Assessment of Wine Adulteration Using Near Infrared Spectroscopy and Laser Backscattering Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Near Infrared Spectroscopy
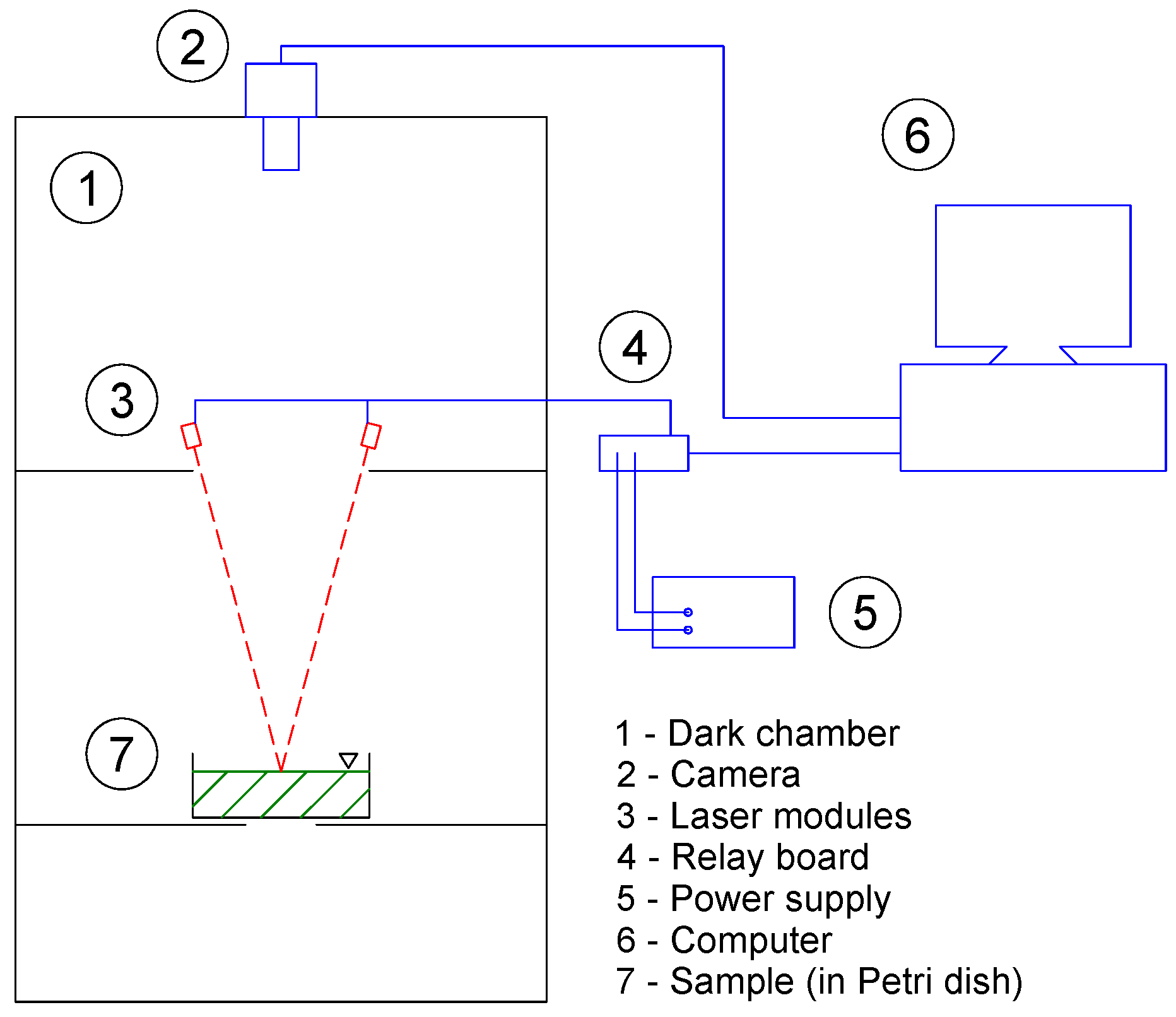
2.3. Laser-Induced Diffuse Reflectance Imaging
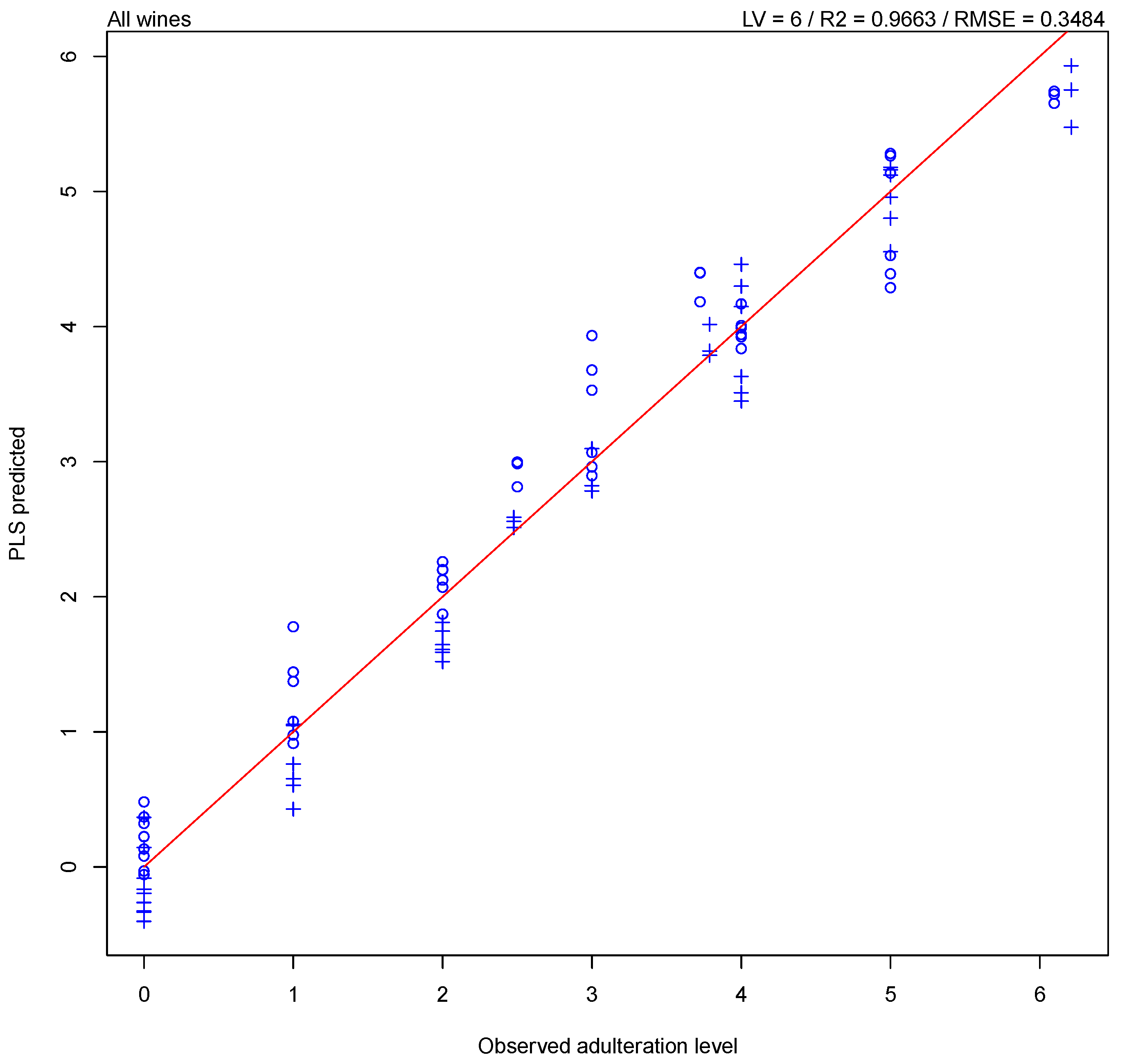
3. Results
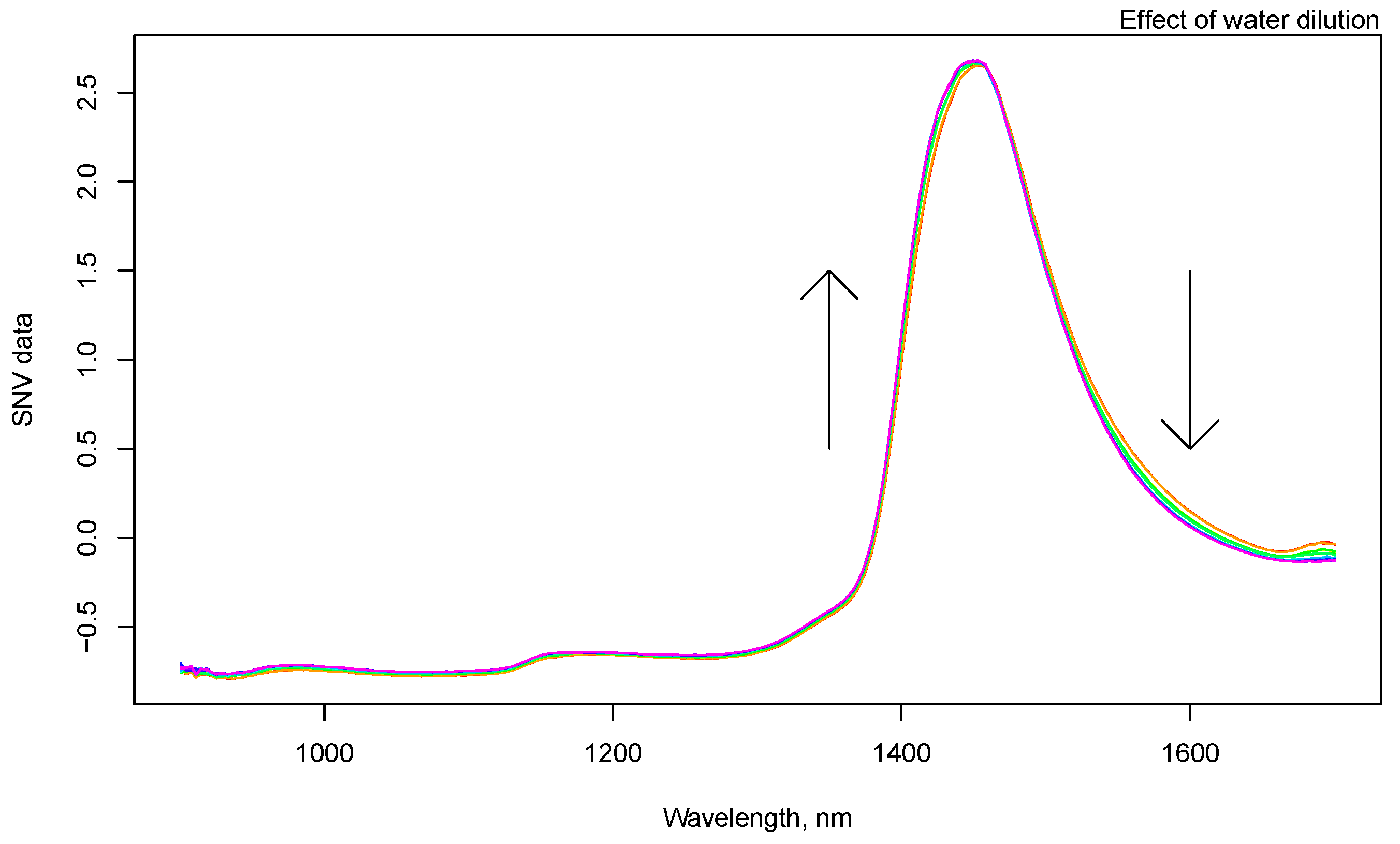
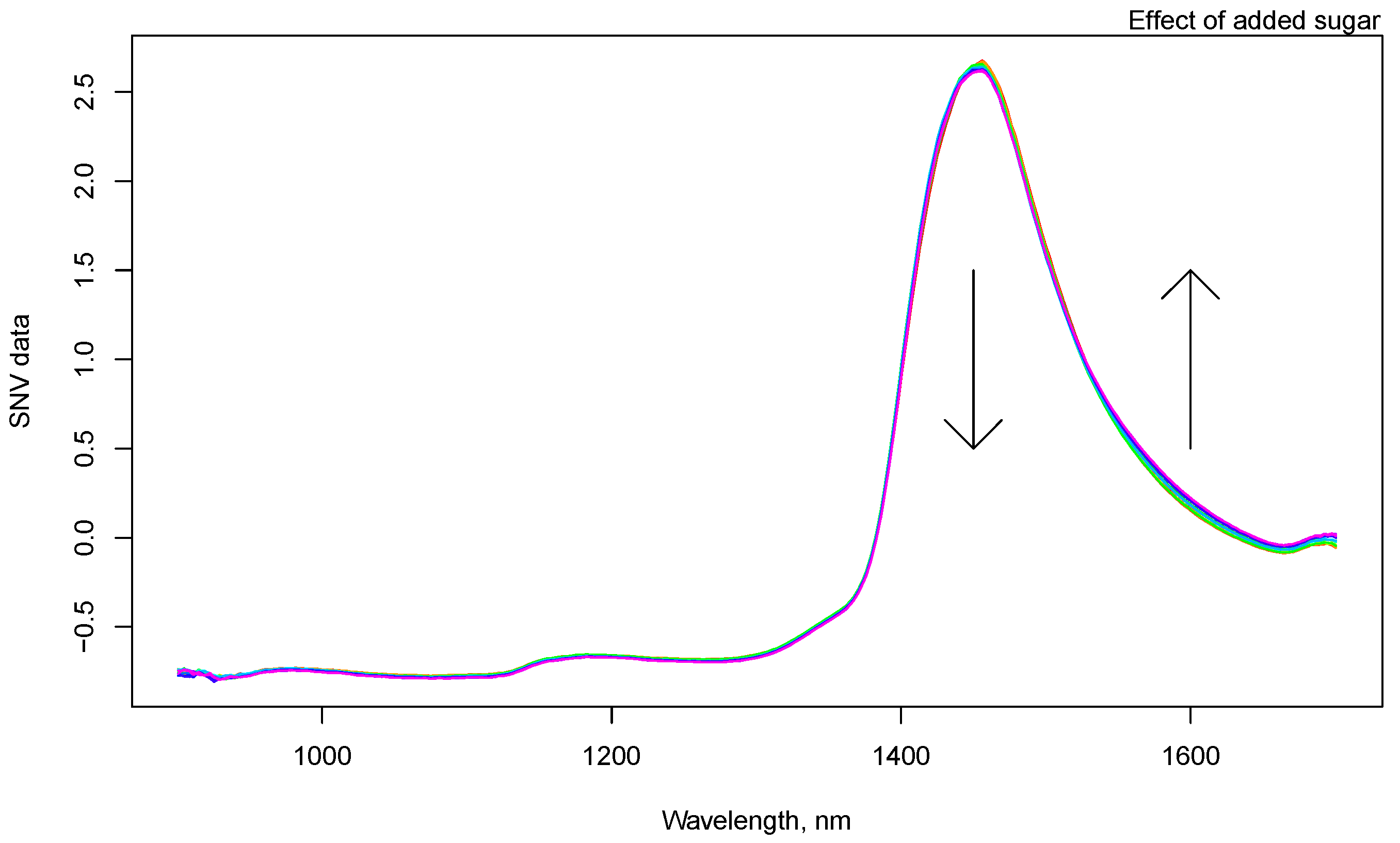
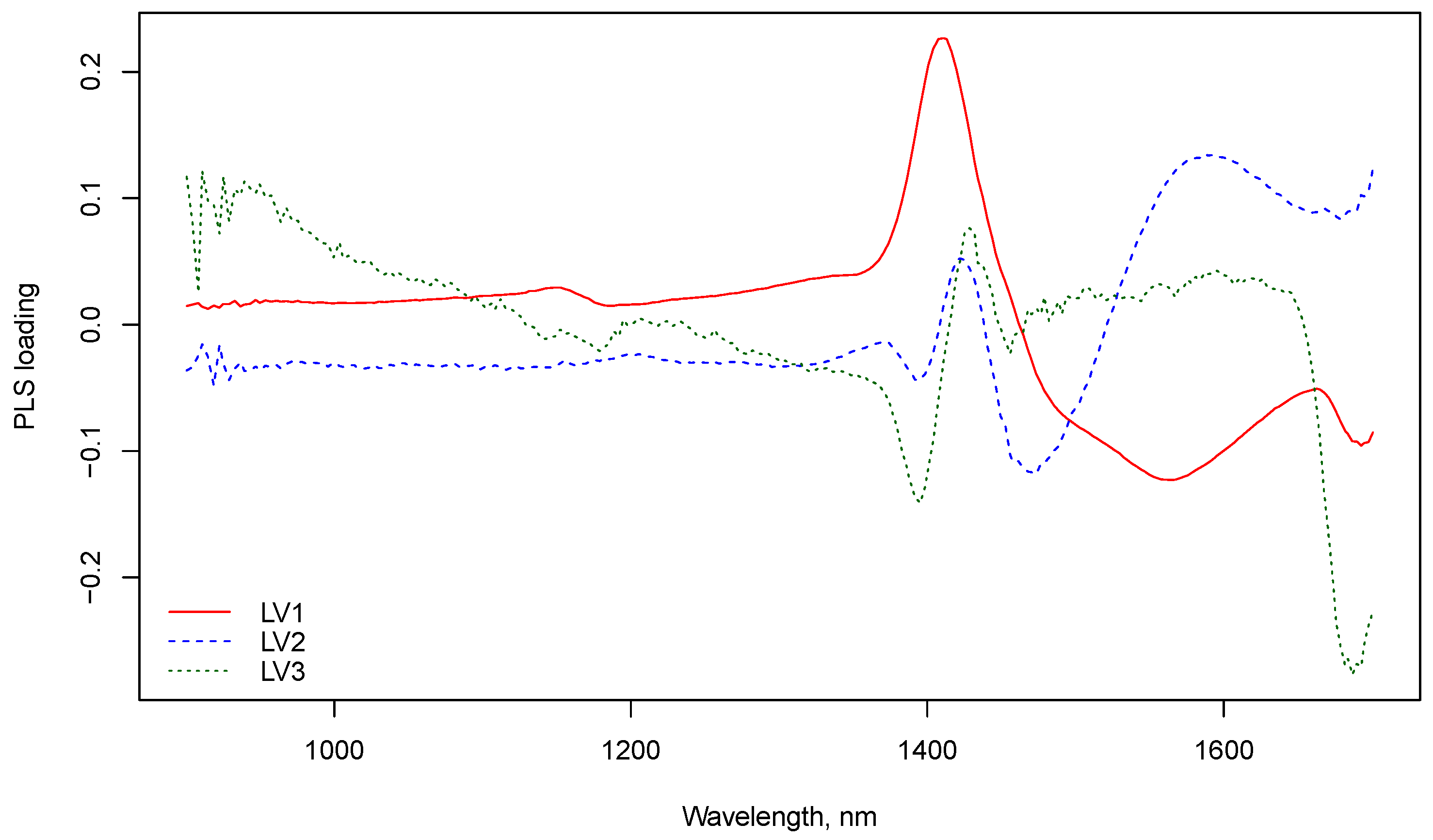
3.1. NIR Spectroscopy
3.2. Diffuse Reflectance Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bánvölgyi, S.; Dusza, E.; Namukwambi, F.K.; Kiss, I.; Stefanovits-Bányai, É.; Vatai, G. Optimization of extraction of phenolic compounds from Tokaji Aszú marc using response surface methodology. Progr. Agric. Eng. Sci. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Christoph, N.; Baratossy, G.; Kubanovic, V.; Kozina, B.; Rossmann, A.; Schlicht, C.; Voerkelius, S. Possibilities and limitations of wine authentication using stable. Mit. Klost. 2004, 54, 144–158. [Google Scholar]
- Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 Establishing a Common Organisation of the Markets in Agricultural Products and Repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Available online: http://data.europa.eu/eli/reg/2013/1308/oj (accessed on 5 December 2021).
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Stefanescu, I.; Ionete, R.E.; Bala, C. Verifying the red wines adulteration through isotopic and chromatographic investigations coupled with multivariate statistic interpretation of the data. Food Cont. 2016, 62, 1–9. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. Wine authenticity, traceability and safety monitoring. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing Limited: Sawston, UK, 2010; pp. 218–270. ISBN 978-1-84569-484-5. [Google Scholar] [CrossRef]
- Cimpoiu, C.; Hosu, A.; Briciu, R.; Miclaus, V. Monitoring the origin of wine by reversed-phase thin-layer chromatography. J. Plan. Chrom. 2007, 20, 407–410. [Google Scholar] [CrossRef]
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern analytical methods for the detection of food fraud and adulteration by food category. J. Sci. Food Agric. 2017, 97, 3877–3896. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Reina, R.; García-González, D.L.; Callejón, R.M.; Amigo, J.M. NIR spectroscopy and chemometrics for the typification of Spanish wine vinegars with a protected designation of origin. Food Cont. 2018, 89, 108–116. [Google Scholar] [CrossRef]
- Bridle, P.; Garcia-Viguera, C. A simple technique for the detection of red wine adulteration with elderberry pigments. Food Chem. 1995, 55, 111–113. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G. Recognition of adulteration of Italian wines by thin-film multisensor array and artificial neural networks. Anal. Chim. Acta 2004, 509, 159–177. [Google Scholar] [CrossRef]
- Zinia Zaukuu, J.L.; Gillay, Z.; Kovács, Z. Standardized extraction techniques for meat analysis with the electronic tongue: A case study of poultry and red meat adulteration. Sensors 2021, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Vitális, F.; Zinia Zaukuu, J.L.; Bodor, Z.; Aouadi, B.; Hitka, G.; Kaszab, T.; Zsom-Muha, V.; Gillay, Z.; Kovács, Z. Detection and quantification of tomato paste adulteration using conventional and rapid analytical methods. Sensors 2020, 20, 6059. [Google Scholar] [CrossRef] [PubMed]
- Power, A.C.; Jones, J.; NiNeil, C.; Geoghegan, S.; Warren, S.; Currivan, S.; Cozzolino, D. What’s in this drink? Classification and adulterant detection in Irish Whiskey samples using near infrared spectroscopy combined with chemometrics. J. Sci. Food Agric. 2021, 101, 5256–5263. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, D.T.; Zinia Zaukuu, J.L.; Bósquez, J.P.A.; Bodor, Z.; Vitalis, F.; Kovács, Z. Near-infrared spectroscopy and aquaphotomics for monitoring mung bean (Vigna radiata) sprout growth and validation of ascorbic acid content. Sensors 2021, 21, 611. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. Detection and quantification of adulterations in aged wine using RGB digital images combined with multivariate chemometric techniques. Food Chem. 2019, 3, 100046. [Google Scholar] [CrossRef]
- Baranyai, L.; Zude, M. Analysis of laser light migration in apple tissue by Monte Carlo simulation. Progr. Agric. Eng. Sci. 2008, 4, 45–59. [Google Scholar] [CrossRef]
- Arango, O.; Trujillo, A.J.; Castillo, M. Inline control of yoghurt fermentation process using a near infrared light backscatter sensor. J. Food Eng. 2020, 277, 109885. [Google Scholar] [CrossRef]
- Sanchez, P.D.C.; Hashim, N.; Shamsudin, R.; Nor, M.Z.M. Laser-light backscattering imaging approach in monitoring and classifying the quality changes of sweet potatoes under different storage conditions. Posth. Biol. Tech. 2020, 164, 111163. [Google Scholar] [CrossRef]

| Portugieser (Red Wine) | Sauvignon Blanc (White Wine) | ||||
|---|---|---|---|---|---|
| Level | Added Water, % | Added Sugar, % | Level | Added Water, % | Added Sugar, % |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 28.57 | 3.62 | 1 | 28.57 | 3.71 |
| 2 | 48.90 | 6.89 | 2 | 48.90 | 7.15 |
| 3 | 63.56 | 10.04 | 3 | 63.56 | 10.44 |
| 4 | 73.97 | 13.06 | 4 | 73.97 | 13.35 |
| 5 | 81.41 | 15.80 | 5 | 81.41 | 16.41 |
| Portugieser (Red Wine) | Sauvignon Blanc (White Wine) | ||||
|---|---|---|---|---|---|
| Level | Added Water, % | Added Sugar, % | Level | Added Water, % | Added Sugar, % |
| 2.50 | 48.98 | 5.08 | 2.48 | 48.98 | 4.92 |
| 3.72 | 28.57 | 11.67 | 3.79 | 28.57 | 11.82 |
| 6.10 | 81.41 | 11.35 | 6.21 | 81.41 | 11.91 |
| Wine | Portugieser (Red Wine) | Sauvignon Blanc (White Wine) | ||||
|---|---|---|---|---|---|---|
| Factor | Water | Sugar | All | Water | Sugar | All |
| Samples | 18 | 18 | 42 | 18 | 18 | 42 |
| LV | 3 | 3 | 6 | 3 | 3 | 6 |
| R2 | 0.9990 | 0.9997 | 0.9891 | 0.9992 | 0.9998 | 0.9913 |
| RMSE | 0.166% | 0.504% | 0.194 | 0.165% | 0.361% | 0.180 |
| Wavelength | D75 | D50 | D25 | D50/D75 | D25/D75 | A50 | A25–75 | A50/A25–75 |
|---|---|---|---|---|---|---|---|---|
| 1064 | s | s | s | - | - | S | s | - |
| 850 | s | - | s | - | - | s | s | - |
| 808 | w | - | s | - | - | - | - | - |
| 780 | - | - | s | - | - | - | - | - |
| 635 | - | - | s | s/w | - | - | s | - |
| 532 | - | - | - | w | - | - | s/w | S/W |
| Diffuse Reflectance | Adulteration Factors | |||
|---|---|---|---|---|
| Wavelength | Parameter | Wine Type | Water | Sugar |
| 532 nm | A50/A25–75 | 0.551 | 7.717 | 2.439 |
| 532 nm | A25–75 | 16.36 | 0.835 | 65.56 |
| 635 nm | D50/D75 | 2.669 | 1.217 | 1.882 |
| 532 nm | A50/A25–75 | Sauvignon Blanc | 1.124 | 28.28 |
| 532 nm | A25–75 | 1.111 | 161.4 | |
| 635 nm | D50/D75 | 0.601 | 1.271 | |
| 532 nm | A50/A25–75 | Portugieser | 21.18 | 1.527 |
| 532 nm | A25–75 | 1.362 | 7.511 | |
| 635 nm | D50/D75 | 1.04 | 1.95 | |
| Wavelength | All Parameters | Selected Parameters | ||||
|---|---|---|---|---|---|---|
| Water | Sugar | All | Water | Sugar | All | |
| 532 nm | 76.67 | 96.67 | 60.00 | 60.00 | 86.67 | 23.33 |
| 635 nm | 66.67 | 100 | 80.00 | 46.67 | 93.33 | 43.33 |
| 650 nm | 56.67 | 93.33 | 73.33 | 50.00 | 83.33 | 33.33 |
| 780 nm | 73.33 | 96.67 | 73.33 | 56.67 | 80.00 | 33.33 |
| 808 nm | 53.33 | 93.33 | 70.00 | 46.67 | 93.33 | 26.67 |
| 850 nm | 63.33 | 100 | 80.00 | 50.00 | 93.33 | 46.67 |
| 1064 nm | 56.67 | 100 | 66.67 | 40.00 | 93.33 | 43.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hencz, A.; Nguyen, L.L.P.; Baranyai, L.; Albanese, D. Assessment of Wine Adulteration Using Near Infrared Spectroscopy and Laser Backscattering Imaging. Processes 2022, 10, 95. https://doi.org/10.3390/pr10010095
Hencz A, Nguyen LLP, Baranyai L, Albanese D. Assessment of Wine Adulteration Using Near Infrared Spectroscopy and Laser Backscattering Imaging. Processes. 2022; 10(1):95. https://doi.org/10.3390/pr10010095
Chicago/Turabian StyleHencz, Anita, Lien Le Phuong Nguyen, László Baranyai, and Donatella Albanese. 2022. "Assessment of Wine Adulteration Using Near Infrared Spectroscopy and Laser Backscattering Imaging" Processes 10, no. 1: 95. https://doi.org/10.3390/pr10010095
APA StyleHencz, A., Nguyen, L. L. P., Baranyai, L., & Albanese, D. (2022). Assessment of Wine Adulteration Using Near Infrared Spectroscopy and Laser Backscattering Imaging. Processes, 10(1), 95. https://doi.org/10.3390/pr10010095








