A Longitudinal Study of Decomposition Odour in Soil Using Sorbent Tubes and Solid Phase Microextraction
Abstract
:1. Introduction
2. Experimental Section
2.1. Field Setup
2.2. Sorbent Tubes
2.3. SPME
2.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis and Data Processing
3. Results
3.1. Carcass Decomposition
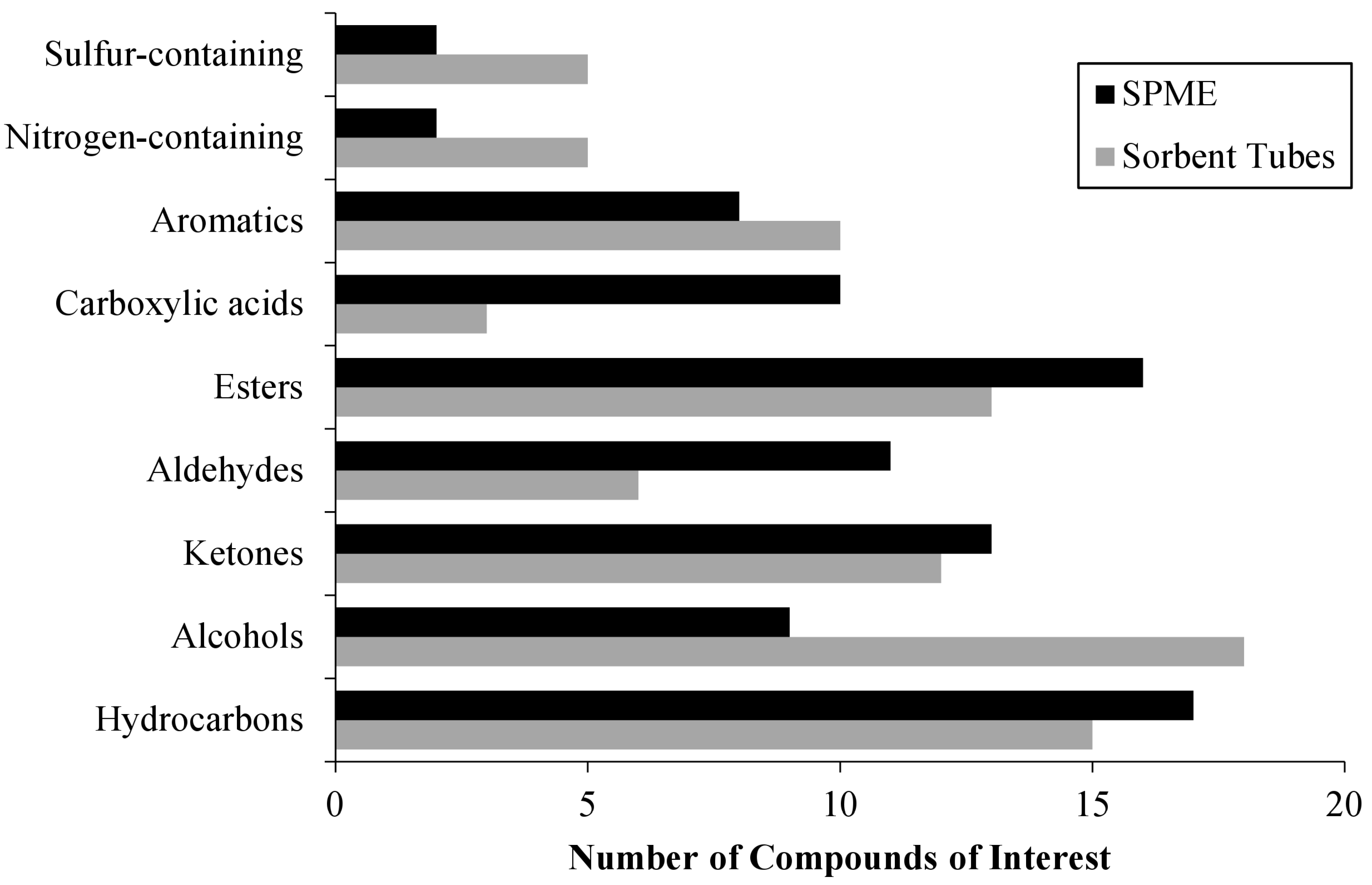
3.2. Decomposition VOC Database
| Day 2 48.2 ADD | Day 4 107.0 ADD | Day 6 149.6 ADD | Day 8 196.8 ADD | Day 10 244.3 ADD | Day 14 340.9 ADD | Day 17 413.7 ADD | Day 21 490.5 ADD | Day 24 557.8 ADD | Day 31 712.62 ADD | Day 45 1078.5 ADD | Day 59 1315.5 ADD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfur-containing | ||||||||||||
| Dimethyl sulfide * [1,4,14,17,18,19,20,28] | ■ | ■ | ||||||||||
| Dimethyl disulfide * [1,2,11,14,17,18,19,20,28] | ■/□ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| Dimethyl trisulfide * [11,13,14,17,18,20,28] | ■/□ | ■ | ■ | ■/□ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |
| 2,4-dithiapentane [28] | ■ | |||||||||||
| 3-methylthiophene [14] | ■ | |||||||||||
| Nitrogen-containing | ||||||||||||
| Trimethylamine [2,3,6,12,14] | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |||||
| 3-methylpyridine [14] | ■ | ■ | ■ | ■ | ■ | ■ | ||||||
| Benzonitrile * [3,11,14,15,19] | ■ | |||||||||||
| Benzylnitrile [14] | ■ | |||||||||||
| Aromatics | ||||||||||||
| Indole * [1,2,12,15,16] | ■ | ■ | ■/□ | ■/□ | ■ | ■/□ | ■/□ | ■ | ||||
| 4-methylindole | □ | |||||||||||
| 2-ethylfuran [17] | ■ | ■ | ||||||||||
| 2-butylfuran | ■ | |||||||||||
| 2-pentylfuran [1,2,12] | □ | ■/□ | ■/□ | ■/□ | ■/□ | ■/□ | ■/□ | □ | ■/□ | ■/□ | ■/□ | ■/□ |
| 2-heptylfuran | □ | □ | □ | □ | □ | |||||||
| 2-methyltetrahydrofuran | ■ | ■ | ■ | ■ | ■ | ■ | ||||||
| 2-butyltetrahydrofuran | ■ | |||||||||||
| Carboxylic Acids | ||||||||||||
| Acetic acid [3,6,13] | ■ | □ | □ | |||||||||
| Propanoic acid [1,2,3,16] | □ | □ | □ | |||||||||
| 2-methylpropanoic acid [14,16,17] | □ | □ | □ | |||||||||
| Butanoic acid [1,2,3,14,15] | □ | □ | ■/□ | □ | □ | |||||||
| 2-methylbutanoic acid [3,12,14,15] | □ | □ | □ | □ | □ | □ | □ | □ | □ | |||
| 3-methylbutanoic acid [3,6,12,14,15] | □ | □ | □ | □ | □ | □ | □ | □ | □ | |||
| Pentanoic acid [1,2,3,14,16] | □ | □ | ||||||||||
| 4-methylpentanoic acid | □ | □ | □ | □ | ■ | |||||||
| Hexanoic acid [1,2,3,14,16] | □ | □ | □ | □ | □ | |||||||
| 2-methylhexanoic acid [12] | □ | □ | ||||||||||
| Benzoic acid [14] | ■ | |||||||||||
| Esters | ||||||||||||
| Acetic acid, methyl ester | ■ | ■ | ||||||||||
| Acetic acid, ethyl ester | ■ | ■ | ||||||||||
| Acetic acid, propyl ester [14,17] | ■ | |||||||||||
| Propanoic acid, 2-methyl-, ethyl ester [17] | ■ | |||||||||||
| Propanoic acid, ethyl ester | ■ | |||||||||||
| Butanoic acid, 1-methyl-, ethyl ester | ■ | ■ | ■ | |||||||||
| Butanoic acid, ethyl ester [1,17] | ■ | ■ | ||||||||||
| Butanoic acid, 2-methyl-, ethyl ester [17] | ■ | |||||||||||
| Butanoic acid, 2-methyl-, pentyl ester | □ | □ | ||||||||||
| Butanoic acid, 3-methyl-, butyl ester [14] | ■/□ | □ | ||||||||||
| Butanoic acid, 3-methyl-, methyl ester | □ | □ | ||||||||||
| Butanoic acid, methyl ester [14] | □ | ■/□ | ■/□ | ■ | ||||||||
| Butanoic acid, propyl ester | ■ | ■ | ■ | |||||||||
| Butanoic acid, butyl ester [1,14] | ■ | ■ | ||||||||||
| Butanoic acid, pentyl ester | □ | |||||||||||
| Butanoic acid, hexyl ester | □ | □ | □ | |||||||||
| Butanoic acid, octyl ester | □ | □ | ||||||||||
| Pentanoic acid, ethyl ester | ■ | ■ | ■ | |||||||||
| Pentanoic acid, methyl ester | □ | □ | ||||||||||
| Pentanoic acid, 4-methyl-, methyl ester | □ | |||||||||||
| Hexanoic acid, methyl ester | □ | □ | □ | □ | □ | □ | □ | □ | □ | |||
| Hexanoic acid, ethyl ester [1] | □ | □ | ||||||||||
| Hexanoic acid, pentyl ester [1] | □ | |||||||||||
| Heptanoic acid, methyl ester | □ | □ | □ | □ | ||||||||
| Octanoic acid, methyl ester [12] | □ | □ | □ | |||||||||
| Nonanoic acid, methyl ester | □ | □ | ||||||||||
| Phenylpropanoic acid, methyl ester | □ | |||||||||||
| Aldehydes | ||||||||||||
| 3-methylbutanal [3,6,18,20] | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |||
| Hexanal * [1,2,4,12,16,17,20] | ■/□ | ■ | □ | ■ | ||||||||
| Heptanal * [1,2,12,13,14,16,20] | □ | □ | □ | |||||||||
| 2-heptenal [1,12] | □ | |||||||||||
| Octanal [1,3,8,16,20] | □ | □ | □ | □ | □ | ■/□ | □ | □ | □ | |||
| 2-octenal [1,12] | □ | □ | ||||||||||
| 2-butyl-2-octenal | □ | |||||||||||
| Nonanal * [4,12,13,20] | ■/□ | ■/□ | □ | ■/□ | ■/□ | ■/□ | □ | ■/□ | □ | |||
| 2,4-nonadienal [1,12] | □ | |||||||||||
| 2-decenal [3] | □ | |||||||||||
| Benzaldehyde * [1,3,6,11,12,13,14,16,19] | □ | |||||||||||
| 2-phenylethanal | □ | □ | □ | □ | ||||||||
| 2-phenylpropenal | ■ | □ | □ | □ | ||||||||
| 2-methyl-prop-2-enal | ■ | |||||||||||
| Ketones | ||||||||||||
| 2-butanone [3,4,6,17,18] | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |||||
| 3-methyl-2-butanone [3,6] | ■ | ■ | ■ | |||||||||
| 2-pentanone [3,6,17] | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ||||
| 2-hexanone [17] | ■ | ■ | ■ | |||||||||
| 2-heptanone [1,3,12,15,17] | □ | ■/□ | □ | ■ | ■/□ | □ | ■/□ | ■/□ | ||||
| 2-octanone [3,15] | ■/□ | □ | □ | ■/□ | ■/□ | |||||||
| 3-octanone [3,15] | □ | □ | □ | □ | ■/□ | ■/□ | ||||||
| 3-octen-2-one | □ | □ | □ | |||||||||
| 3,5-octadien-2-one [12] | □ | □ | □ | |||||||||
| 2-nonanone * [3,11,12,15,18,20] | ■ | □ | ■/□ | □ | ■/□ | ■/□ | ||||||
| 2-decanone [3,12,20] | □ | |||||||||||
| 2-undecanone * [3,11,12,15] | ■ | □ | ■/□ | □ | ||||||||
| 2-nonadecanone | ■ | |||||||||||
| 1-phenylethanone * [6,11,14,15] | ■ | □ | □ | ■/□ | □ | ■/□ | ■/□ | |||||
| Ketones (continued) | ||||||||||||
| 3-methyl-3-buten-2-one | ■ | |||||||||||
| 2-piperidinone [14] | □ | |||||||||||
| 1,7,7-trimethylbicyclo[2.2.1]heptan-2-one | □ | □ | □ | □ | ■ | |||||||
| 6,6-dimethylbicyclo[3.1.1]heptan-2-one | □ | |||||||||||
| 2,6,6-trimethylbicyclo[3.1.1]heptan-2-one | □ | □ | □ | |||||||||
| Alcohols | ||||||||||||
| Ethanol * [4,14,17,18,20] | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |||||
| 1-propanol [3,15] | ■ | ■ | ■ | ■ | ■ | ■ | ||||||
| 2-propanol [15,18] | ■ | ■ | ■ | |||||||||
| 2-methyl-1-propanol [6,14,17] | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ||||
| 2-propen-1-ol | ■ | |||||||||||
| 1-butanol [3,6,15,16,17] | ■ | ■ | ■ | ■ | ■ | |||||||
| 2-butanol [3,6,14] | ■ | |||||||||||
| 2-methyl-1-butanol | ■ | ■ | ■ | ■ | ■ | ■ | ||||||
| 3-methyl-1-butanol [3,14] | ■ | ■/□ | ■ | ■/□ | ■ | |||||||
| 1-pentanol [1,2,3,6,12,14,17] | ■ | ■ | ■ | ■ | ||||||||
| 2-pentanol [6,17] | ■ | ■ | ||||||||||
| 2-hexanol | ■ | □ | □ | ■ | ||||||||
| 1-heptanol [3,20] | □ | |||||||||||
| 2-heptanol | ■ | ■ | ||||||||||
| 1-octanol [1,3,16] | □ | ■ | ■ | |||||||||
| 3,5-octadien-2-ol | □ | |||||||||||
| 1-nonanol | ■/□ | ■ | ■ | ■ | ■ | |||||||
| Phenol * [3,6,11,13,14,15,18] | ■ | □ | □ | □ | ■/□ | |||||||
| 4-methylphenol [3,14,15,18] | ■ | ■ | ■ | ■/□ | ■/□ | □ | ||||||
| 2-phenylethanol [14,16] | ■ | □ | □ | □ | □ | |||||||
| Hydrocarbons | ||||||||||||
| Pentane [4] | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ||
| Hexane * [4,6,12,17,18,20] | □ | □ | □ | ■ | ■ | ■ | ■/□ | ■/□ | ■/□ | ■ | ■/□ | |
| 1-hexene [6,17] | ■ | |||||||||||
| Heptane * [4,6,17,18,19] | ■ | ■ | ■ | ■ | ■ | ■ | ■/□ | ■ | ■ | |||
| 1-heptene [6,17] | ■ | ■ | ||||||||||
| 4-ethyl-3-heptene | □ | |||||||||||
| Octane [3,4,17,18] | ■ | ■ | ■ | ■/□ | ■ | ■ | □ | ■/□ | ■ | ■ | ||
| 1-octene [3] | □ | |||||||||||
| 1-decene | ■ | |||||||||||
| 5-undecene | ■ | ■ | □ | ■ | ||||||||
| Tridecane | ■ | ■/□ | ■ | ■/□ | ||||||||
| 1-tridecene | □ | □ | ||||||||||
| Pentadecane [6,13] | □ | □ | □ | ■/□ | ■/□ | ■/□ | □ | |||||
| 1-pentadecene | ■/□ | □ | ||||||||||
| Heptadecane | □ | □ | ||||||||||
| 8-heptadecene | □ | ■ | ■/□ | |||||||||
| α-pinene [6,17] | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ||||
| β-pinene | ■ | |||||||||||
| Copaene | □ | □ | ||||||||||
| 1-methyl-2-pentylcyclopropane | ■ | □ | □ | |||||||||
| 1,3-Hexadiene, 3-ethyl-2-methyl- | □ | |||||||||||
| 1-methyl-4-(1-methylethyl)-1,4-cyclohexadiene | □ | ■ | ■ | ■/□ | ||||||||
| 2,2-dimethyl-3-methylene-bicyclo[2.2.1]heptane | □ |
3.3. PCA Analysis
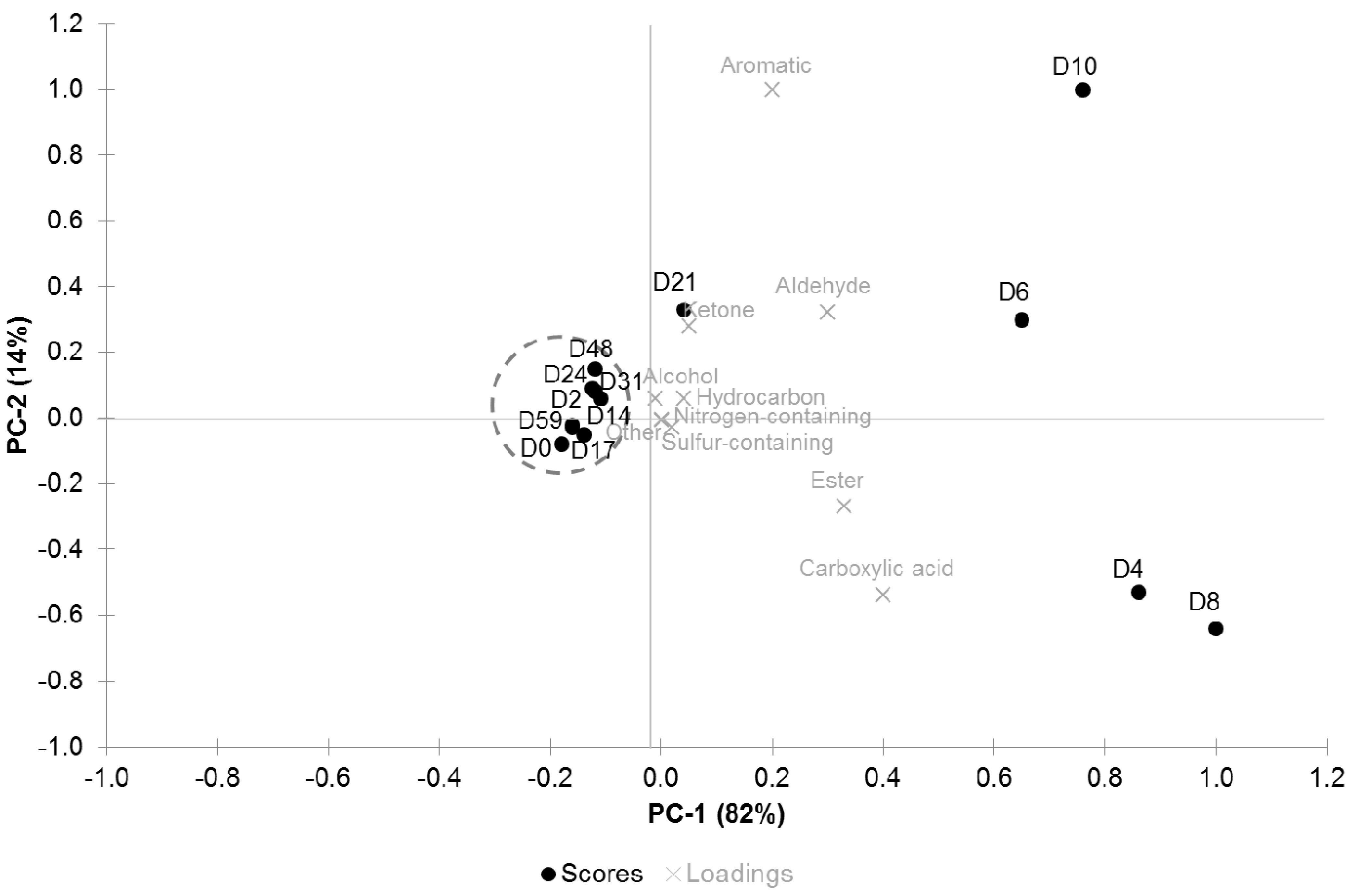
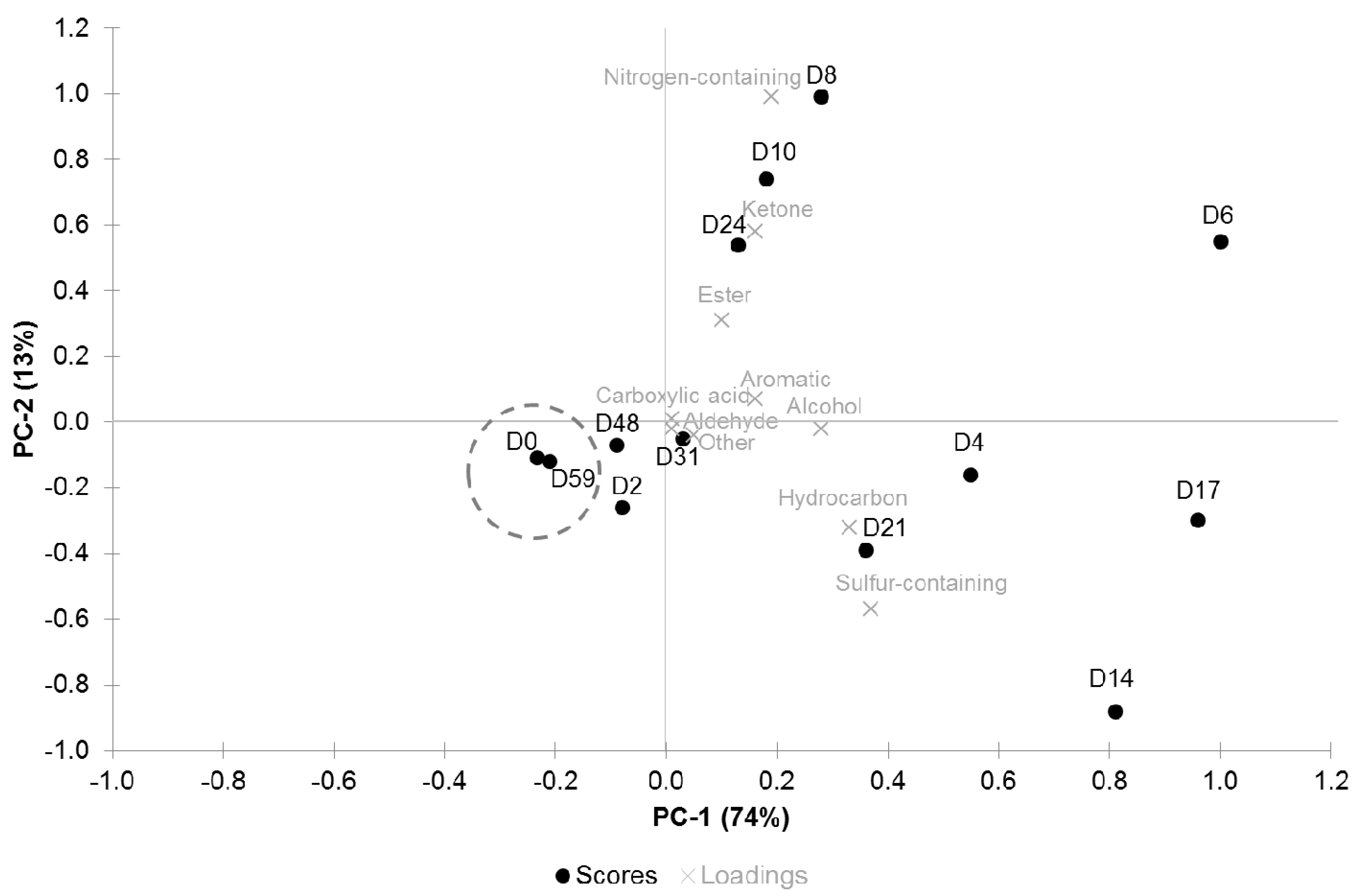
4. Discussion
4.1. Decomposition VOC Profile in Soil
4.2. Collection Technique Considerations
4.3. Practical Considerations
4.4. Longitudinal VOC Data in Forensic Casework
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoffman, E.M.; Curran, A.M.; Dulgerian, N.; Stockham, R.A.; Eckenrode, B.A. Characterization of the volatile organic compounds present in the headspace of decomposing human remains. Forensic Sci. Int. 2009, 186, 6–13. [Google Scholar] [CrossRef]
- Lorenzo, N.; Wan, T.; Harper, R.J.; Hsu, Y.-L.; Chow, M.; Rose, S.; Furton, K.G. Laboratory and field experiments used to identify Canis lupus var. familiaris active odor signature chemicals from drugs, explosives, and humans. Anal. Bioanal. Chem. 2003, 376, 1212–1224. [Google Scholar] [CrossRef]
- Stadler, S.; Stefanuto, P.-H.; Brokl, M.; Forbes, S.L.; Focant, J.-F. Characterization of volatile organic compounds from human analogue decomposition using thermal desorption coupled to comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Anal. Chem. 2013, 85, 998–1005. [Google Scholar] [CrossRef]
- Vass, A.A. Odor mortis. Forensic Sci. Int. 2012, 221, 234–241. [Google Scholar] [CrossRef]
- Paczkowski, S.; Schütz, S. Post-mortem volatiles of vertebrate tissue. Appl. Microbiol. Biotechnol. 2011, 91, 917–935. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Agapiou, A.; Zorba, E.; Mikedi, K.; Karma, S.; Pallis, G.C.; Eliopoulos, C.; Spiliopoulou, C. Combined chemical and optical methods for monitoring the early decay stages of surrogate human models. Forensic Sci. Int. 2011, 210, 154–163. [Google Scholar] [CrossRef]
- Hädrich, C.; Ortmann, C.; Reisch, R.; Liebing, G.; Ahlers, H.; Mall, G. An electronic body-tracking dog? Int. J. Legal Med. 2010, 124, 43–47. [Google Scholar] [CrossRef]
- Mochalski, P.; Rudnicka, J.; Agapiou, A.; Statheropoulos, M.; Amann, A.; Buszewski, B. Near real-time VOCs analysis using an aspiration ion mobility spectrometer. J. Breath Res. 2013, 7. [Google Scholar] [CrossRef]
- Anderson, G. Insect succession on carrion and its relationship to determining time of death. In Forensic Entomology: The Utility of Arthropods in Legal Investigation; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 143–175. [Google Scholar]
- LeBlanc, H.N.; Logan, J.G. Exploiting insect olfaction in forensic entomology. In Current Concepts in Forensic Entomology; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 205–221. [Google Scholar]
- Brasseur, C.; Dekeirsschieter, J.; Schotsmans, E.M.J.; de Koning, S.; Wilson, A.S.; Haubruge, E.; Focant, J.-F. Comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the forensic study of cadaveric volatile organic compounds released in soil by buried decaying pig carcasses. J. Chromatogr. A 2012, 1255, 163–170. [Google Scholar] [CrossRef]
- Cablk, M.E.; Szelagowski, E.E.; Sagebiel, J.C. Characterization of the volatile organic compounds present in the headspace of decomposing animal remains, and compared with human remains. Forensic Sci. Int. 2012, 220, 118–125. [Google Scholar] [CrossRef]
- DeGreeff, L.E.; Furton, K.G. Collection and identification of human remains volatiles by non-contact, dynamic airflow sampling and SPME-GC/MS using various sorbent materials. Anal. Bioanal. Chem. 2011, 401, 1295–1307. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Verheggen, F.J.; Gohy, M.; Hubrecht, F.; Bourguignon, L.; Lognay, G.; Haubruge, E. Cadaveric volatile organic compounds released by decaying pig carcasses (Sus domesticus L.) in different biotopes. Forensic Sci. Int. 2009, 189, 46–53. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Stefanuto, P.-H.; Brasseur, C.; Haubruge, E.; Focant, J.-F. Enhanced characterization of the smell of death by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GCxGC-TOFMS). PLoS One 2012, 7, e39005. [Google Scholar]
- Kasper, J.; Mumm, R.; Ruther, J. The composition of carcass volatile profiles in relation to storage time and climate conditions. Forensic Sci. Int. 2012, 223, 64–71. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Spiliopoulou, C.; Agapiou, A. A study of volatile organic compounds evolved from the decaying human body. Forensic Sci. Int. 2005, 153, 147–155. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Agapiou, A.; Spiliopoulou, C.; Pallis, G.C.; Sianos, E. Environmental aspects of VOCs evolved in the early stages of human decomposition. Sci. Total Environ. 2007, 385, 221–227. [Google Scholar] [CrossRef]
- Vass, A.A.; Smith, R.R.; Thompson, C.V, Burnett; Wolf, D.A.; Synstelien, J.A.; Dulgerian, N.; Eckenrode, B.A. Decompositional odor analysis database. J. Forensic Sci. 2004, 49, 1–10. [Google Scholar]
- Vass, A.A.; Smith, R.R.; Thompson, C.V.; Burnett, M.N.; Dulgerian, N.; Eckenrode, B.A. Odor analysis of decomposing buried human remains. J. Forensic Sci. 2008, 53, 384–391. [Google Scholar] [CrossRef]
- Lewis, T.; Crockett, A.B.; Siegrist, R. Soil sampling and analysis for volatile organic compounds. Environ. Monit. Assess. 1994, 30, 213–246. [Google Scholar] [CrossRef]
- Forbes, S.L.; Perrault, K.A. Decomposition odour profiling in the air and soil surrounding vertebrate carrion. PLoS One 2014, 9, e95107. [Google Scholar] [CrossRef]
- Schoenly, K.G.; Haskell, N.H.; Mills, D.K.; Bieme-Ndi, C. Using pig carcasses as model corpses. Science 2006, 68, 402–410. [Google Scholar]
- Stadler, S.; Desaulniers, J.-P.; Forbes, S.L. Inter-year repeatability study of volatile organic compounds from surface decomposition of human analogues. Int. J. Legal Med. 2014. [Google Scholar] [CrossRef]
- Rosier, E.; Cuypers, E.; Dekens, M.; Verplaetse, R.; Develter, W.; van de Voorde, W.; Maes, D.; Tytgat, J. Development and validation of a new TD-GC/MS method and its applicability in the search for human and animal decomposition products. Anal. Bioanal. Chem. 2014, 406, 3611–3619. [Google Scholar] [CrossRef]
- Payne, J. A Summer Carrion Study of the Baby Pig Sus Scrofa Linnaeus. Ecology 1965, 46, 592–602. [Google Scholar] [CrossRef]
- Megyesi, M.S.; Nawrocki, S.P.; Haskell, N.H. Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. J. Forensic Sci. 2005, 50, 618–626. [Google Scholar]
- Kalinová, B.; Podskalská, H.; Růzicka, J.; Hoskovec, M. Irresistible bouquet of death—How are burying beetles (Coleoptera: Silphidae: Nicrophorus) attracted by carcasses. Naturwissenschaften 2009, 96, 889–899. [Google Scholar]
- McNeal, K.S.; Herbert, B.E. Volatile organic metabolites as indicators of soil microbial activity and community composition shifts. Soil Sci. Soc. Am. J. 2009, 73, 579–588. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Perrault, K.A.; Stuart, B.H.; Forbes, S.L. A Longitudinal Study of Decomposition Odour in Soil Using Sorbent Tubes and Solid Phase Microextraction. Chromatography 2014, 1, 120-140. https://doi.org/10.3390/chromatography1030120
Perrault KA, Stuart BH, Forbes SL. A Longitudinal Study of Decomposition Odour in Soil Using Sorbent Tubes and Solid Phase Microextraction. Chromatography. 2014; 1(3):120-140. https://doi.org/10.3390/chromatography1030120
Chicago/Turabian StylePerrault, Katelynn A., Barbara H. Stuart, and Shari L. Forbes. 2014. "A Longitudinal Study of Decomposition Odour in Soil Using Sorbent Tubes and Solid Phase Microextraction" Chromatography 1, no. 3: 120-140. https://doi.org/10.3390/chromatography1030120
APA StylePerrault, K. A., Stuart, B. H., & Forbes, S. L. (2014). A Longitudinal Study of Decomposition Odour in Soil Using Sorbent Tubes and Solid Phase Microextraction. Chromatography, 1(3), 120-140. https://doi.org/10.3390/chromatography1030120