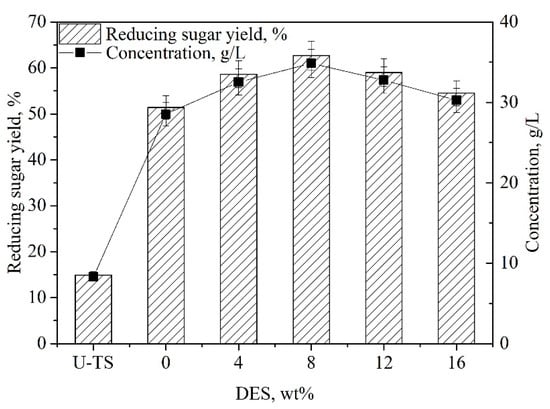
Figure 2.
Effects of deep eutectic solvent (DES) concentration on the enzymatic saccharification of TS. [Pretreatment condition: NaOH (7 wt%)–ChCl:UA-TA (1−16 wt%), 75 °C, 60 min; Enzymatic hydrolysis condition: cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS), 50 °C, pH 4.8]. [U-TS represents untreated TS].
Pretreatment temperature is a key parameter for enhancing the enzymatic saccharification of biomass [,]. To promote enzymatic hydrolysis of TS, the effects of pretreatment temperature (−25 °C, 0 °C, 25 °C, 50 °C, 75 °C, 100 °C, and 125 °C) were investigated after 1 h of pretreatment. As revealed in Figure 3, the yield of reducing sugars was raised from 31.0% to 62.5% by increasing the pretreatment temperature from −25 °C to 75 °C. Upon increasing the temperature from 75 °C to 125 °C, the saccharification yield decreased significantly. Obviously, the appropriate pretreatment temperature was 75 °C. High pretreatment temperature (over 75 °C) could disrupt and remove cellulose and hemicellulose, which would reduce the reducing sugar yield. Several efficient alkaline pretreatments had been carried out at high-performance temperature (100 °C) and/or concentrated alkali [], which required a higher performance cost and complicated control system. Our work provided an effective alkaline pretreatment, which could be conducted at 75 °C and give high enzymatic saccharification (62.5%).
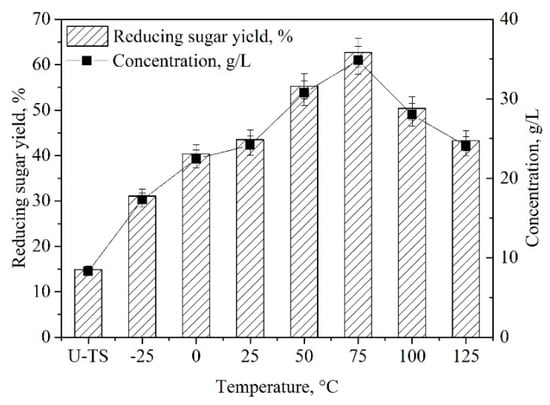
Figure 3.
Effects of TS pretreatment temperature on the enzymatic saccharification of TS. [Pretreatment condition: NaOH (7 wt%)–ChCl:UA-TA (8 wt%), −25−75 °C, 60 min; Enzymatic hydrolysis condition: cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS), 50 oC, pH 4.8]. [U-TS represents untreated TS].
Recently, various alkaline pretreatments have been utilized to improve the enzymatic hydrolysis of lignocellulosic biomass [,]. Long pretreatment time was required at low-performance temperature []. In addition to the performance temperature [], the pretreatment time is also a very important factor influencing biomass pretreatment and enzymatic saccharification []. As revealed in Figure 4, by increasing the pretreatment time from 10 to 60 min, the yield of reducing sugar was raised from 45.9% to 62.5%. As the pretreatment time rose from 1 to 2 h, the yield of reducing sugar only increased by ~2%. A long pretreatment duration was not economical for biomass pretreatment. Hence, the optimal pretreatment time was 60 min.
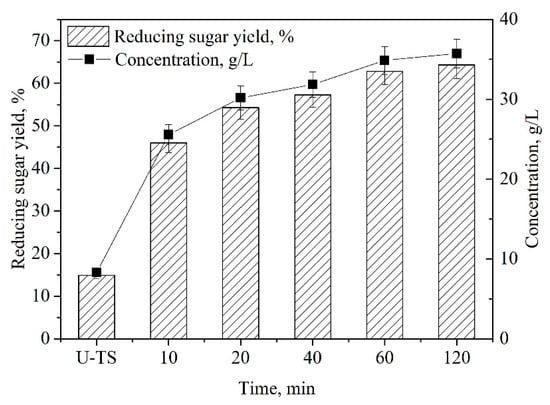
Figure 4.
Effects of TS pretreatment duration on the enzymatic saccharification of TS. [Pretreatment condition: NaOH (7 wt%)–ChCl:UA-TA (8 wt%), 75 °C, 10−120 min; Enzymatic hydrolysis condition: cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS), 50 °C, pH 4.8]. [U-TS represents untreated TS].
Overall, combination pretreatment of TS was conducted in dilute NaOH (7 wt%)–ChCl:UA-TA (8 wt%) at 75 °C for 60 min in the solid-to-liquid of 1:10 (wt:wt). This established pretreatment was easy to operate under the relatively mild performance condition. The obtained DNC-TS could be enzymatically saccharified into reducing sugars in a yield of 62.5% after 48 h of enzymatic hydrolysis.
3.2. Comparison of Different Pretreatments on the Enzymatic Saccharification of TS
The recalcitrance of biomass can hinder the enzymatic hydrolysis of lignocellulose []. Prior to enzymatic saccharification, it is necessary to establish an appropriate pretreatment system for disrupting the tight structure of biomass [,]. In this work, dilute NaOH (7 wt%), ChCl:UA-TA (8 wt%), and their combination [dilute NaOH (7 wt%)–ChCl:UA-TA (8 wt%)] were separately used as pretreatment media to treat TS. U-TS (untreated TS), DN-TS (dilute NaOH-pretreated TS), C-TS (ChCl:UA-TA-pretreated TS), and DNC-TS (dilute NaOH–ChCl:UA-TA pretreated TS) were individually utilized as substrates for enzymatic hydrolysis. As presented in Figure 5a, pretreatment with dilute NaOH (7 wt%), ChCl:UA-TA, and their combination (dilute NaOH–ChCl:UA-TA) could give the delignification of 71.3%, 41.2%, and 82.1%, respectively. DNC-TS had the lowest solid recovery (30.2%) than those of DN-TS (32.9%) and C-TS (57.0%). It was observed that the relationship about delignification and saccharification rate (48 h) had a significant linear correction [Y(saccharification rate) = 0.692 × X(Delignification) + 4.13, R2 = 0.98] after NaOH–ChCl:UA-TA pretreatment. With the increase in delignification, the saccharification rate had an increasing trend. The yield of reducing sugars from DNC-TS reached 62.5% after 48 h, which was highest than those of DN-TS (51.4%), C-TS (33.2%), and U-TS (14.9%). U-TS gave the lowest saccharification, which might be attributed to its highly tight structure. In addition, the lignin and hemicellulose in U-TS may have an inhibitory effect on enzymatic hydrolysis. The linear relationships of solid sorghum straw recovery-xylan removal (R2 = 0.94) and solid sorghum straw recovery-reducing sugar yield (R2 = 0.81) were observed in the NaOH–ChCl:LAC pretreatment []. Tandem pretreatment with NaOH (0.75 wt%) at 121 °C for 1 h and ChCl:LAC at 140 °C for 40 min could remove lignin (78.4%) and xylan (67.6%). Time courses for the enzymatic hydrolysis of U-TS, C-TS, DN-TS, and DNC-TS (50 g/L) were monitored at 50 °C and pH 4.8 (Figure 5b). These pretreated TS samples had higher saccharification rates than U-TS. Saccharification for 30 min, the reducing sugar yield of U-TS, C-TS, DN-TS, and DNC-TS could reach 2.8%, 7.7%, 13.6%, and 19.5%, respectively. By increasing the saccharification time from 30 min to 48 h, the reducing sugar yield increased gradually. DNC-TS had the highest yield of reducing sugar (62.5%) after 48 h of enzymatic saccharification, which was 4.2 times higher than that of U-TS. Therefore, the combination pretreatment (NaOH–ChCl:UA-TA) had the highest pretreatment efficiency to pretreat TS.
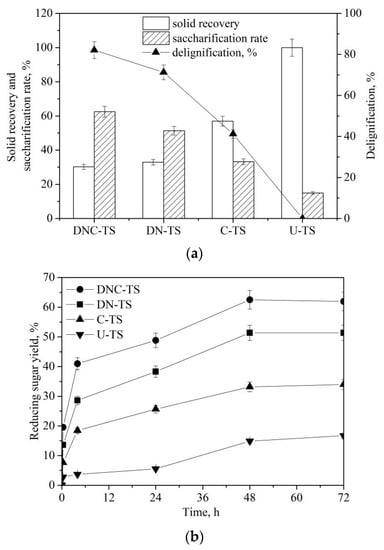
Figure 5.
Effects of dilute NaOH (7 wt%), ChCl:UA-TA and their combination (dilute NaOH–ChCl:UA-TA) on the pretreatment and enzymatic saccharification of TS [Pretreatment condition: NaOH (7 wt%)–ChCl:UA-TA (1–16 wt%), 75 °C, 60 min; Enzymatic hydrolysis condition: cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS), 50 °C, pH 4.8] (a); Time courses for the enzymatic hydrolysis of U-TS (untreated TS), DN-TS (dilute NaOH-pretreated TS), C-TS (ChCl:UA-TA-pretreated TS), and DNC-TS (dilute NaOH–ChCl:UA-TA pretreated TS) (50 g/L) with cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS) at 50 °C and pH 4.8 (b).
To visualize the change in cellulose structure, the SEM micrographs were used to characterize U-TS and DNC-TS (Figure 6). It showed that there were significant surface changes in the morphology of TS before and after pretreatment. U-TS was relatively smooth and continuous and had few pores for the hydrolysis with cellulases. However, the NaOH–ChCl:UA-TA-treated TS (DNC-TS) had looser and rougher surfaces and presented more porosity than U-TS. After U-TS was pretreated with NaOH–ChCl:UA-TA, the rigidity of the cellulose in TS decreased due to the lignin removal and disruption of surface structure.
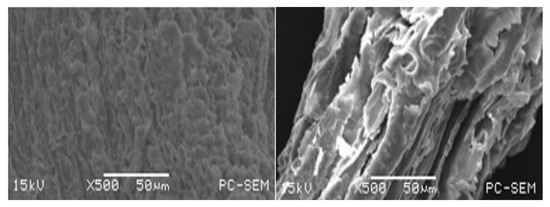
Figure 6.
SEM micrographs of U-TS (left) and DNC-TS (right). [U-TS represents untreated TS; DNC-TS represents dilute NaOH–ChCl:UA-TA pretreated TS].
3.3. Reuse of Pretreatment Medium NaOH–ChCl:UA-TA
Industrially, efficient recovery and reuse of pretreatment medium have gained great interest to reduce the pretreatment costs and avoid environmental pollution [,]. After each use, the solids were separated from the pretreatment slurry by filtration, and the pretreatment liquor was reused for the next batch of pretreatment. After each batch, the solid recovery, delignification and enzymatic saccharification were determined. As presented in Figure 7, the saccharification rate and delignification decreased gradually with the increase in the recycling number. The reducing sugar yield dropped from 62.5% to 40.5% from the first to sixth batch, maintaining a high yield of reducing sugars (>40%). The good reusability of pretreatment medium NaOH–ChCl:UA-TA would potentially reduce the pretreatment cost. Although NaOH–ChCl:UA-TA could be used for efficient pretreatment of TS, potential pretreatment mechanisms involving the action of ChCl:UA-TA with alkali and the stability of ChCl:UA-TA in an alkaline medium can be explored in the future.
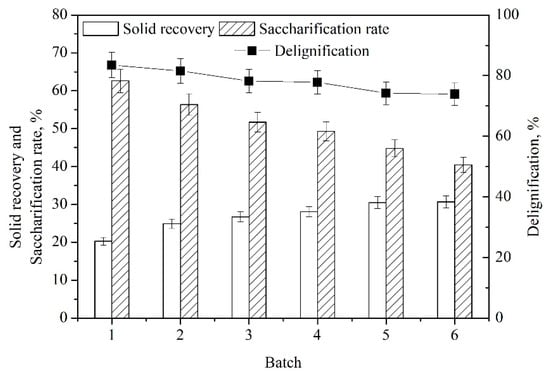
Figure 7.
Reuse of pretreatment medium NaOH–ChCl:UA-TA [Pretreatment condition: NaOH (7 wt%)–ChCl:UA-TA (8 wt%), 75 °C, 60 min; Enzymatic hydrolysis condition: cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS), 50 °C, pH 4.8].
3.4. Enzymatic Hydrolysis of DNC-TS
An effective pretreatment can facilitate the enzymatic saccharification of lignocellulosic materials [,,]. To enhance the saccharification efficiency, the effects of DNC-TS concentration on enzymatic hydrolysis were examined. As illustrated in Figure 8a, time courses for converting different concentrations of DNC-TS (10–100 g/L) were monitored at 50 °C and pH 4.8. Within 48 h, the saccharification rates increased by raising the saccharification time. Over 48 h, the reducing sugar yield had no significant change. Saccharification for 48 h, 10 g/L, 20 g/L, 40 g/L, 50 g/L, 60 g/L, 80 g/L, and 100 g/L of DNC-TS could be saccharified to reducing sugars in the yield of 56.2%, 58.8%, 60.2%, 62.5%, 54.7%, 43.3%, and 29.1% (Figure 8b), respectively. The highest concentration of reducing sugars was also obtained by using 50 g/L of DNC-TS as feedstock. Significantly, the appropriate DNC-TS concentration for enzymatic hydrolysis was 50 g/L. This work provided an effective pretreatment strategy for enhancing the enzymatic saccharification of waste TS.
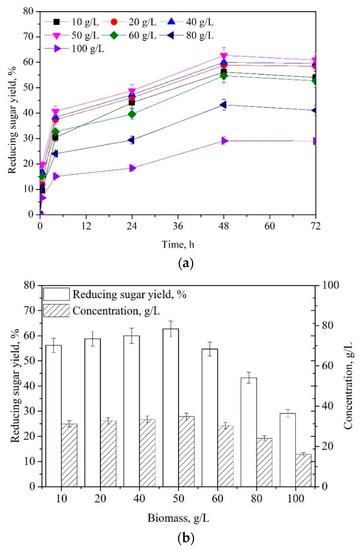
Figure 8.
Time courses for the enzymatic hydrolysis of DNC-TS concentration (10–100 g/L) (a); Effects of DNC-TS concentration (10–100 g/L) on the enzymatic saccharification (b). [Enzymatic hydrolysis condition: cellulase (10.0 FPU/g TS) and xylanase (30.0 CBU/g TS), 50 °C, pH 4.8].
Lignocellulosic biomass consists of highly crystalline cellulose and hemicellulose polymers whereas lignin interacts with hemicellulose and cellulose to form a complex matrix [,,,,,,]. NaOH (1 wt%) could remove 60.4% of lignin in corn stover at 70 °C for 1 h []. A combined ethylene glycol-HClO4-H2O (88.8:1.2:10, wt/wt/wt) soaking at 130 °C for 0.5 h with NaOH (1 wt%) extraction at 90 °C for 1 h was utilized to treat chestnut shell, achieving a 77.5% of delignification and 77.8% of enzymatic saccharification yield []. DES can be used to pretreat biomass in a high delignification (Table 1). Various ChCl-based DESs (e.g., ChCl:FA, ChCl:Gly, ChCl:LA, ChCl:FA:AA, and ChCl:MEA) have been used to remove lignin in a series of biomasses (e.g., corncob, lettuce leave, peach endocarp, rice straw, and wheat straw) at 70–150 °C for 0.5–16 h [,,,,]. The delignification rates could reach 40.0–71.4%. Although ChCl:MEA could be used for pretreating wheat straw in a high delignification (71.4%), a high pretreatment time (9 h) was required. Combination pretreatment could enhance the delignification significantly []. Sorghum straw could be pretreated by sequential pretreatment with NaOH (0.75 wt%) at 121 °C for 1 h and ChCl:LAC at 140 °C for 40 min, achieving the highest delignification (78.4%). However, this combination pretreatment required high temperatures (≥121 °C) and cumbersome performances. In our study, it was firstly reported that NaOH (7 wt%)–ChCl:UA-TA (8 wt%) pretreated TS at a relatively mild performance temperature (75 °C) within 1 h, and a good delignification reached 82.1%. In addition, this pretreatment medium could be recovered and reused, potentially reducing the pretreatment cost. The development of a cost-efficient and sustainable pretreatment strategy by using green solvent as a pretreatment medium is still in progress.

Table 1.
Related works about DES pretreatment of biomass.
4. Conclusions
In this work, a feasible combination pretreatment was developed under a relatively mild performance condition. And a significant enhancement of enzymatic saccharification of TS was achieved by combination pretreatment with a mixture of NaOH (7 wt%) and ChCl:Urea-Thioure (8 wt%) in a one-pot manner at 75 °C for 60 min. The pretreated TS (DNC-TS) had a looser structure, more porosity, and more cracks than raw TS. High delignification was obtained at 82.1%, and the highest yield of reducing sugars (62.5%) was obtained. Overall, the NaOH–ChCl:UA-TA pretreatment has high potential in the valorization of lignocellulose.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10101905/s1. Figure S1: 1H NMR spectra of DES (ChCl:UA-TA); Figure S2: 13C NMR spectra of DES (ChCl:UA-TA); Figure S3: FT-IR spectra of DES (ChCl:UA-TA).
Author Contributions
Conceptualization, Methodology, and Writing original draft, B.F., J.N. and Q.L.; Data curation, Software, Supervision, Review and revising manuscript, Y.H. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the National-Local Joint Engineering Research Center of Biomass Refining and High-Quality Utilization (Changzhou University) for kindly analysis of biomass with SEM.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| LCB | Lignocellulosic biomass |
| TS | Tomato stalk |
| DES | deep eutectic solvent |
| ChCl | Choline chloride |
| UA | urea |
| TA | thiourea |
| DI water | deionized water |
References
- Gu, T.; Wang, B.; Zhang, Z.; Wang, Z.; Chong, G.; Ma, C.; Tang, Y.-J.; He, Y. Sequential pretreatment of bamboo shoot shell and biosynthesis of ethyl (R)-4-chloro-3-hydroxybutanoate in aqueous-butyl acetate media. Process Biochem. 2019, 80, 112–118. [Google Scholar] [CrossRef]
- Wu, J.-W.; Dong, L.-L.; Liu, B.-F.; Xing, D.-F.; Zhou, C.-S.; Wang, Q.; Wu, X.-K.; Feng, L.-P.; Cao, G.-L. A novel integrated process to convert cellulose and hemicellulose in rice straw to biobutanol. Environ. Res. 2020, 186, 109580. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shukla, A.; Tiwari, S.; Srivastava, M. A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sustain. Energ. Rev. 2014, 32, 713–728. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energ. Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Pandiyan, K.; Tiwari, R.; Rana, S.; Arora, A.; Singh, S.; Saxena, A.-K.; Nain, L. Comparative efficiency of different pretreatment methods on enzymatic digestibility of Parthenium sp. World J. Microbiol. Biotechnol. 2014, 30, 55–64. [Google Scholar] [CrossRef]
- He, Y.-C.; Liu, F.; Di, J.-H.; Ding, Y.; Zhu, Z.-Z.; Wu, Y.-Q.; Chen, L.; Wang, C.; Xue, Y.-F.; Chong, G.-G. Effective enzymatic saccharification of dilute NaOH extraction of chestnut shell pretreated by acidified aqueous ethylene glycol media. Ind. Crop. Prod. 2016, 81, 129–138. [Google Scholar] [CrossRef]
- Modenbach, A.-A.; Nokes, S.-E. Enzymatic hydrolysis of biomass at high-solids loading—A review. Biomass. Bioeng. 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Cheng, W.-K.; Li, Y.-L.; Wang, T.; Xia, Q.-Q.; Liu, Y.-Z.; Yu, H.-P. Tailored one-pot lignocellulose fractionation to maximize biorefinery toward versatile xylochemicals and nanomaterials. Green. Chem. 2022, 24, 3257. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.-V. Multi-product biorefinery with sugarcane bagasse: Process development for nanocellulose, lignin and biohydrogen production and lifecycle analysis. Chem. Eng. J. 2022, 446, 137233. [Google Scholar] [CrossRef]
- Haykir, N.-I.; Bakir, U. Ionic liquid pretreatment allows utilization of high substrate loadings in enzymatic hydrolysis of biomass to produce ethanol from cotton stalks. Ind. Crop. Prod. 2013, 51, 408–414. [Google Scholar] [CrossRef]
- Pińkowska, H.; Wolak, P.; Złocińska, A. Hydrothermal decomposition of xylan as a model substance for plant biomass waste–Hydrothermolysis in subcritical water. Biomass Bioenerg. 2011, 35, 3502–3912. [Google Scholar] [CrossRef]
- Strassberger, Z.; Prinsen, P.; Klis, F.; Es, D.; Tanase, S.; Rothenberg, G. Lignin solubilisation and gentle fractionation in liquid ammonia. Green Chem. 2015, 17, 325–334. [Google Scholar] [CrossRef]
- Kim, S.-B.; Lee, S.-J.; Lee, J.-H.; Jung, Y.-R.; Thapa, L.-P.; Kim, J.-S.; Um, Y.; Park, C.; Kim, S.-W. Pretreatment of rice straw with combined process using dilute sulfuric acid and aqueous ammonia. Biotechnol. Biofuels 2013, 6, 109. [Google Scholar] [CrossRef]
- Jiang, C.-X.; He, Y.-C.; Chong, G.-G.; Di, J.-H.; Tang, Y.-J.; Ma, C.-L. Enzymatic in situ saccharification of sugarcane bagasse pretreated with low loading of alkalic salts Na2SO3/Na3PO4 by autoclaving. J. Biotechnol. 2017, 259, 73–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Su, J.; Lin, Y.; Huang, Z.; Lu, Y.; Sun, G.; Yang, M.; Huang, A.; Hu, H.; et al. A green and efficient technology for the degradation of cellulosic materials: Structure changes and enhanced enzymatic hydrolysis of natural cellulose pretreated by synergistic interaction of mechanical activation and metal salt. Bioresour. Technol. 2015, 177, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Barmana, D.-N.; Haquea, M.-A.; Kang, T.-H.; Kim, G.-H.; Kim, T.-Y.; Kim, M.-K.; Yun, H.-D. Effect of mild alkali pretreatment on structural changes of reed (Phragmites communis Trinius) straw. Environ. Technol. 2014, 35, 232–241. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, T.; Liu, J.; Gao, H.; Bian, H.; Wang, R.; Huang, C.; Sha, J.; Dai, H. Recyclable deep eutectic solvent coupling sodium hydroxide post-treatment for boosting woody/herbaceous biomass conversion at mild condition. Bioresour. Technol. 2021, 320, 124327. [Google Scholar] [CrossRef]
- Zhou, J.-P.; Zhang, L.-N.; Cai, J. Behavior of cellulose in NaOH/urea aqueous solution characterized by light scattering and viscometry. J. Polym. Sci. Pol. Phys. 2004, 42, 347–353. [Google Scholar] [CrossRef]
- Jin, H.-J.; Zha, C.-X.; Gu, L.-X. Direct dissolution of cellulose in NaOH/thiourea/urea aqueous solution. Carbohyd. Res. 2007, 342, 851–858. [Google Scholar] [CrossRef]
- Dong, L.-L.; Cao, G.-L.; Wu, J.-W.; Liu, B.-F.; Xing, D.-F.; Zhao, L.; Zhou, C.-S.; Feng, L.-P.; Ren, N.-Q. High-solid pretreatment of rice straw at cold temperature using NaOH/Urea for enhanced enzymatic conversion and hydrogen production. Bioresour. Technol. 2019, 287, 121399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Ruan, D.; Gao, J. Dissolution and regeneration of cellulose in NaOH/thiourea aqueous solution. J. Polym. Sci. Pol. Phys. 2002, 40, 1521–1529. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Jeihanipour, A.; Karimia, K.; Taherzadeh, M.-J. Alkali pretreatment of softwood spruce and hardwood birch by NaOH/thiourea, NaOH/urea, NaOH/urea/thiourea, and NaOH/PEG to improve ethanol and biogas production. J. Chem Technol. Biotechnol. 2012, 87, 1209–1214. [Google Scholar] [CrossRef]
- Liang, X.-Q.; Zhu, Y.; Qi, B.-K.; Li, S.-Q.; Luo, J.-Q.; Wan, Y.-H. Structure-property-performance relationships of lactic acid-based deep eutectic solvents with different hydrogen bond acceptors for corn stover pretreatment. Bioresour. Technol. 2021, 336, 125312. [Google Scholar] [CrossRef]
- Pan, L.; Li, Q.; Tao, Y.; Ma, C.; Chai, H.; Ai, Y.; He, Y.-C. An efficient chemoenzymatic strategy for valorisation of corncob to furfuryl alcohol in CA:Betaine-water. Ind. Crop Prod. 2022, 186, 115203. [Google Scholar] [CrossRef]
- Ling, R.-X.; Wu, W.-J.; Yuan, Y.-F.; Wei, W.-Q.; Jin, Y.-C. Investigation of choline chloride-formic acid pretreatment and Tween 80 to enhance sugarcane bagasse enzymatic hydrolysis. Bioresour. Technol. 2021, 326, 124748. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Li, J.; Zhu, P.; Liang, B. Exploring dynamics and associations of dominant lignocellulose degraders in tomato stalk composting. J. Environ. Manage. 2021, 294, 113162. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structural Carbohydrates and Lignin in Biomass; Report No. TP-510-42618; National Renweable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- He, Y.-C.; Liu, F.; Gong, L.; Zhu, Z.-Z.; Ding, Y.; Wang, C.; Xue, Y.-F.; Rui, H.; Tao, Z.-C.; Zhang, D.-P.; et al. Significantly improving enzymatic saccharification of high crystallinity index’s corn stover by combining ionic liquid [Bmim]Cl-HCl-water media with dilute NaOH pretreatment. Bioresour. Technol. 2015, 189, 421–425. [Google Scholar] [CrossRef]
- He, Y.-C.; Ding, Y.; Xue, Y.-F.; Yang, B.; Liu, F.; Wang, C.; Zhu, Z.-Z.; Qing, Q.; Wu, H.; Zhu, C.; et al. Enhancement of enzymatic saccharification of corn stover with sequential Fenton pretreatment and dilute NaOH extraction. Bioresour. Technol. 2015, 193, 324–330. [Google Scholar] [CrossRef]
- Chong, G.-G.; He, Y.-C.; Liu, Q.-X.; Kou, X.-Q.; Huang, X.-J.; Di, J.-H.; Ma, C.-L. Effective enzymatic in situ saccharification of bamboo shoot shell pretreated by dilute alkalic salts sodium hypochlorite/sodium sulfide pretreatment under the autoclave system. Bioresour. Technol. 2017, 241, 726–734. [Google Scholar] [CrossRef]
- Wu, M.-J.; Gong, L.; Ma, C.-L.; He, Y.-C. Enhanced enzymatic saccharification of sorghum straw by effective delignification via combined pretreatment with alkali extraction and deep eutectic solvent soaking. Bioresour. Technol. 2021, 340, 125695. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.-G.; He, Y.-C.; Liu, Q.-X.; Kou, X.-Q.; Qing, Q. Sequential aqueous ammonia extraction and LiCl/N,N-dimethyl formamide pretreatment for enhancing enzymatic saccharification of winter bamboo shoot shell. Appl. Biochem. Biotechnol. 2017, 182, 1341–1357. [Google Scholar] [CrossRef]
- Dai, Y.; Si, M.; Chen, Y.; Zhang, N.; Zhou, M.; Liao, Q.; Shi, D.; Liu, Y. Combination of biological pretreatment with NaOH/Urea pretreatment at cold temperature to enhance enzymatic hydrolysis of rice straw. Bioresour. Technol. 2015, 198, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Shahabazuddin, M.; Chandra, T.-S.; Meena, S.; Sukumaran, R.-K.; Shetty, N.-P.; Mudliar, S.-N. Thermal assisted alkaline pretreatment of rice husk for enhanced biomass deconstruction and enzymatic saccharification: Physico-chemical and structural characterization. Bioresour. Technol. 2018, 263, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.-M.; Seo, J.-W.; Kim, C.-H. Sequential acid-/alkali-pretreatment of empty palm fruit bunch fiber. Bioresour. Technol. 2012, 109, 229–233. [Google Scholar] [CrossRef]
- Shao, L.-Y.; Chen, H.; Li, Y.-L.; Li, J.-N.; Chen, G.; Wang, G. Pretreatment of corn stover via sodium hydroxide-urea solutions to improve the glucose yield. Bioresour. Technol. 2020, 307, 123191. [Google Scholar] [CrossRef]
- Xu, G.-C.; Ding, J.-C.; Han, R.-Z.; Dong, J.-J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2015, 203, 364–369. [Google Scholar] [CrossRef]
- Lin, W.; Xing, S.; Jin, Y.; Lu, X.; Huang, C.; Yong, Q. Insight into understanding the performance of deep eutectic solvent pretreatment on improving enzymatic digestibility of bamboo residues. Bioresour. Technol. 2020, 306, 123163. [Google Scholar] [CrossRef]
- Wang, W.-C.; Zhang, P.; Zhang, S.; Li, F.-X.; Yu, J.-Y.; Lin, J.-Y. Structure and properties of novel regenerated cellulose fibers prepared in NaOH complex solution. Carbohyd. Polym. 2013, 98, 1031–1038. [Google Scholar] [CrossRef]
- Chong, G.-G.; Huang, X.-J.; Di, J.-H.; Xu, D.-Z.; He, Y.-C.; Pei, Y.-N.; Tang, Y.-J.; Ma, C.-L. Biodegradation of alkali lignin by a newly isolated Rhodococcus pyridinivorans CCZU-B16. Bioproc Biosyst Eng. 2018, 41, 501–510. [Google Scholar] [CrossRef]
- He, Y.-C.; Liu, F.; Gong, L.; Lu, T.; Ding, Y.; Zhang, D.-P.; Qing, Q.; Zhang, Y. Improving enzymatic hydrolysis of corn stover pretreated by ethylene glycol-perchloric acid-water mixture. Appl. Biochem. Biotechnol. 2015, 175, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zhang, Q.; Liu, Z.-Y.; Li, F.-L.; Lu, M.; Fang, X.-C. Emerging technologies for the pretreatment of lignocellulosic materials for bio-based products. Appl. Microbiol. Biotechnol. 2020, 104, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zha, J.; Pan, L.; Ma, C.; He, Y.-C. Highly efficient conversion of sunflower stalk-hydrolysate to furfural by sunflower stalk residue-derived carbonaceous solid acid in deep eutectic solvent/organic solvent system. Bioresour. Technol. 2021, 351, 126945. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.-K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.-F.; Mohammadi, A.-A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.-J. Pretreatment technologies for an efficient bioethanol production pro-cess based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, L.; Tang, Z.; Chen, L.; He, Y. Enhanced saccharification of purple alfalfa via sequential pretreatment with acidified ethylene glycol and Urea/NaOH. Processes 2021, 10, 61. [Google Scholar] [CrossRef]
- Hanhikoski, S.; Warsta, E.; Varhimo, A.; Niemelä, K.; Vuorinen, T. Sodium sulphite pulping of Scots pine under neutral and mildly alkaline conditions (NS pulping). Holzforschung 2016, 70, 603–609. [Google Scholar] [CrossRef]
- Xu, G.-C.; Li, H.; Xing, W.-R.; Gong, L.; Dong, J.-J.; Ni, Y. Facilely reducing recalcitrance of lignocellulosic biomass by a newly developed ethylamine-based deep eutectic solvent for biobutanol fermentation. Biotechnol. Biofuels 2020, 13, 166. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.-E.; Rehmann, L.; Marzocchella, A. Low-energy biomass pretreatment with deep eutectic solvents for bio-butanol production. Bioresour. Technol. 2017, 243, 464–473. [Google Scholar] [CrossRef]
- Li, W.-Q.; Amos, K.; Li, M.; Pu, Y.-Q.; Debolt, S.; Ragauskas, A.-J.; Shi, J. Fractionation and characterization of lignin streams from unique high-lignin content endocarp feedstocks. Biotechnol. Biofuels 2018, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.-R.; Xu, G.-C.; Dong, J.-J.; Han, R.-Z.; Ni, Y. Novel dihydrogen-bonding deep eutectic solvents: Pretreatment of rice straw for butanol fermentation featuring enzyme recycling and high solvent yield. Chem. Eng. J. 2018, 333, 712–720. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Ali, M.; Abdeltawab, A.; Yakout, S.; Yu, G. Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Qing, Q.; Guo, Q.; Zhou, L.; He, Y.; Wang, L.; Zhang, Y. Enhancement of In Situ Enzymatic Saccharification of Corn Stover by a Stepwise Sodium Hydroxide and Organic Acid Pretreatment. Appl. Biochem. Biotechnol. 2017, 181, 350–364. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).