Impact of Silica Additions on the Phase Composition and Electrical Transport Properties of Ruddlesden-Popper La2NiO4+δ Mixed Conducting Ceramics
Abstract
:1. Introduction
2. Materials and Methods
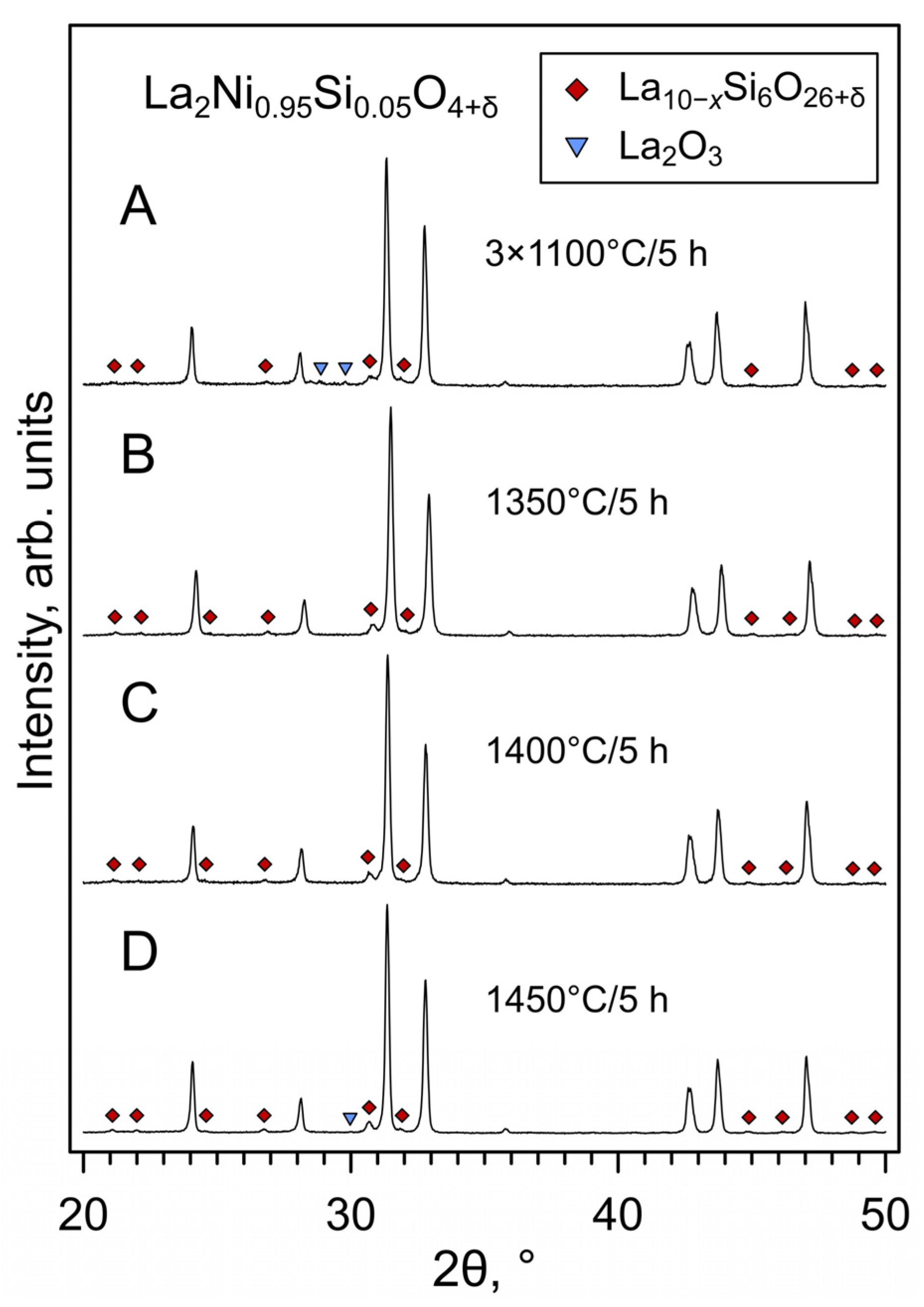
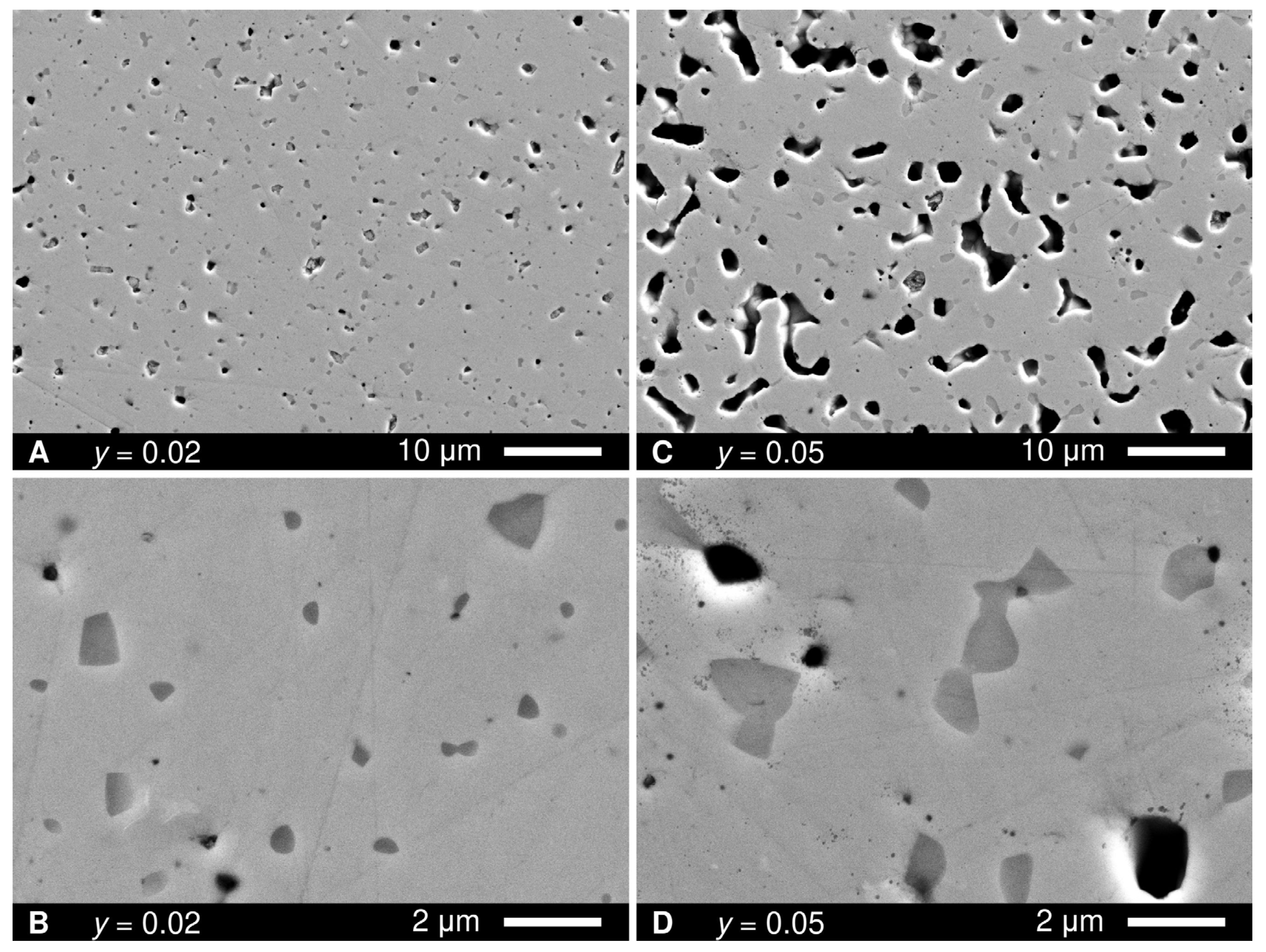
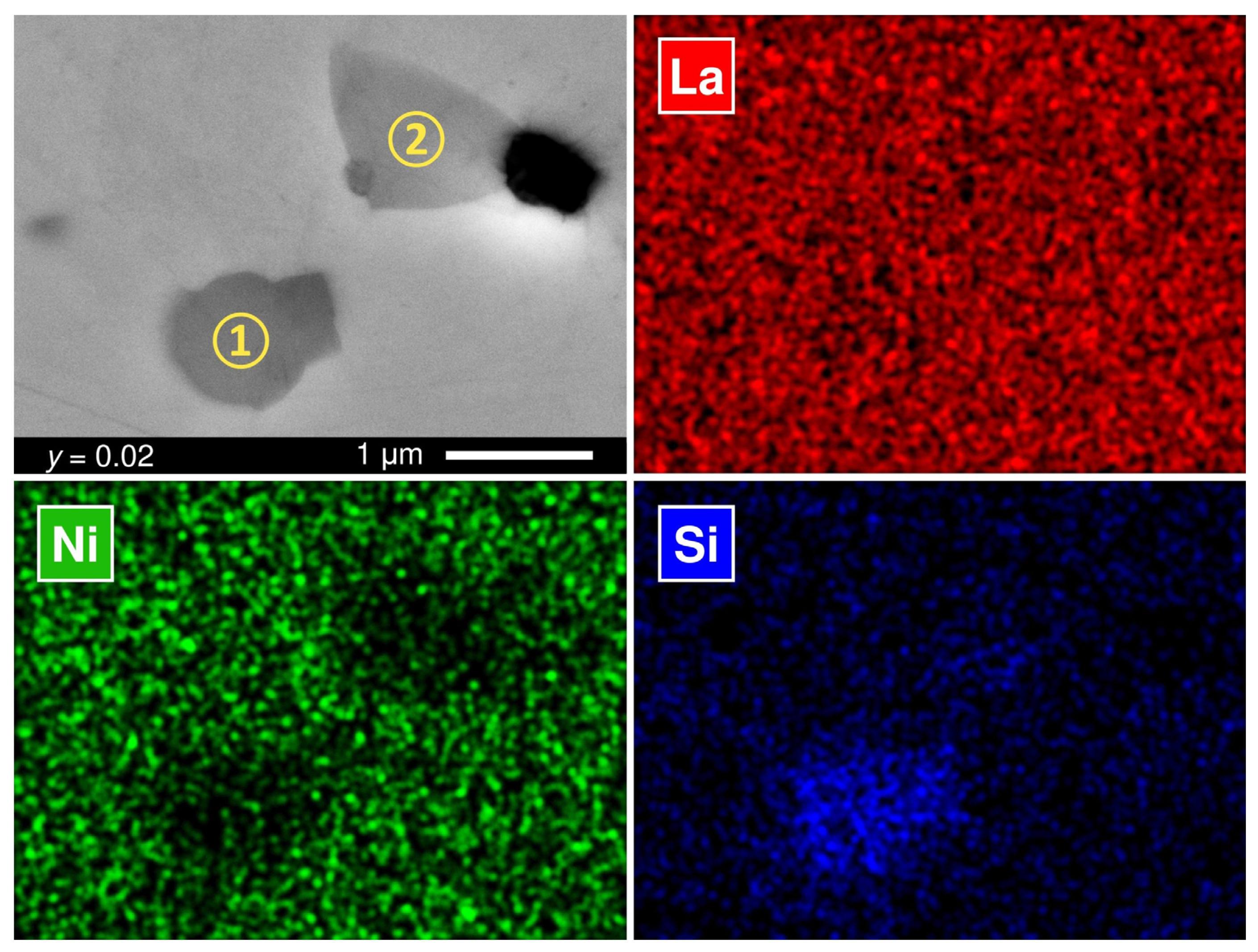
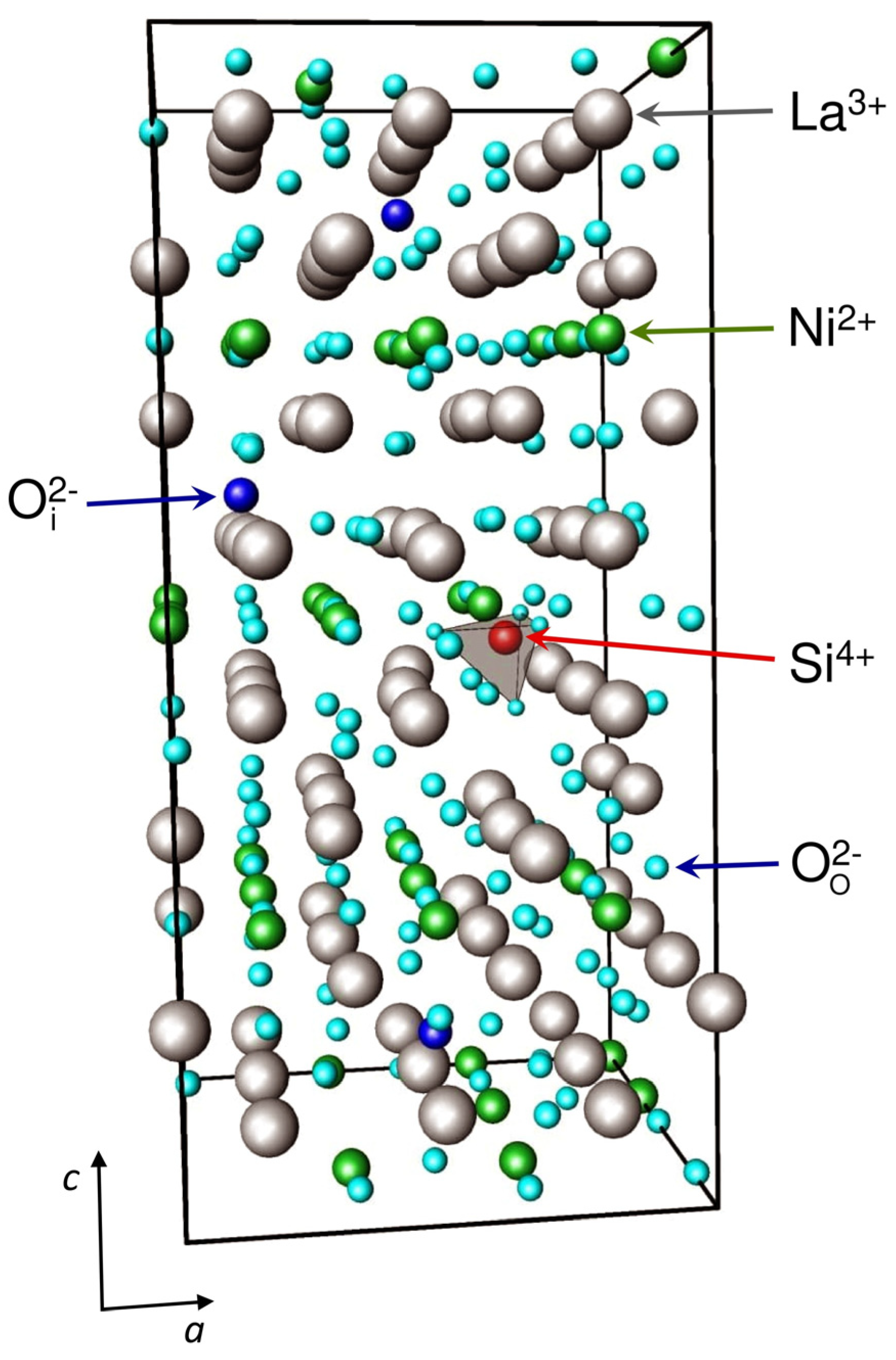
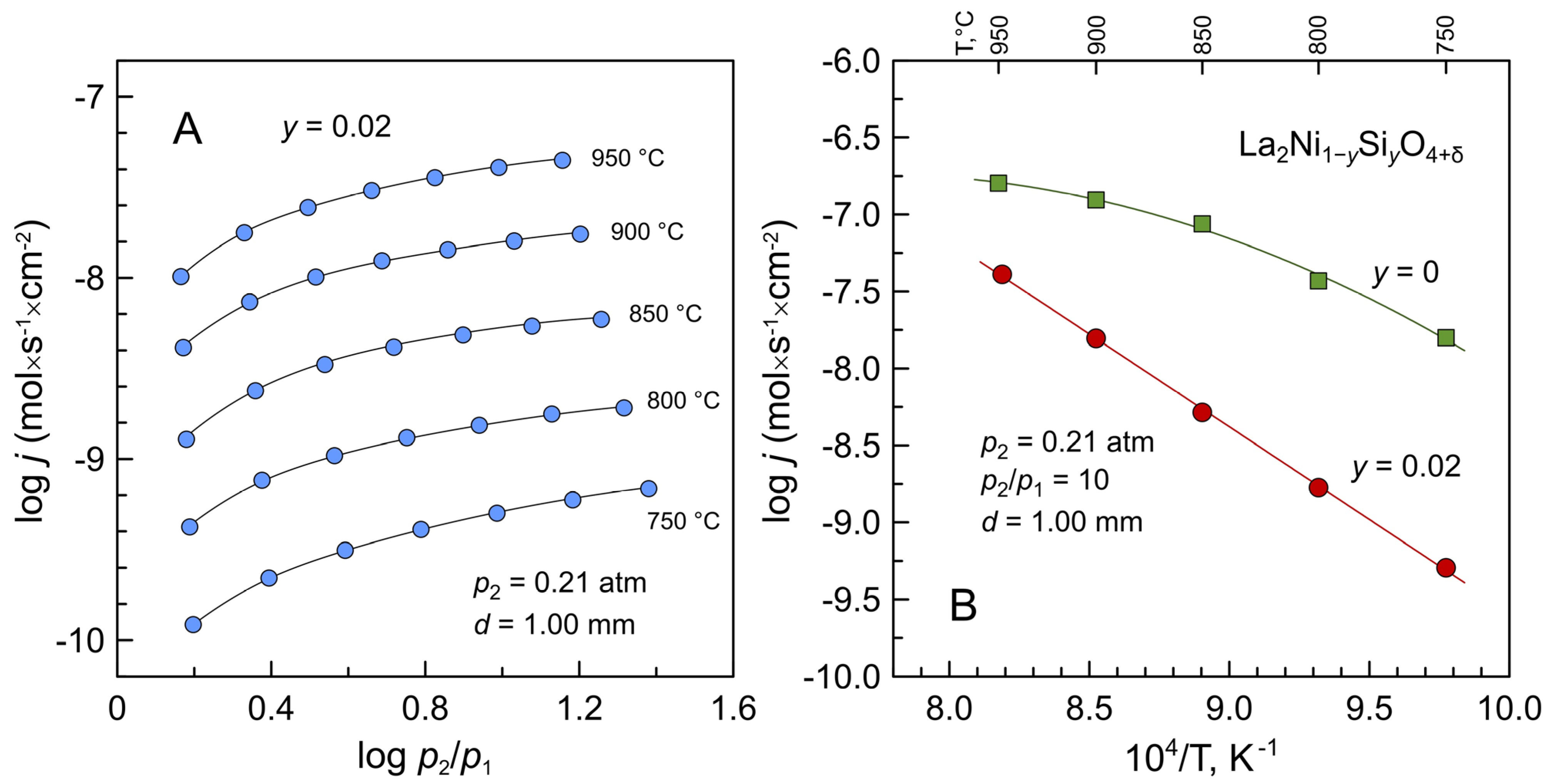
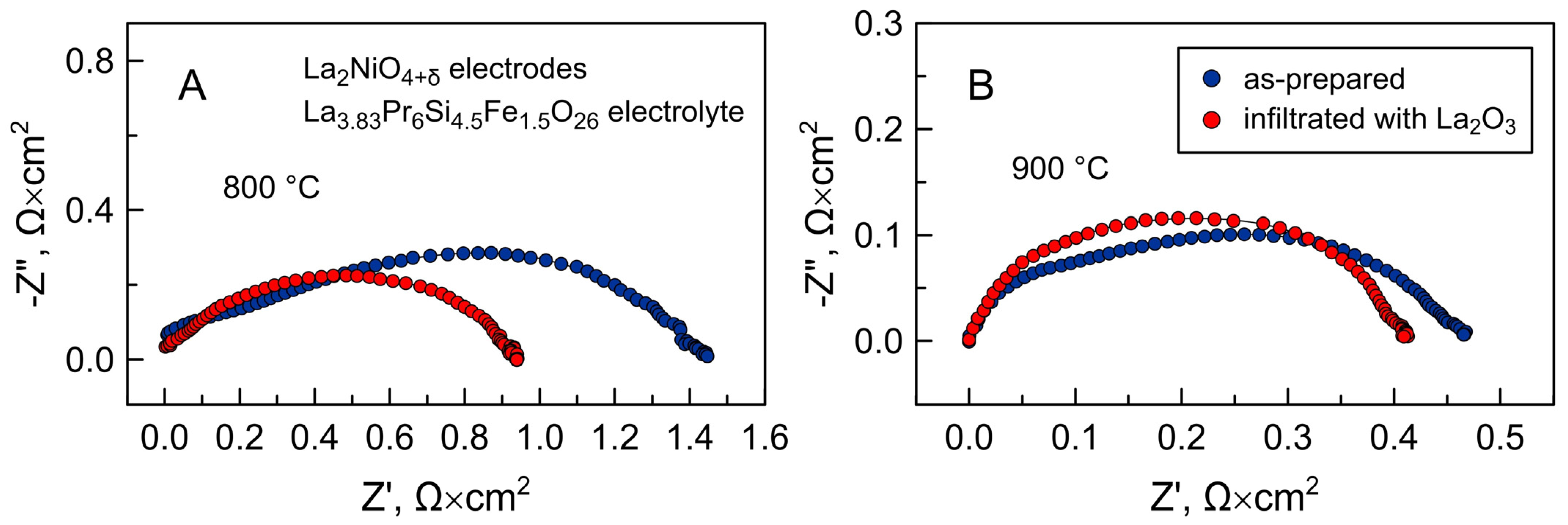
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive review on fuel cell technology for stationary applications as sustainable and efficient poly-generation energy systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Horri, B.A. Progress in material development for low-temperature solid oxide fuel cells: A review. Energies 2021, 14, 1280. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Mineshige, A.; Saito, A.; Kobayashi, M.; Hayakawa, H.; Momai, M.; Yazawa, T.; Yoshioka, H.; Sakao, M.; Mori, R.; Takayama, Y.; et al. Lanthanum silicate-based layered electrolyte for intermediate-temperature fuel cell application. J. Power Sources 2020, 475, 228543. [Google Scholar] [CrossRef]
- Wang, S.F.; Hsu, Y.F.; Hsia, P.; Hung, W.K.; Jasinski, P. Design and characterization of apatite La9.8Si5.7Mg0.3O26±δ-based micro-tubular solid oxide fuel cells. J. Power Sources 2020, 460, 228072. [Google Scholar] [CrossRef]
- Marrero-López, D.; Martín-Sedeño, M.C.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Evaluation of apatite silicates as solid oxide fuel cell electrolytes. J. Power Sources 2010, 195, 2496–2506. [Google Scholar] [CrossRef]
- Malavasi, L.; Fisher, C.A.J.; Islam, M.S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: Structural and mechanistic features. Chem. Soc. Rev. 2010, 39, 4370–4387. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, E.; Islam, M.S.; Slater, P.R. Developing apatites for solid oxide fuel cells: Insight into structural, transport and doping properties. J. Mater. Chem. 2007, 17, 3104–3111. [Google Scholar] [CrossRef]
- Shaula, A.L.; Kharton, V.V.; Marques, F.M.B. Oxygen ionic and electronic transport in apatite-type La10-x(Si,Al)6O27+δ. J. Solid State Chem. 2005, 178, 2050–2061. [Google Scholar] [CrossRef]
- Kharton, V.V.; Shaula, A.L.; Patrakeev, M.V.; Waerenborgh, J.C.; Rojas, D.P.; Vyshatko, N.P.; Tsipis, E.V.; Yaremchenko, A.A.; Marques, F.M.B. Oxygen ionic and electronic transport in apatite-type solid electrolytes. J. Electrochem. Soc. 2004, 151, A1236–A1246. [Google Scholar] [CrossRef]
- Yoshioka, H.; Nojiri, Y.; Tanase, S. Ionic conductivity and fuel cell properties of apatite-type lanthanum silicates doped with Mg and containing excess oxide ions. Solid State Ion. 2008, 179, 2165–2169. [Google Scholar] [CrossRef]
- Mineshige, A.; Hayakawa, H.; Nishimoto, T.; Heguri, A.; Yazawa, T.; Takayama, Y.; Kagoshima, Y.; Takano, H.; Takeda, S.; Matsui, J. Preparation of lanthanum silicate electrolyte with high conductivity and high chemical stability. Solid State Ion. 2018, 319, 223–227. [Google Scholar] [CrossRef]
- Nakayama, S.; Aono, H.; Sadaoka, Y. Ionic conductivity of Ln10(SiO4)6O3 (Ln = La, Nd, Sm, Gd and Dy). Chem. Lett. 1995, 431–432. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Kovalevsky, A.V.; Kharton, V.V. Mixed conductivity, stability and electrochemical behavior of perovskite-type (Sr0.7Ce0.3)1-xMn1-yCryO3-δ. Solid State Ion. 2008, 179, 2181–2191. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Bannikov, D.O.; Kovalevsky, A.V.; Cherepanov, V.A.; Kharton, V.V. High-temperature transport properties, thermal expansion and cathodic performance of Ni-substituted LaSr2Mn2O7-δ. J. Solid State Chem. 2008, 181, 3024–3032. [Google Scholar] [CrossRef]
- Brisse, A.; Sauvet, A.L.; Barthet, C.; Beaudet-Savignat, S.; Fouletier, J. Electrochemical characterizations of Ni/doped lanthanum silicates cermets deposited by spin coating. Fuel Cells 2006, 6, 59–63. [Google Scholar] [CrossRef]
- Gasparyan, H.; Argirusis, C.; Szepanski, C.; Sourkouni, G.; Stathopoulos, V.; Kharlamova, T.; Sadykov, V.; Bebelis, S. Electrochemical characterization of a La0.8Sr0.2Ni0.4Fe0.6O3-δ electrode interfaced with La9.83Si5Al0.75Fe0.25O26±δ apatite-type electrolyte. ECS Trans. 2009, 25, 2681–2688. [Google Scholar] [CrossRef]
- Philippeau, B.; Mauvy, F.; Mazataud, C.; Fourcade, S.; Grenier, J.C. Comparative study of electrochemical properties of mixed conducting Ln2NiO4+δ (Ln = La, Pr and Nd) and La0.6Sr0.4Fe0.8Co0.2O3-δ as SOFC cathodes associated to Ce0.9Gd0.1O2-δ, La0.8Sr0.2Ga0.8Mg0.2O3-δ and La9Sr1Si6O26.5 electrolytes. Solid State Ion. 2013, 249-250, 17–25. [Google Scholar] [CrossRef]
- Zhou, J.; Ye, X.F.; Li, J.L.; Wang, S.R.; Wen, T.L. Synthesis and characterization of apatite-type La9.67Si6-xAlxO26.5-x/2 electrolyte materials and compatible cathode materials. Solid State Ion. 2011, 201, 81–86. [Google Scholar] [CrossRef]
- Bonhomme, C.; Beaudet-Savignat, S.; Chartier, T.; Geffroy, P.M.; Sauvet, A.L. Evaluation of the La0.75Sr0.25Mn0.8Co0.2O3-δ system as cathode material or IT-SOFCs with La9Sr1Si6O26.5 apatite as electrolyte. J. Eur. Ceram. Soc. 2009, 29, 1781–1788. [Google Scholar] [CrossRef]
- Tsipis, E.V.; Kharton, V.V.; Frade, J.R. Electrochemical behavior of mixed-conducting oxide cathodes in contact with apatite-type La10Si5AlO26.5 electrolyte. Electrochim. Acta 2007, 52, 4428–4435. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Kharton, V.V.; Bannikov, D.O.; Znosak, D.V.; Frade, J.R.; Cherepanov, V.A. Performance of perovskite-related oxide cathodes in contact with lanthanum silicate electrolyte. Solid State Ion. 2009, 180, 878–885. [Google Scholar] [CrossRef]
- Cao, X.G.; Jiang, S.P. Identification of oxygen reduction processes at (La,Sr)MnO3 electrode/La9.5Si6O26.25 apatite electrolyte interface of solid oxide fuel cells. Int. J. Hydrogen Energy 2013, 38, 2421–2431. [Google Scholar] [CrossRef]
- McFarlane, J.; Barth, S.; Swaffer, M.; Sansom, J.E.H.; Slater, P.R. Synthesis and conductivities of the apatite-type systems, La9.33+xSi6-yMyO26+z (M = Co, Fe, Mn) and La8Mn2Si6O26. Ionics 2002, 8, 149–154. [Google Scholar] [CrossRef]
- Antonova, E.P.; Osinkin, D.A.; Bogdanovich, N.M.; Gorshkov, M.Y.; Bronin, D.I. Electrochemical performance of Ln2NiO4+δ (Ln–La, Nd, Pr) and Sr2Fe1.5Mo0.5O6-δ oxide electrodes in contact with apatite-type La10(SiO6)4O3 electrolyte. Solid State Ion. 2019, 329, 82–89. [Google Scholar] [CrossRef]
- Robles-Fernandez, A.; Orera, A.; Merino, R.I.; Slater, P.R. Suitability of strontium and cobalt-free perovskite cathodes with La9.67Si5AlO26 apatite electrolyte for intermediate temperature solid oxide fuel cells. Dalton Trans. 2020, 49, 14280–14289. [Google Scholar] [CrossRef]
- Das, A.; Xhafa, E.; Nikolla, E. Electro- and thermal-catalysis by layered, first series Ruddlesden-Popper oxides. Catal. Today 2016, 277, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden-Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, J.G.; Medvedev, D.A.; Bi, L.; Yaremchenko, A.A. Recent advances in layered Ln2NiO4+δ nickelates: Fundamentals and prospects of their applications in protonic ceramic fuel and electrolysis cells. J. Mater. Chem. A 2021, 9, 154–195. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.N. Controlling oxygen mobility in Ruddlesden-Popper oxides. Materials 2017, 10, 368. [Google Scholar] [CrossRef]
- Ding, P.; Li, W.; Zhao, H.; Wu, C.; Zhao, L.; Dong, B.; Wang, S. Review on Ruddlesden-Popper perovskites as cathode for solid oxide fuel cells. J. Phys. Mater. 2021, 4, 022002. [Google Scholar] [CrossRef]
- Hancock, C.A.; Porras-Vazquez, J.M.; Keenan, P.J.; Slater, P.R. Oxyanions in perovskites: From superconductors to solid oxide fuel cells. Dalton Trans. 2015, 44, 10559–10569. [Google Scholar] [CrossRef] [Green Version]
- Hancock, C.A.; Slater, P.R. Synthesis of silicon doped SrMO3 (M = Mn, Co): Stabilization of the cubic perovskite and enhancement in conductivity. Dalton Trans. 2011, 40, 5599–5603. [Google Scholar] [CrossRef]
- Porras-Vazquez, J.M.; Pike, T.; Hancock, C.A.; Marco, J.F.; Berrya, F.J.; Slater, P.R. Investigation into the effect of Si doping on the performance of SrFeO3-δ SOFC electrode materials. J. Mater. Chem. A 2013, 1, 11834–11841. [Google Scholar] [CrossRef] [Green Version]
- Merkulov, O.V.; Markova, A.A.; Patrakeev, M.V.; Chukin, A.V.; Leonidov, I.A.; Kozhevnikov, V.L. Structural features and high-temperature properties of SrFe1-xSixO3-δ. Solid State Ion. 2016, 292, 83–87. [Google Scholar] [CrossRef]
- Porras-Vazquez, J.M.; Losilla, E.R.; Keenan, P.J.; Hancock, C.A.; Kemp, T.F.; Hanna, J.V.; Slater, P.R. Investigation into the effect of Si doping on the performance of Sr1-yCayMnO3-δ SOFC cathode materials. Dalton Trans. 2013, 42, 5421–5429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porras-Vazquez, J.M.; Smith, R.I.; Slater, P.R. Investigation into the effect of Si doping on the cell symmetry and performance of Sr1-yCayFeO3-δ SOFC cathode materials. J. Solid State Chem. 2014, 213, 132–137. [Google Scholar] [CrossRef]
- Smith, A.D.; James, M.S.; Slater, P.R. Effect of Si-doping on the structure and conductivity of (Sr/Ca)2MnFeO6-δ systems. ECS Trans. 2019, 91, 1425–1436. [Google Scholar] [CrossRef] [Green Version]
- Porras-Vazquez, J.M.; Slater, P.R. Synthesis and characterization of oxyanion-doped cobalt containing perovskites. Fuel Cells 2012, 12, 1056–1063. [Google Scholar] [CrossRef]
- Kharton, V.V.; Tikhonovich, V.N.; Shuangbao, L.; Naumovich, E.N.; Kovalevsky, A.V.; Viskup, A.P.; Bashmakov, I.A.; Yaremchenko, A.A. Ceramic microstructure and oxygen permeability of SrCo(Fe,M)O3-δ (M = Cu or Cr) perovskite membranes. J. Electrochem. Soc. 1998, 145, 1363–1374. [Google Scholar] [CrossRef]
- Kovalevsky, A.V.; Yaremchenko, A.A.; Kolotygin, V.A.; Shaula, A.L.; Kharton, V.V.; Snijkers, F.M.M.; Buekenhoudt, A.; Frade, J.R.; Naumovich, E.N. Processing and oxygen permeation studies of asymmetric multilayer Ba0.5Sr0.5Co0.8Fe0.2O3-δ membranes. J. Membr. Sci. 2011, 380, 68–80. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Shaula, A.L.; Kharton, V.V.; Waerenborgh, J.C.; Rojas, D.P.; Vyshatko, N.P.; Patrakeev, M.V.; Marques, F.M.B. Ionic and electronic conductivity of La9.83-xPrxSi4.5Fe1.5O26±δ apatites. Solid State Ion. 2004, 171, 51–59. [Google Scholar] [CrossRef]
- Gale, J.D. GULP—A computer program for the symmetry adapted simulation of solids. J. Chem. Soc. Faraday Trans. 1997, 93, 629–637. [Google Scholar] [CrossRef]
- Gale, J.D.; Rohl, A.L. The general utility lattice program (GULP). Mol. Simul. 2003, 29, 291–341. [Google Scholar] [CrossRef]
- Purton, J.A.; Crabtree, J.C.; Parker, S.C. DL_MONTE: A general purpose program for parallel Monte Carlo simulation. Mol. Simul. 2013, 39, 1240–1252. [Google Scholar] [CrossRef]
- Brukhno, A.V.; Grant, J.; Underwood, T.L.; Stratford, K.; Parker, S.C.; Purton, J.A.; Wilding, N.B. DL_MONTE: A multipurpose code for Monte Carlo simulation. Mol. Simul. 2021, 47, 131–151. [Google Scholar] [CrossRef] [Green Version]
- Tolchard, J.R.; Islam, M.S.; Slater, P.R. Defect chemistry and oxygen ion migration in the apatite-type materials La9.33Si6O26 and La8Sr2Si6O26. J. Mater. Chem. 2003, 13, 1956–1961. [Google Scholar] [CrossRef] [Green Version]
- DFRL-UCL Potentials Library: Ni. Available online: https://www.ucl.ac.uk/klmc/Potentials/Ni/index.html (accessed on 16 November 2021).
- DFRL-UCL Potentials Library: Silicon. Available online: https://www.ucl.ac.uk/klmc/Potentials/Si/index.html (accessed on 16 November 2021).
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Kang, S.J.L. Sintering. Densification, Grain Growth, and Microstructure; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands, 2005; pp. 95–103. [Google Scholar]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2-xNiO4+δ oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kolchugin, A.A.; Sadykov, V.A.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Bogdanovich, N.M. Structure, transport properties and electrochemical behavior of the layered lanthanide nickelates doped with calcium. Int. J. Hydrogen Energy 2018, 43, 17373–17386. [Google Scholar] [CrossRef]
- Ternero, F.; Rosa, L.G.; Urban, P.; Montes, J.M.; Cuevas, F.G. Influence of the total porosity on the properties of sintered materials—A review. Metals 2021, 11, 730. [Google Scholar] [CrossRef]
- Shaula, A.L.; Naumovich, E.N.; Viskup, A.P.; Pankov, V.V.; Kovalevsky, A.V.; Kharton, V.V. Oxygen transport in La2NiO4+δ: Assessment of surface limitations and multilayer membrane architectures. Solid State Ion. 2009, 180, 812–816. [Google Scholar] [CrossRef]
- Nakamura, T.; Yashiro, K.; Sato, K.; Mizusaki, J. Electronic state of oxygen nonstoichiometric La2-xSrxNiO4+δ at high temperatures. Phys. Chem. Chem. Phys. 2009, 11, 3055–3062. [Google Scholar] [CrossRef]
- Merkulov, O.V.; Naumovich, E.N.; Patrakeev, M.V.; Markov, A.A.; Shalaeva, E.V.; Kharton, V.V.; Tsipis, E.V.; Waerenborgh, J.C.; Leonidov, I.A.; Kozhevnikov, V.L. Defect formation, ordering, and transport in SrFe1-xSixO3-δ (x = 0.05–0.20). J. Solid State Electrochem. 2018, 22, 727–737. [Google Scholar] [CrossRef]
- Viitanen, M.M.; Welzenis, R.G.v.; Brongersma, H.H.; van Berkel, F.P.F. Silica poisoning of oxygen membranes. Solid State Ion. 2002, 150, 223–228. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Li, M.; Li, W.; Yang, W. Degradation and stabilization of perovskite membranes containing silicon impurity at low temperature. J. Membr. Sci. 2015, 492, 173–180. [Google Scholar] [CrossRef]
- Carvalho, T.; Antunes, E.; Calado, J.; Figueiredo, F.M.; Frade, J.R. Lanthanum oxide as a scavenging agent for zirconia electrolytes. Solid State Ion. 2012, 225, 484–487. [Google Scholar] [CrossRef]
- Ivanova, D.; Lima, E.M.C.L.G.P.; Kovalevsky, A.; Figueiredo, F.M.L.; Kharton, V.V.; Marques, F.M.B. Heterogeneous ceramics formed by grain boundary engineering. Ionics 2008, 14, 349–356. [Google Scholar] [CrossRef]


| Interaction | Buckingham Term | Core-Shell Term | Three-Body Term | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| A, eV | ρ, Å | C, Å−6 | Y, e | ksp, eV Å−2 | k3b, eV rad−2 | θ0, ° | ||
| O2−-O2− | 22,764.3 | 0.149 | 27.89 | −2.860 | 74.92 | [48] | ||
| La3+-O2− | 4579.23 | 0.30437 | - | −0.250 | 145.00 | [48] | ||
| Ni2+-O2− | 1582.50 | 0.28820 | - | - | - | [49] | ||
| Si4+-O2− | 1283.91 | 0.32052 | 10.66 | - | - | [48] | ||
| O2−-Si4+-O2− | 2.097240 | 109.47 | [50] | |||||
| Composition | Unit Cell Parameters | Density ρexp, g/cm3 | Relative Density ρexp/ρtheor, % 1 | ||
|---|---|---|---|---|---|
| a, Å | c, Å | V, Å3 | |||
| 0 | 3.8636(1) | 12.6662(2) | 189.07(1) | 6.82 | 96.3 |
| 0.02 | 3.8611(1) | 12.6784(4) | 189.02(1) | 6.73 | 95.2 |
| 0.05 | 3.8610(1) | 12.6831(4) | 189.08(1) | 5.60 | 79.5 |
| Cation Composition | Supercell | Lattice Energy, eV/f.u. | Formation Energy |
|---|---|---|---|
| La2O3 | 1 × 1 × 1 | −129.92 | |
| La2NiO4 | 3 × 3 × 2 | −171.40 | |
| La9.33Si6O26 | 3 × 3 × 3 | −1395.06 | |
| La2Ni0.972Si0.028O4.028 | 3 × 3 × 2, config.(i) | −173.66 1 | 0.24 |
| 3 × 3 × 2, config.(ii) | −173.64 | 0.26 | |
| 3 × 3 × 2, config.(iii) | −173.61 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharchuk, K.; Bamburov, A.; Naumovich, E.N.; Vieira, M.A.; Yaremchenko, A.A. Impact of Silica Additions on the Phase Composition and Electrical Transport Properties of Ruddlesden-Popper La2NiO4+δ Mixed Conducting Ceramics. Processes 2022, 10, 82. https://doi.org/10.3390/pr10010082
Zakharchuk K, Bamburov A, Naumovich EN, Vieira MA, Yaremchenko AA. Impact of Silica Additions on the Phase Composition and Electrical Transport Properties of Ruddlesden-Popper La2NiO4+δ Mixed Conducting Ceramics. Processes. 2022; 10(1):82. https://doi.org/10.3390/pr10010082
Chicago/Turabian StyleZakharchuk, Kiryl, Aleksandr Bamburov, Eugene N. Naumovich, Miguel A. Vieira, and Aleksey A. Yaremchenko. 2022. "Impact of Silica Additions on the Phase Composition and Electrical Transport Properties of Ruddlesden-Popper La2NiO4+δ Mixed Conducting Ceramics" Processes 10, no. 1: 82. https://doi.org/10.3390/pr10010082
APA StyleZakharchuk, K., Bamburov, A., Naumovich, E. N., Vieira, M. A., & Yaremchenko, A. A. (2022). Impact of Silica Additions on the Phase Composition and Electrical Transport Properties of Ruddlesden-Popper La2NiO4+δ Mixed Conducting Ceramics. Processes, 10(1), 82. https://doi.org/10.3390/pr10010082








