Computational Analysis of Zingiber officinale Identifies GABAergic Signaling as a Potential Therapeutic Mechanism in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. CC Cohort Data Collection and Acquisition
2.2. Gene Expression Analysis and DEGs Identification
2.3. Ginger Chemical Components and Target Selection
2.4. DEGs Overlapping of CC and Ginger Targets Using Venn Diagrams
2.5. Biological Function and Pathway Analysis
2.6. Modular Clustering Analysis
2.7. Protein–Protein Network Construction and Hub-Gene Selection
2.8. Network Construction of Ginger-Component Target Interactions
2.9. Verification by Molecular Docking Simulation
3. Results
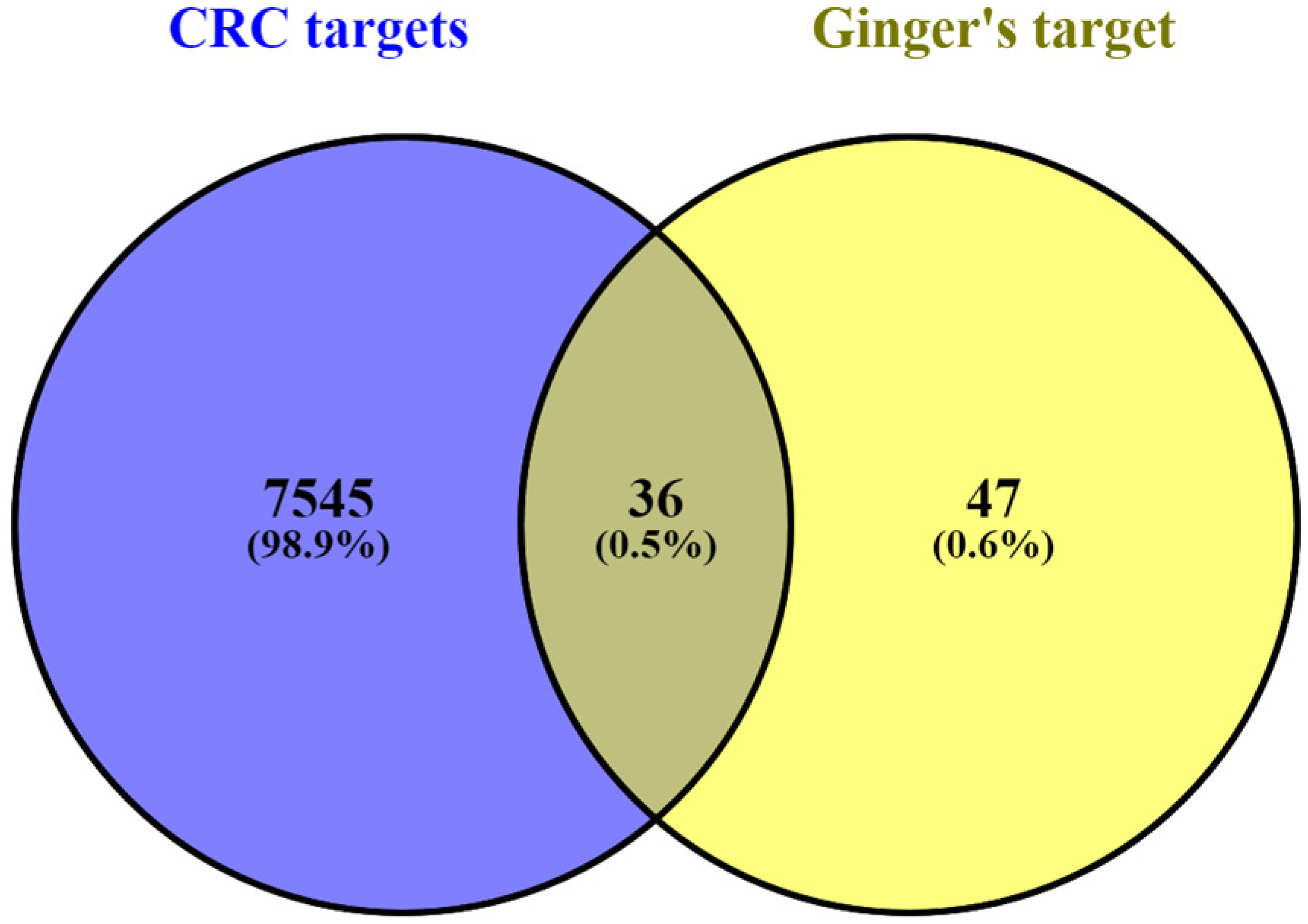
3.1. Screening and Collection of Ginger Target Genes
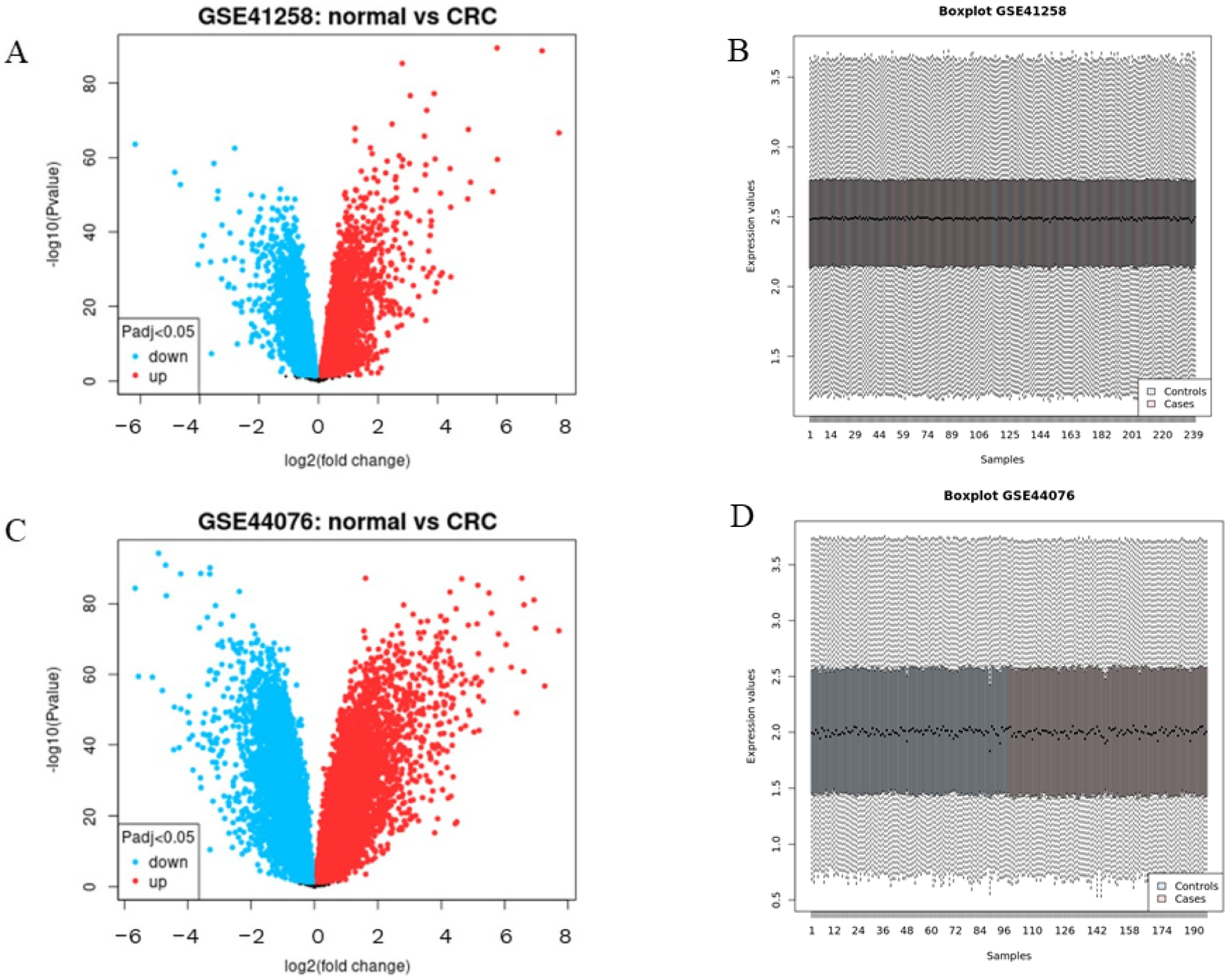
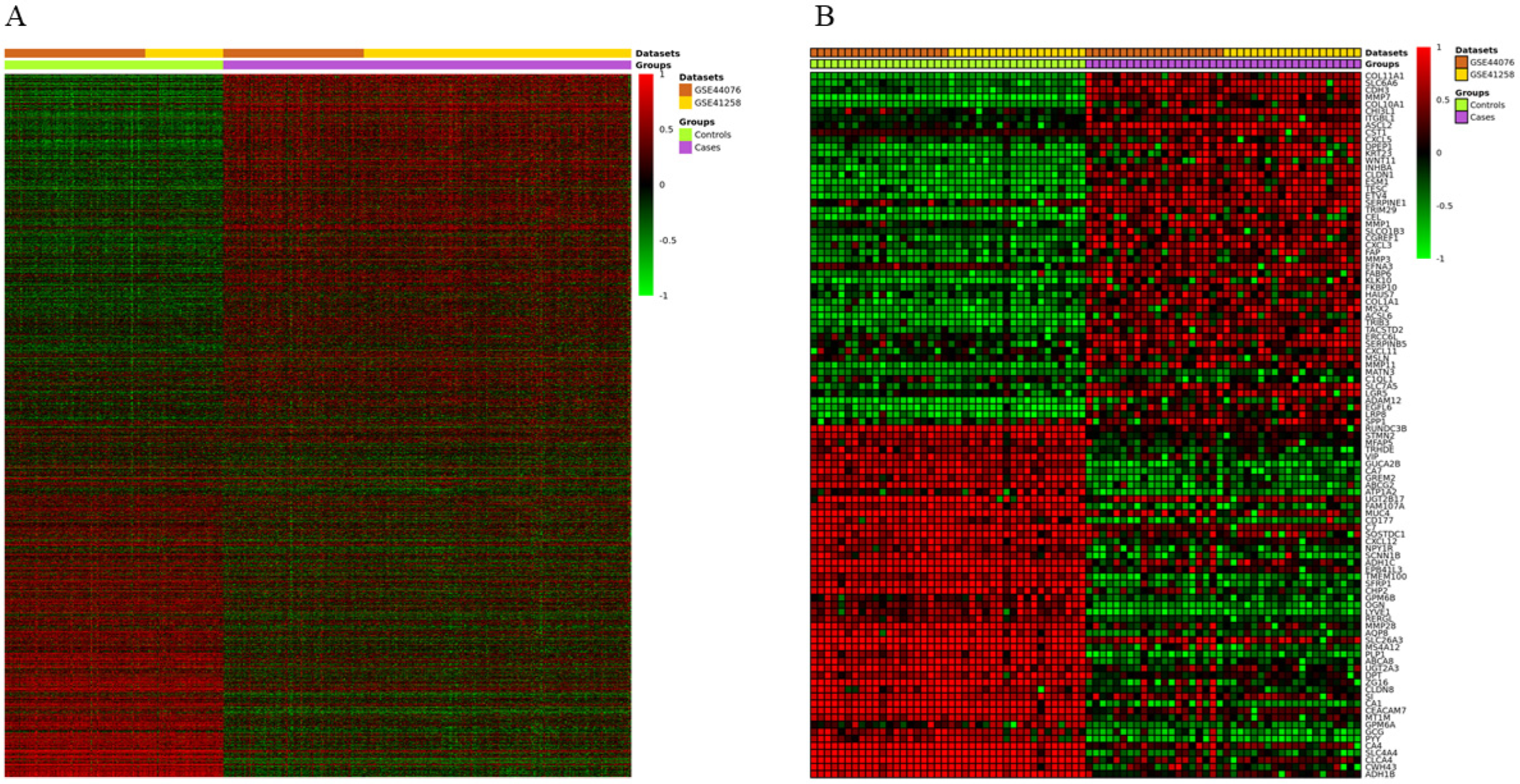
3.2. Identification of DEGs in CC
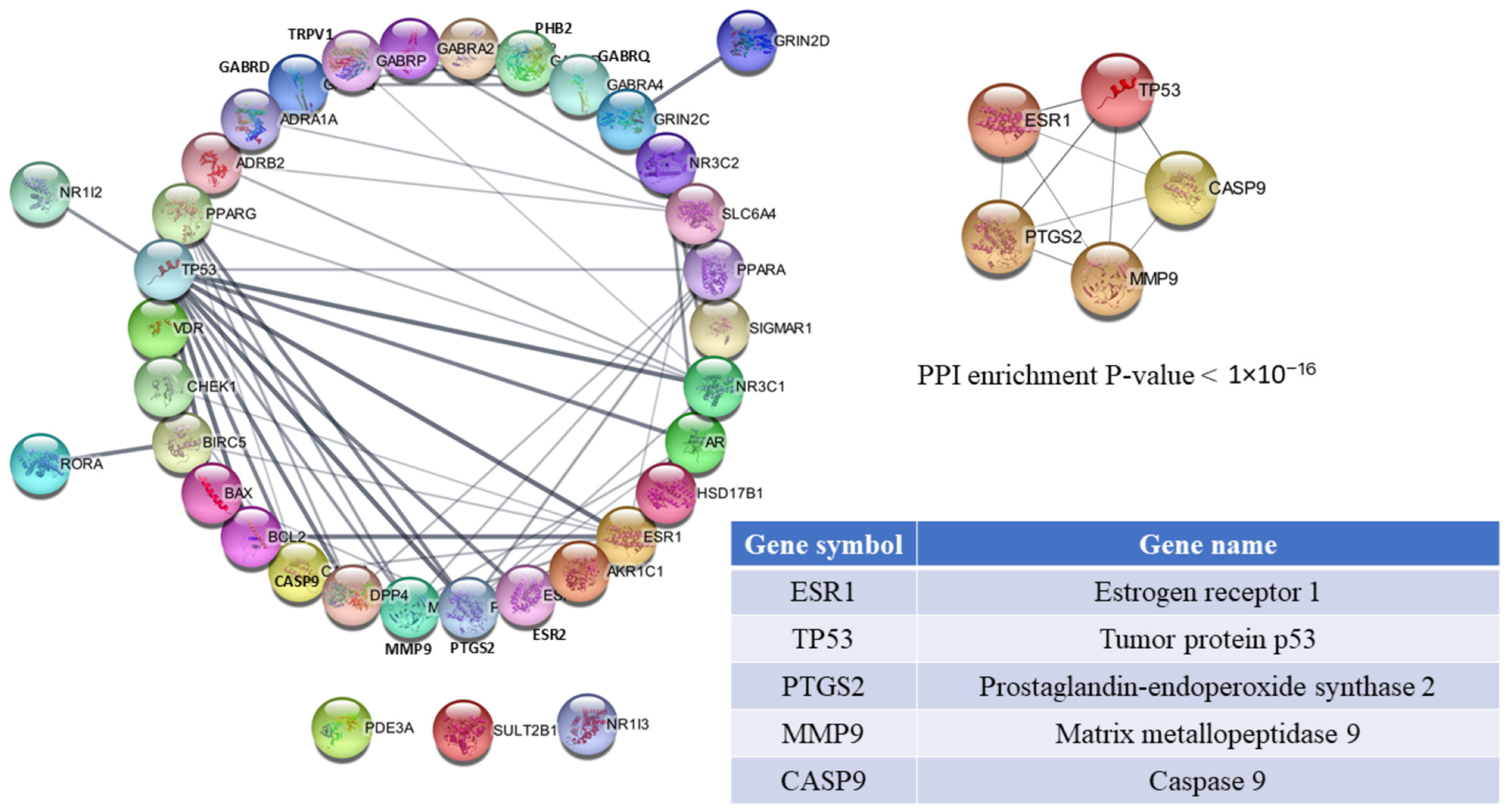
3.3. PPI Network Analysis and Hub Gene Selection
3.4. Gene Ontology Enrichment Analysis
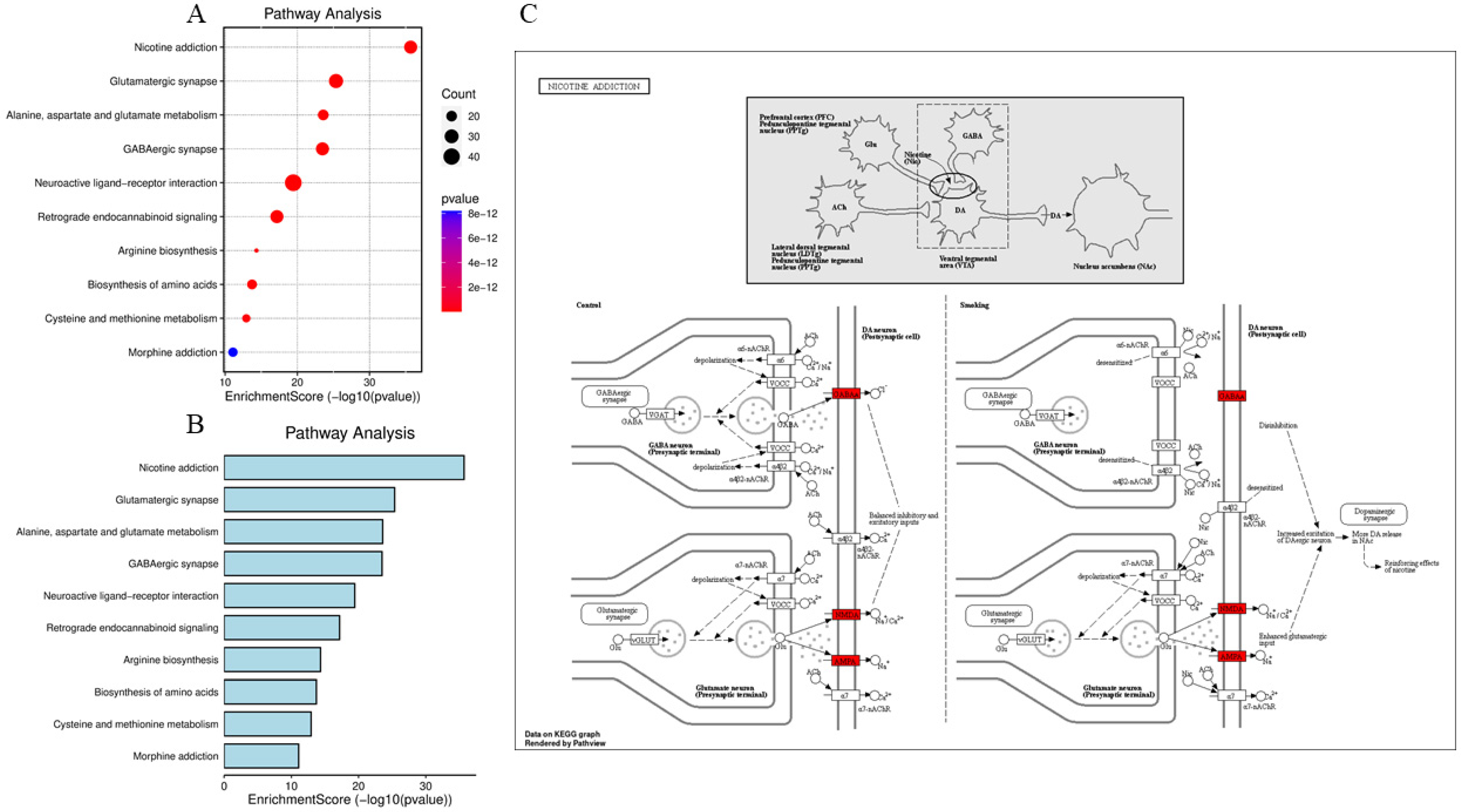
3.5. KEGG Pathway Analysis
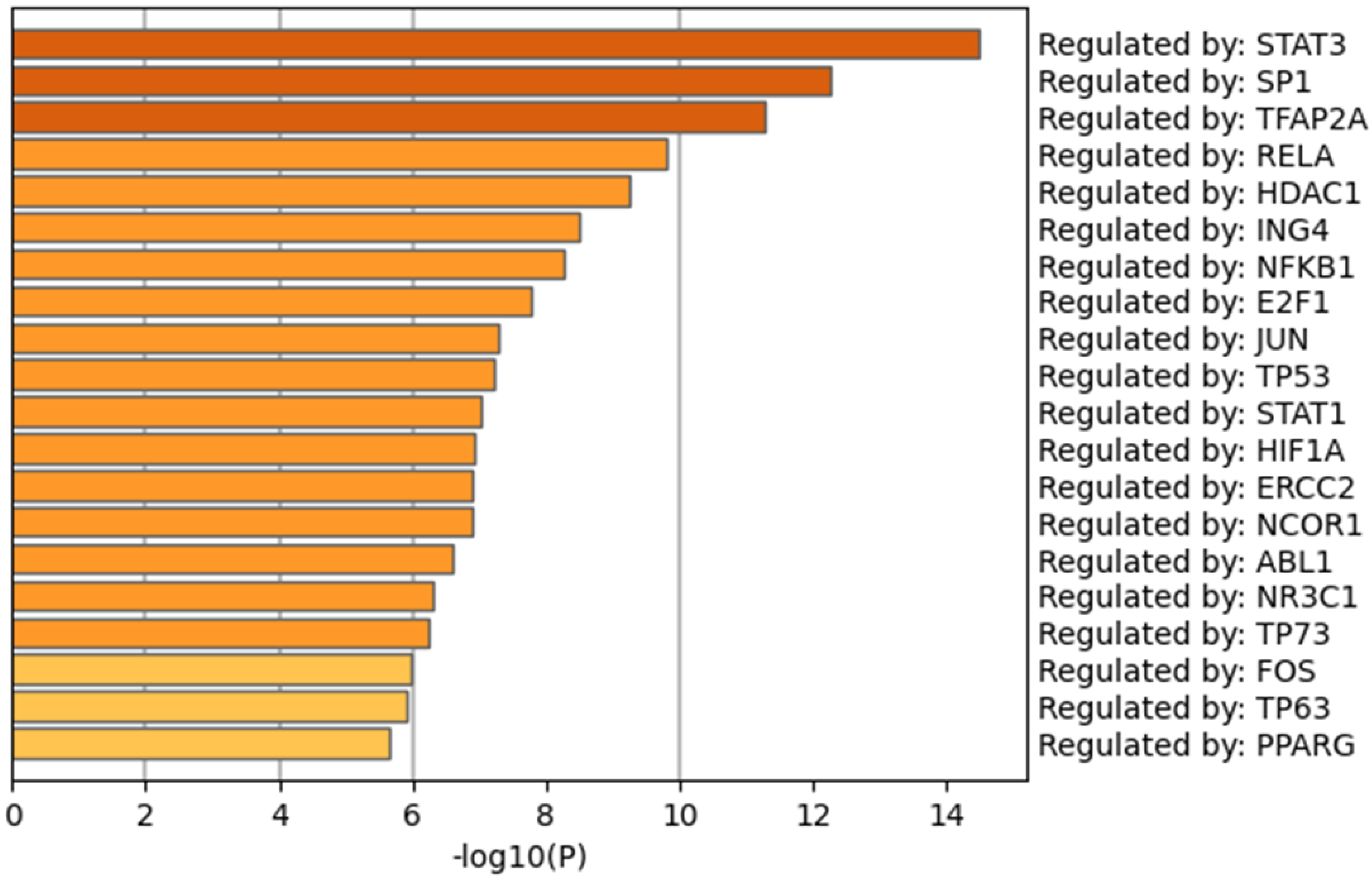
3.6. Transcription Factors (TFs) Analysis
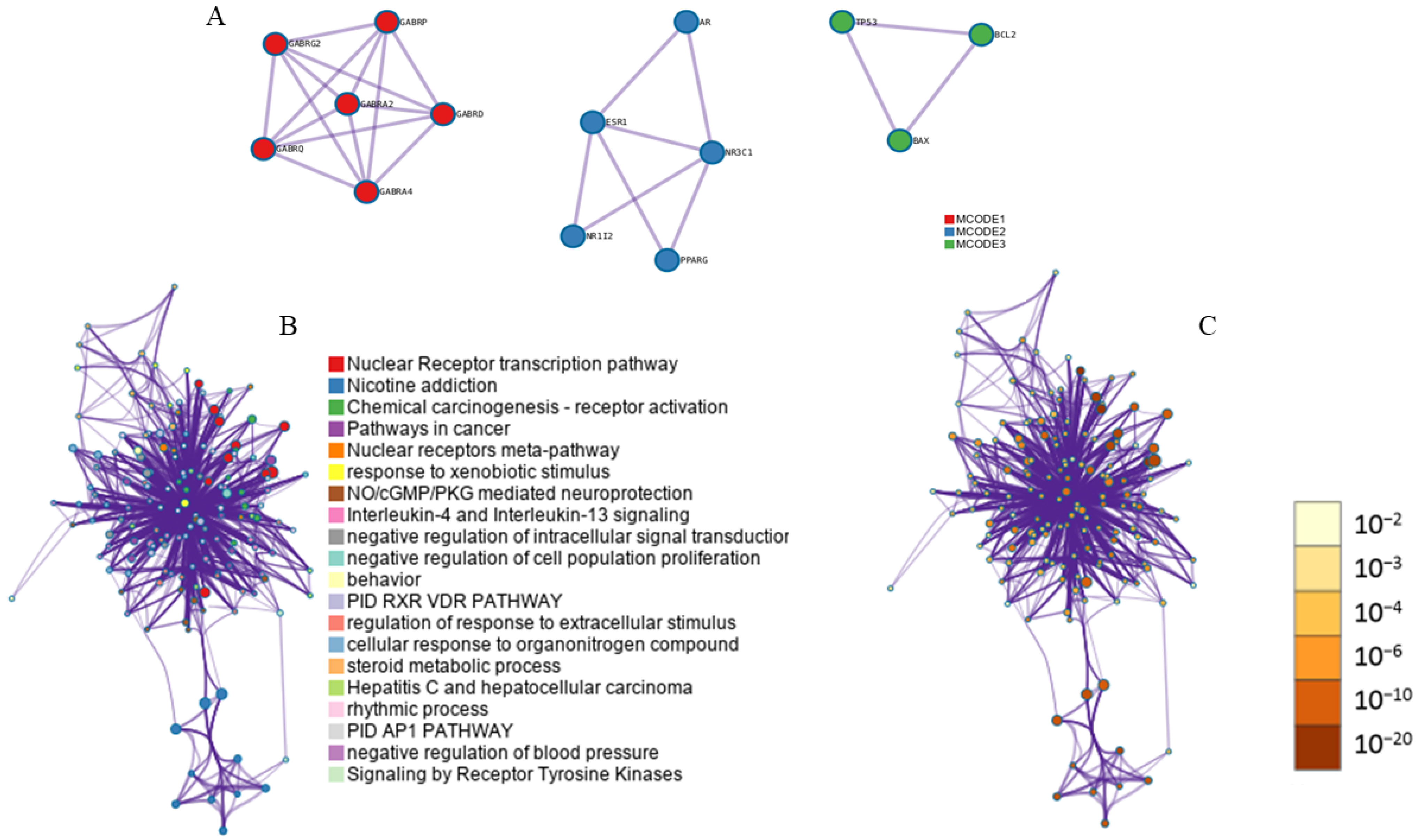
3.7. Module-Based Network Analysis
3.8. Construction of Putative Components-Targets-Pathways-Diseases
3.9. Validation Using Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General Insight into Cancer: An Overview of Colorectal Cancer. Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- Sifaki-Pistolla, D.; Poimenaki, V.; Fotopoulou, I.; Saloustros, E.; Mavroudis, D.; Vamvakas, L.; Lionis, C. Significant Rise of Colorectal Cancer Incidence in Younger Adults and Strong Determinants: 30 Years Longitudinal Differences between Under and Over 50s. Cancers 2022, 14, 4799. [Google Scholar] [CrossRef] [PubMed]
- Didkowska, J.; Wojciechowska, U.; Michalek, I.M.; Caetano dos Santos, F.L. Cancer Incidence and Mortality in Poland in 2019. Sci. Rep. 2022, 12, 10875. [Google Scholar] [CrossRef]
- Winner, M.; Mooney, S.J.; Hershman, D.L.; Feingold, D.L.; Allendorf, J.D.; Wright, J.D.; Neugut, A.I.M. Management and Outcomes of Bowel Obstruction in Patients with Stage IV Colon Cancer: A Population-Based Cohort Study. Dis. Colon Rectum 2013, 56, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Drummond, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.J.; Chen, B.; Kumar, N. Prospective of Colon Cancer Treatments and Scope for Combinatorial Approach to Enhanced Cancer Cell Apoptosis. Crit. Rev. Oncol. Hematol. 2013, 86, 232–250. [Google Scholar] [CrossRef]
- Pissarra, A.; Malheiro, M.; Matos, L.V.; Plácido, A.N. Severe Rhabdomyolysis Related to Oxaliplatin Adjuvant Therapy for Colorectal Cancer. BMJ Case Rep. 2019, 12, e228673. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Shi, D.; Khan, F.; Abagyan, R. Extended Multitarget Pharmacology of Anticancer Drugs. J. Chem. Inf. Model. 2019, 59, 3006–3017. [Google Scholar] [CrossRef]
- Marshall, A.C. Traditional Chinese Medicine and Clinical Pharmacology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Zhang, Q.Y.; Wang, F.X.; Jia, K.K.; Kong, L.D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Masuku, N.P.; Paimo, O.K.; Lebelo, S.L. Ginger from Farmyard to Town: Nutritional and Pharmacological Applications. Front. Pharmacol. 2021, 12, 779352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kou, X.; Zhao, H.; Mak, K.K.; Balijepalli, M.K.; Pichika, M.R. Zingiber officinale var. rubrum: Red Ginger’s Medicinal Uses. Molecules 2022, 27, 775. [Google Scholar] [CrossRef]
- Zhang, G.B.; Li, Q.Y.; Chen, Q.L.; Su, S.B. Network Pharmacology: A New Approach for Chinese Herbal Medicine Research. Evid.-Based Complement. Altern. Med. 2013, 2013, 621423. [Google Scholar] [CrossRef]
- Chu, X.; Sun, B.; Huang, Q.; Peng, S.; Zhou, Y.; Zhang, Y. Quantitative Knowledge Presentation Models of Traditional Chinese Medicine (TCM): A Review. Artif. Intell. Med. 2020, 103, 101810. [Google Scholar] [CrossRef]
- Sheffer, M.; Bacolod, M.D.; Zuk, O.; Giardina, S.F.; Pincas, H.; Barany, F.; Paty, P.B.; Gerald, W.L.; Notterman, D.A.; Domany, E. Association of Survival and Disease Progression with Chromosomal Instability: A Genomic Exploration of Colorectal Cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 7131–7136. [Google Scholar] [CrossRef]
- Díez-Villanueva, A.; Sanz-Pamplona, R.; Solé, X.; Cordero, D.; Crous-Bou, M.; Guinó, E.; Lopez-Doriga, A.; Berenguer, A.; Aussó, S.; Paré-Brunet, L. COLONOMICS-Integrative Omics Data of One Hundred Paired Normal-Tumoral Samples from Colon Cancer Patients. Sci. Data 2022, 9, 595. [Google Scholar] [CrossRef]
- Toro-Domínguez, D.; Martorell-Marugán, J.; López-Domínguez, R.; García-Moreno, A.; González-Rumayor, V.; Alarcón-Riquelme, M.E.; Carmona-Sáez, P. ImaGEO: Integrative Gene Expression Meta-Analysis from GEO Database. Bioinformatics 2019, 35, 880–882. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A Web Server for Target Prediction of Bioactive Small Molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W. An Automated Method for Finding Molecular Complexes in Large Protein Interaction Networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a Protein-Small Molecule Docking Web Service Based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Han, X.; Zhang, A.; Meng, Z.; Wang, Q.; Liu, S.; Wang, Y.; Tan, J.; Guo, L.; Li, F. Bioinformatics Analysis Based on Extracted Ingredients Combined with Network Pharmacology, Molecular Docking and Molecular Dynamics Simulation to Explore the Mechanism of Jinbei Oral Liquid in the Therapy of Idiopathic Pulmonary Fibrosis. Heliyon 2024, 10, e38173. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F. Colon Cancer, Version 2.2018: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2018, 16, 359. [Google Scholar] [CrossRef]
- Cho, E.; Lee, J.E.; Rimm, E.B.; Fuchs, C.S.; Giovannucci, E.L. Alcohol Consumption and the Risk of Colon Cancer by Family History of Colorectal Cancer. Am. J. Clin. Nutr. 2012, 95, 413–419. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese Medicine as a Cancer Treatment: Modern Perspectives of Ancient but Advanced Science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial Intelligence in Drug Discovery and Development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-S.; Wu, C.C. Systems Biology as an Integrated Platform for Bioinformatics, Systems Synthetic Biology, and Systems Metabolic Engineering. Cells 2013, 2, 635–688. [Google Scholar] [CrossRef]
- Chandran, U.; Mehendale, N.; Patil, S.; Chaguturu, R.; Patwardhan, B. Network Pharmacology. In Innovative Approaches in Drug Discovery; Academic Press: Cambridge, MA, USA, 2017; pp. 127–164. [Google Scholar] [CrossRef]
- Man, X.; Sai, Z. Study on the Prognosis Effect of Traditional Chinese Medicine Treatment in DR Patients Based on the Perspective of Network Pharmacology. CMMI 2022, 2022, 3528732. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, X.; Xu, J.; Li, C.; Yu, Y.; Wang, W.; Zhu, L. The Protective Effect of Dietary Phytosterols on Cancer Risk: A Systematic Meta-Analysis. J. Oncol. 2019, 2019, 7479518. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, Z.; Liu, J.; Hong, L. β-Sitosterol as a Promising Anticancer Agent for Chemoprevention and Chemotherapy: Mechanisms of Action and Future Prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef]
- Rajavel, T.; Packiyaraj, P.; Suryanarayanan, V.; Singh, S.K.; Ruckmani, K.; Pandima Devi, K. β-Sitosterol Targets Trx/Trx1 Reductase to Induce Apoptosis in A549 Cells via ROS Mediated Mitochondrial Dysregulation and p53 Activation. Sci. Rep. 2018, 8, 2071. [Google Scholar] [CrossRef]
- Awad, A.; Chen, Y.C.; Fink, C.; Hennessey, T. Beta-Sitosterol Inhibits HT-29 Human Colon Cancer Cell Growth and Alters Membrane Lipids. Anticancer Res. 1996, 16, 2797–2804. [Google Scholar]
- Mohan, J.; Gandhi, A.A.; Bhavya, B.C.; Rashmi, R.; Karunagaran, D.; Indu, R.; Santhoshkumar, T.R. Caspase-2 Triggers Bax-Bak-Dependent and -Independent Cell Death in Colon Cancer Cells Treated with Resveratrol. J. Biol. Chem. 2006, 281, 17599–17611. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yuan, H.B.; Zhao, S.; Liu, F.F.; Jiang, Y.Q.; Guo, Y.X.; Wan, X.L. Activation of GABAB Receptor Suppresses Diabetic Neuropathic Pain through Toll-Like Receptor 4 Signaling Pathway in the Spinal Dorsal Horn. Mediat. Inflamm. 2018, 2018, 6016272. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Sun, Z.; Chen, W.; Miao, C. GABAB Receptor Inhibits Tumor Progression and Epithelial-Mesenchymal Transition via the Regulation of Hippo/YAP1 Pathway in Colorectal Cancer. Int. J. Biol. Sci. 2021, 17, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Jiang, J.; Tang, Y.L.; Liang, X.H. Insights into the Leveraging of GABAergic Signaling in Cancer Therapy. Cancer Med. 2023, 12, 14498–14510. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, L.; Li, W.; Zhang, Y.; Zhang, S.; Ge, B.; Yang, H.; Du, G.; Tang, B.; Wang, H.; et al. GABAergic Signaling as a Potential Therapeutic Target in Cancers. Biomed. Pharmacother. 2023, 161, 114410. [Google Scholar] [CrossRef]
- Waggas, A.M. Neuroprotective Evaluation of Extract of Ginger (Zingiber officinale) Root in Monosodium Glutamate-Induced Toxicity in Different Brain Areas Male Albino Rats. Pak. J. Biol. Sci. 2009, 12, 201–212. [Google Scholar] [CrossRef]
- Schepici, G.; Contestabile, V.; Valeri, A.; Mazzon, E. Ginger, a Possible Candidate for the Treatment of Dementias? Molecules 2021, 26, 5700. [Google Scholar] [CrossRef]
- Schuller, H.M. Is Cancer Triggered by Altered Signalling of Nicotinic Acetylcholine Receptors? Nat. Rev. Cancer 2009, 9, 195–205. [Google Scholar] [CrossRef]
- West, K.A.; Brognard, J.; Clark, A.S.; Linnoila, I.R.; Yang, X.; Swain, S.M.; Harris, C.; Belinsky, S.; Dennis, P.A. Rapid Akt Activation by Nicotine and A Tobacco Carcinogen Modulates the Phenotype of Normal Human Airway Epithelial Cells. J. Clin. Investig. 2003, 111, 81–90. [Google Scholar] [CrossRef]
- Schuller, H.M.; Al-Wadei, H.A.; Majidi, M. GABA B Receptor is a Novel Drug Target for Pancreatic Cancer. Cancer 2008, 112, 767–778. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA Receptors in the Gastrointestinal Tract: From Motility to Inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Majidi, M.; Al-Wadei, H.A.; Takahashi, T.; Schuller, H.M. Nongenomic Beta Estrogen Receptors Enhance Nicotine-Induced Proliferation in Human Small Cell Lung Carcinoma Cells. Lung Cancer 2009, 65, 97–104. [Google Scholar]
- Al-Wadei, H.A.; Schuller, H.M. Nicotine Stimulates Pancreatic Cancer Xenografts by Systemic Increase in Stress Neurotransmitters and Suppression of GABA. Oncol. Rep. 2012, 28, 2225–2229. [Google Scholar]
- Gargalionis, A.N.; Papavassiliou, K.A.; Papavassiliou, A.G. Targeting STAT3 Signaling Pathway in Colorectal Cancer. Biomedicines 2021, 9, 1016. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Z.G.; Tian, X.Q.; Sun, D.-F.; Liang, Q.-C.; Zhang, Y.-J.; Lu, R.; Chen, Y.-X.; Fang, J.-Y. Inhibition of JAK1, 2/STAT3 Signaling Induces Apoptosis, Cell Cycle Arrest, and Reduces Tumor Cell Invasion in Colorectal Cancer Cells. Neoplasia 2008, 10, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Rhode, J.; Fogoros, S.; Zick, S.; Wahl, H.; A Griffith, K.; Huang, J.; Liu, J.R. Ginger Inhibits Cell Growth and Modulates Angiogenic Factors in Ovarian Cancer Cells. BMC Complement Altern. Med. 2007, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Yang, H.; Ta, S.H.; Chui, W.K.; Chew, E.H. 6-Shogaol, an Active Constituent of Ginger, Inhibits Breast Cancer Cell Invasion by Reducing Matrix Metalloproteinase-9 Expression via Blockade of Nuclear Factor-κB Activation. Br. J. Pharmacol. 2010, 161, 1763–1777. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Nachvak, S.M.; Soleimani, D.; Rahimi, M.; Azizi, A.; Moradinazar, M.; Rouhani, M.H.; Halashi, B.; Abbasi, A.; Miryan, M. Ginger as an Anticolorectal Cancer Spice: A Systematic Review of In Vitro to Clinical Evidence. Food Sci. Nutr. 2022, 11, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.V.; Damerla, R.R.; Dikhit, P.S.; Kumar, N.A. Prostaglandin-endoperoxide synthase 2 (PTGS2) gene expression and its association with genes regulating the VEGF signaling pathway in head and neck squamous cell carcinoma. J. Oral Biol. Craniofacial Res. 2023, 13, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; El-Rayes, B.F. Cyclooxygenase-2 in Gastrointestinal Malignancies. Cancer 2019, 125, 1221–1227. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, H.; Yu, F.B.; Li, B.; Zhang, Y.; Zhu, Y.T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Abdul Rahim, M.H.; Roosli, R.A.J.; Sani, M.H.M.; Omar, M.H.; Tohid, S.F.M.; Othman, F.; Ching, S.M.; Kadir, A.A. Antinociceptive Activity of Methanolic Extract of Clinacanthus nutans Leaves: Possible Mechanisms of Action Involved. Pain Res. Manag. 2018, 2018, 9536406. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Shukla, A.; Makhal, P.N.; Kaki, V.R. Natural Product-Driven Dual COX-LOX Inhibitors: Overview of Recent Studies on the Development of Novel Anti-Inflammatory Agents. Heliyon 2023, 9, e14569. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in Gastrointestinal Disorders: A Systematic Review of Clinical Trials. Food Sci. Nutr. 2018, 7, 96–108. [Google Scholar] [CrossRef]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of Estrogen Receptors in Health and Disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Ye, S.B.; Cheng, Y.K.; Deng, R.; Deng, Y.; Li, P.; Zhang, L.; Lan, P. The Predictive Value of Estrogen Receptor 1 on Adjuvant Chemotherapy in Locally Advanced Colorectal Cancer: A Retrospective Analysis with Independent Validation and Its Potential Mechanism. Front. Oncol. 2020, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, F.; Ryan, E.J.; Doherty, G.; Winter, D.C.; Sheahan, K. Estrogen Receptors and Their Implications in Colorectal Carcinogenesis. Front. Oncol. 2015, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.F.; Stoner, G.D. Gingers and Their Purified Components as Cancer Chemopreventative Agents. Molecules 2019, 24, 2859. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.M.; McConnery, J.R.; Hoskin, D.W. [10]-Gingerol, a Major Phenolic Constituent of Ginger Root, Induces Cell Cycle Arrest and Apoptosis in Triple-Negative Breast Cancer Cells. Exp. Mol. Pathol. 2017, 102, 370–376. [Google Scholar] [CrossRef]



| MOL ID | Molecular Name | OB% | DL | Structure |
|---|---|---|---|---|
| MOL000449 | Stigmasterol | 43.83 | 0.76 |  |
| MOL000358 | beta-sitosterol | 36.91 | 0.75 | 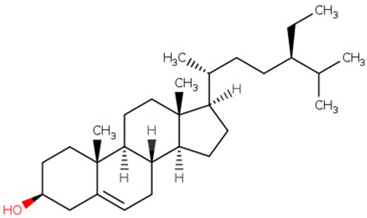 |
| MOL001771 | poriferast-5-en-3beta-yl beta-D-glucopyranoside | 36.91 | 0.75 |  |
| MOL006129 | Methyl diacetoxy-6-gingerdiol | 48.73 | 0.32 |  |
| MOL008698 | Dihydrocapsaicin | 47.07 | 0.19 |  |
| MOL003358 | Euxanthone | 92.98 | 0.16 |  |
| Compounds | Interacted Genes |
|---|---|
| Stigmasterol | AKR1C1, AKR1C2, AR, CLEC4E, ESR1, ESR2, GABRA1, GABRA2, GABRA3, GABRA4, GABRA5, GABRA6, GABRB1, GABRB2, GABRB3, GABRD, GABRE, GABRG1, GABRG2, GABRG3, GABRP, GABRQ, GRIN1, GRIN2A, GRIN2B, GRIN2C, GRIN2D, GRIN3A, GRIN3B, HSD17B1, LIP3, LSS, NCOA2, NR1I2, NR1I3, NR3C1, NR3C2, PGR, PPARA, RORA, SIGMAR1, SULT2A1, SULT2B1, VDR |
| beta-sitosterol | AKR1C1, AKR1C2, AR, CLEC4E, ESR1, ESR2, GABRA1, GABRA2, GABRA3, GABRA4, GABRA5, GABRA6, GABRB1, GABRB2, GABRB3, GABRD, GABRE, GABRG1, GABRG2, GABRG3, GABRP, GABRQ, GRIN1, GRIN2A, GRIN2B, GRIN2C, GRIN2D, GRIN3A, GRIN3B, HSD17B1, LIP3, LSS, NCOA2, NR1I2, NR1I3, NR3C1, NR3C2, PGR, PPARA, RORA, SIGMAR1, SULT2A1, SULT2B1, VDR |
| poriferast-5-en-3beta-yl beta-D-glucopyranoside | AKR1C1, AKR1C2, AR, CLEC4E, ESR1, ESR2, GABRA1, GABRA2, GABRA3, GABRA4, GABRA5, GABRA6, GABRB1, GABRB2, GABRB3, GABRD, GABRE, GABRG1, GABRG2, GABRG3, GABRP, GABRQ, GRIN1, GRIN2A, GRIN2B, GRIN2C, GRIN2D, GRIN3A, GRIN3B, HSD17B1, LIP3, LSS, NCOA2, NR1I2, NR1I3, NR3C1, NR3C2, PGR, PPARA, RORA, SIGMAR1, SULT2A1, SULT2B1, VDR |
| Methyl diacetoxy-6-gingerdiol | ESR1, PTGS2, CaM |
| Dihydrocapsaicin | PHB2, TRPV1 |
| Euxanthone | COX1, F2, PPARG, COX2, eNOS, PDE3A, DPP4, Hsp90, LTA4H, MAO, CHEK1, PRKACA, NCOA2, PKA |
| Color | MCODE | GO | Description | Log10(P) |
|---|---|---|---|---|
| ■ | MCODE_1 | WP4159 | GABA receptor signaling | −18.2 |
| ■ | MCODE_1 | WP4829 | mBDNF and proBDNF regulation of GABA neurotransmission | −17.6 |
| ■ | MCODE_1 | hsa05033 | Nicotine addiction | −17.4 |
| ■ | MCODE_2 | R-HSA-4090294 | SUMOylation of intracellular receptors | −15.2 |
| ■ | MCODE_2 | WP170 | Nuclear receptors | −14.6 |
| ■ | MCODE_2 | R-HSA-383280 | Nuclear Receptor transcription pathway | −13.9 |
| ■ | MCODE_3 | WP1742 | TP53 network | −9.7 |
| ■ | MCODE_3 | GO:0001836 | release of cytochrome c from mitochondria | −9.6 |
| ■ | MCODE_3 | GO:0070231 | T cell apoptotic process | −9.2 |
| Name. | CASP9 | MMP9 | PTGS2 (COX2) | ESR1 | TP53 |
|---|---|---|---|---|---|
| PDB code | 1JXQ | 1GKC | 3LN1 | 2OCF | 3LH0 |
| Euxanthone | −5.812 | −7.72 | −7.26 | −7.97 | 5.63 |
| Dihydrocapsaicin | −5.278 | −6.72 | −8.08 | −7.01 | −7.89 |
| Methyl diacetoxy-6-gingerdiol | −5.112 | −6.91 | −9.20 | −6.73 | −6.22 |
| poriferast-5-en-3beta-yl beta-D-glucopyranoside | −5.676 | −6.42 | −7.71 | −8.05 | −5.91 |
| beta-sitosterol | −5.678 | −6.14 | −7.69 | −7.18 | −6.07 |
| Stigmasterol | −5.732 | −6.38 | −7.04 | −7.39 | −6.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chujan, S.; Vajeethaveesin, N.; Satayavivad, J. Computational Analysis of Zingiber officinale Identifies GABAergic Signaling as a Potential Therapeutic Mechanism in Colorectal Cancer. Informatics 2025, 12, 116. https://doi.org/10.3390/informatics12040116
Chujan S, Vajeethaveesin N, Satayavivad J. Computational Analysis of Zingiber officinale Identifies GABAergic Signaling as a Potential Therapeutic Mechanism in Colorectal Cancer. Informatics. 2025; 12(4):116. https://doi.org/10.3390/informatics12040116
Chicago/Turabian StyleChujan, Suthipong, Nutsira Vajeethaveesin, and Jutamaad Satayavivad. 2025. "Computational Analysis of Zingiber officinale Identifies GABAergic Signaling as a Potential Therapeutic Mechanism in Colorectal Cancer" Informatics 12, no. 4: 116. https://doi.org/10.3390/informatics12040116
APA StyleChujan, S., Vajeethaveesin, N., & Satayavivad, J. (2025). Computational Analysis of Zingiber officinale Identifies GABAergic Signaling as a Potential Therapeutic Mechanism in Colorectal Cancer. Informatics, 12(4), 116. https://doi.org/10.3390/informatics12040116






