Hypermanganesemia with Dystonia Type 2: A Potentially Treatable Neurodegenerative Disorder: A Case Series in a Tertiary University Hospital
Abstract
:1. Introduction
2. Methods
2.1. Patient 1
2.2. Patient 2
2.3. Patient 3
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat. Commun. 2016, 7, 11601. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, D.S.; Ghaisas, S.; Zenitsky, G.; Jin, H.; Kanthasamy, A.; Anantharam, V.; Kanthasamy, A.G. Manganese-Induced Neurotoxicity: New Insights into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.B.; Kwakye, G.F.; Hernández, E.H.; Aschner, M. Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol. 2011, 25, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Marti-Sanchez, L.; Ortigoza-Escobar, J.D.; Darling, A.; Villaronga, M.; Baide, H.; Molero-Luis, M.; Batllori, M.; Vanegas, M.I.; Muchart, J.; Aquino, L.; et al. Hypermanganesemia due to mutations in SLC39A14: Further insights into Mn deposition in the central nervous system. Orphanet J. Rare Dis. 2018, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Tuschl, K.; Gregory, A.; Meyer, E.; Clayton, P.T.; Hayflick, S.J.; Mills, P.B.; Kurian, M.A. SLC39A14 Deficiency. In GeneReviews® [Internet]; University of Washington: Seattle, DC, USA, 2017. [Google Scholar]
- Mukhtiar, K.; Ibrahim, S.; Tuschl, K.; Mills, P. Hypermanganesemia with Dystonia, Polycythemia and Cirrhosis (HMDPC) due to mutation in the SLC30A10 gene. Brain Dev. 2016, 38, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Avelino, M.A.; Fusão, E.F.; Pedroso, J.L.; Arita, J.H.; Ribeiro, R.T.; Pinho, R.S.; Tuschl, K.; Barsottini, O.G.; Masruha, M.R. Inherited manganism: The "cock-walk" gait and typical neuroimaging features. J. Neurol. Sci. 2014, 341, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Tuschl, K.; Clayton, P.T.; Gospe, S.M., Jr.; Gulab, S.; Ibrahim, S.; Singhi, P.; Aulakh, R.; Ribeiro, R.T.; Barsottini, O.G.; Zaki, M.S.; et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 2012, 90, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Tavasoli, A.; Rafsanjani, K.A.; Hemmati, S.; Mojbafan, M.; Zarei, E.; Hosseini, S. A case of dystonia with polycythemia and hypermanganesemia caused by SLC30A10 mutation: A treatable inborn error of manganese metabolism. BMC Pediatrics 2019, 19, 229. [Google Scholar] [CrossRef] [PubMed]
- Anazi, S.; Maddirevula, S.; Faqeih, E.; AlSedairy, H.; Alzahrani, F.; E Shamseldin, H.; Patel, N.; Hashem, M.; Ibrahim, N.; Abdulwahab, F.; et al. Clinical genomics expands the morbid genome of intellectual disability and offers a high diagnostic yield. Mol. Psychiatry 2017, 22, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Syversen, T.; Aschner, J.L.; Aschner, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ. Toxicol. Pharmacol. 2005, 19, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Aschner, M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem. Int. 2003, 43, 475–480. [Google Scholar] [CrossRef]
- Aschner, M.; Guilarte, T.R.; Schneider, J.S.; Zheng, W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007, 221, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.G.; Cowley, M.J.; Gayevskiy, V.; Roscioli, T.; Thorburn, D.R.; Prelog, K.; Bahlo, M.; Sue, C.M.; Balasubramaniam, S.; Christodoulou, J. A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J. Inherit. Metab. Dis. 2017, 40, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hogrebe, M.; Grüneberg, M.; Duchesne, I.; Von Der Heiden, A.L.; Reunert, J.; Schlingmann, K.P.; Boycott, K.M.; Beaulieu, C.L.; Mhanni, A.A.; et al. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am. J. Hum. Genet. 2015, 97, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Erikson, K.M.; Hernández, E.H.; Tjalkens, R. Manganese and its Role in Parkinson’s Disease: From Transport to Neuropathology. NeuroMolecular Med. 2009, 11, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Calne, D.B.; Chu, N.S.; Huang, C.C.; Lu, C.S.; Olanow, W. Manganism and idiopathic parkinsonism: Similarities and differences. Neurology 1994, 44, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Namnah, M.; Bauer, M.; Mor-Shaked, H.; Bressman, S.B.; Ms, D.R.; Ozelius, L.J.; Arkadir, D. Benign SLC39A14 Course of Dystonia-Parkinsonism Secondary to Inherited Manganese Accumulation. Mov. Disord. Clin. Pract. 2020, 7, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Stanwood, G.D.; Leitch, D.B.; Savchenko, V.; Wu, J.; Fitsanakis, V.A.; Anderson, D.J.; Stankowski, J.N.; Aschner, M.; McLaughlin, B. Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J. Neurochem. 2009, 110, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Stamelou, M.; Tuschl, K.; Chong, W.K.; Burroughs, A.K.; Mills, P.B.; Bhatia, K.P.; Clayton, P.T. Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: A new treatable disorder. Mov. Disord. 2012, 27, 1317–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
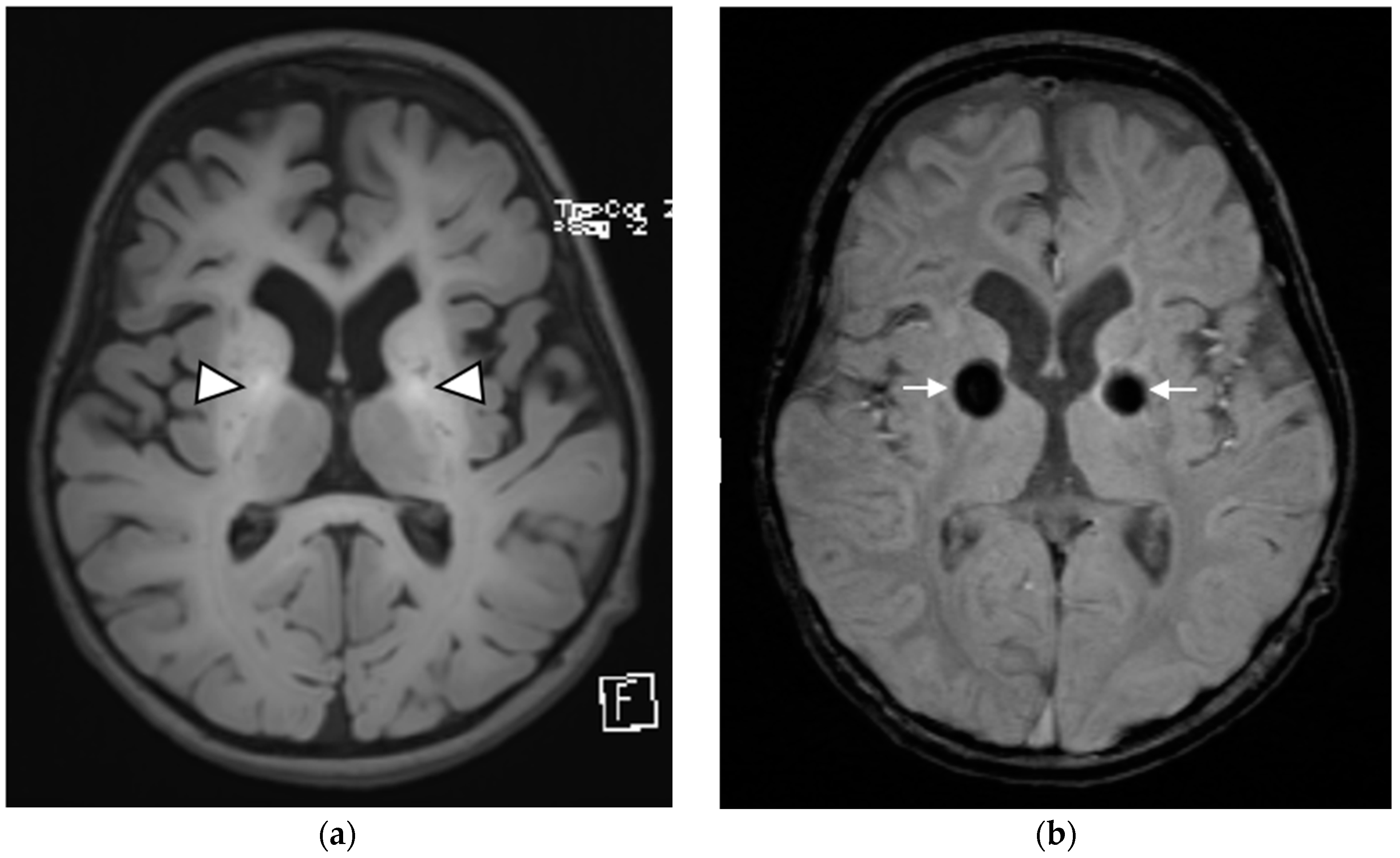
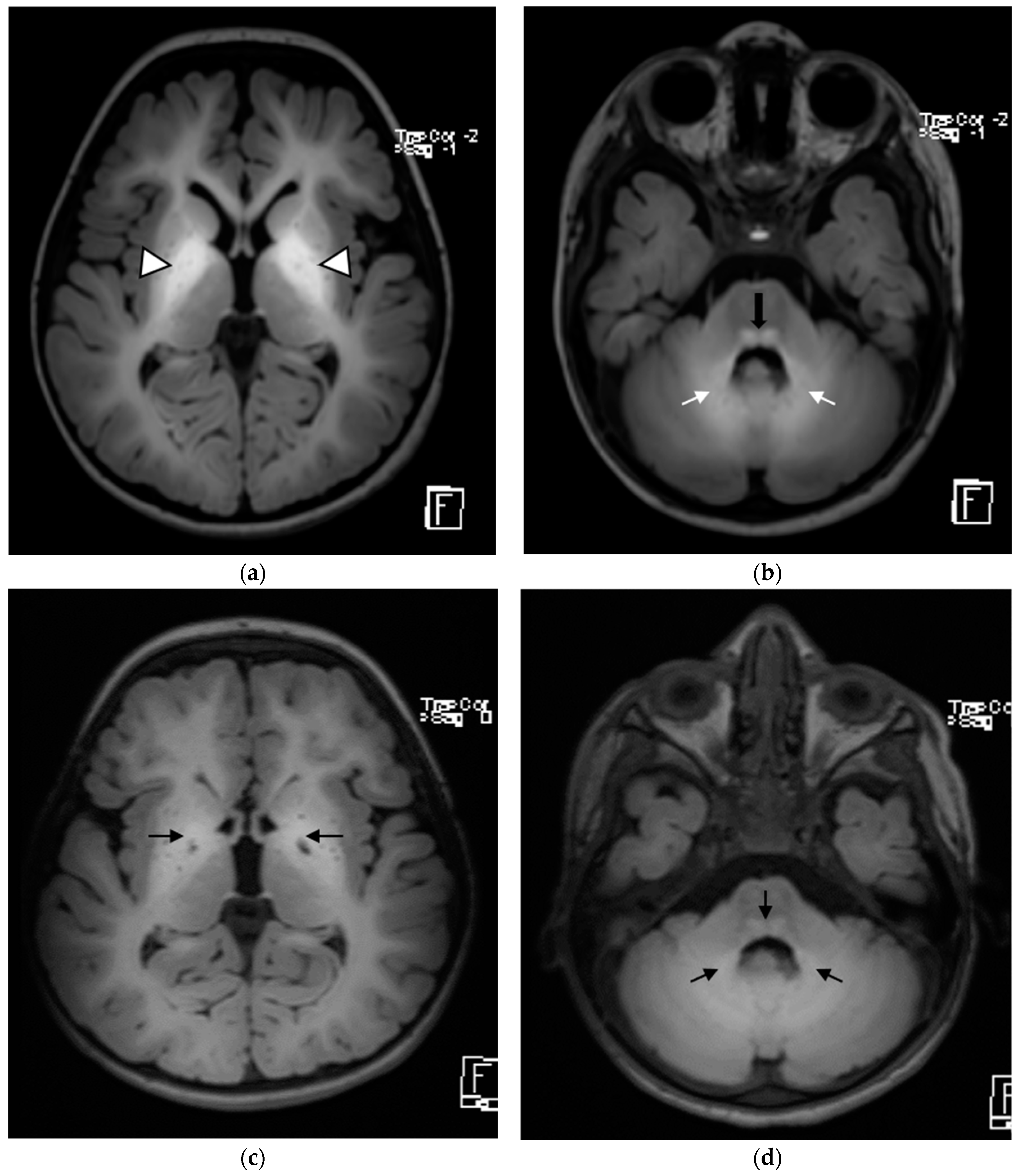
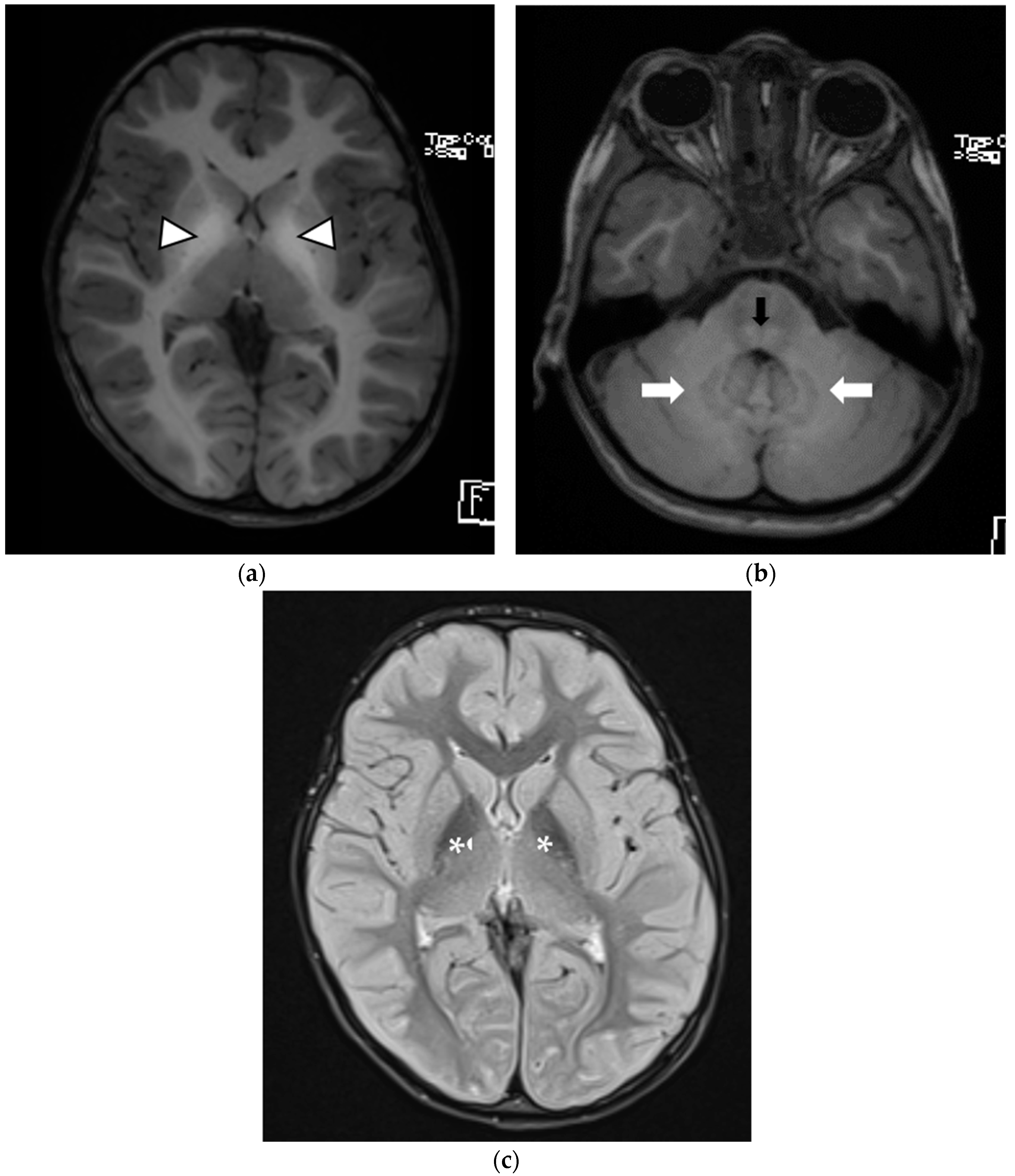
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age at presentation/Sex | 9 months/F | 8 months/M | 30 months/F |
| Clinical features at diagnosis | Regression of acquired milestones Dystonia Spasticity | Regression of acquired milestones Dystonia Spasticity | Regression of acquired milestones Dystonia Spasticity |
| Age at diagnosis | 5 years | 16 months | 3 years |
| Gene Mutation | SLC39A14 rs879253764;NM_001351660.2):c.313G > T (p.Glu105Ter) Homozygous nonsense | SLC39A14 c.776_777insAT NM_015359.6):c.776_777insAT (p.Glu261LeufsTer14) Homozygous frameshift | SLC39A14 rs774860376; NM_001351657.2):c.1096G > A (p.Gly366Ser) Homozygous missense |
| Age at the start of chelation therapy | 5 years | 16 months | 3 years |
| Brain MRI findings at the time of diagnosis | Symmetrical involvement of the basal ganglia, with decreased T2 and increased T1 signal intensity Mild global atrophy Mildly enlarged eye globes | Symmetrical involvement of the basal ganglia, with decreased T2 and increased T1 signal intensity, and the involvement of cerebellar white matter and posterior brain stem to a lesser degree | Symmetrical involvement of the basal ganglia, tegmentum of the midbrain, posterior tegmentum of the pons, both superior cerebellar peduncles and dentate nuclei, with decreased T2 and increased T1 signal intensity |
| Neurological manifestation before chelation therapy | Regular wheelchair use No social interaction Inability to sit without support No words spoken Generalized spasticity Brisk deep tendon reflexes Dystonia | Feeding difficulties Unable to roll over Able to recognize parents and siblings, and demonstrated stranger anxiety No words spoken Central hypotonia Bilateral lower limb spasticity Brisk deep tendon reflexes and sustained ankle clonus Dystonia in the right upper limb | Stuttering Unable to sit without support Preserved social developmental milestones Spasticity in all the limbs Brisk deep tendon reflexes Mild intentional tremors |
| Neurological manifestation after chelation therapy | Only 2 doses received, did not show significant changes | Decreased dystonia and improved spasticity Able to roll over (after 8 cycles) Started speaking few words | Improvement in hand function Decreased spasticity |
| Patient 1 | Patient 2 | Patient 3 | |
| Manganese level before the first session | 780 (ug/L) N: <2.5 | 119.2 (ug/L) N: <2.5 | 4900 (ug/L) N: <2.5 |
| Manganese level after the latest session | 55.2 ug/L | 19.1 ug/L | n/d |
| Follow-up brain MRI | Not performed | Similar changes to those observed at initial MRI, with no new lesions and no extension of previous lesions | Similar changes to those observed at initial MRI, with no new lesions and no extension of previous lesions |
| Hepatic impairment with chelation therapy | None | None | None |
| Renal impairment with chelation therapy | None | None | None |
| Changes in trace mineral levels in the serum (copper, zinc, iron, and lead) | None | None | None |
| Total sessions completed | 2 | 18 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhasan, K.A.; Alshuaibi, W.; Hamad, M.H.; Salim, S.; Jamjoom, D.Z.; Alhashim, A.H.; AlGhamdi, M.A.; Kentab, A.Y.; Bashiri, F.A. Hypermanganesemia with Dystonia Type 2: A Potentially Treatable Neurodegenerative Disorder: A Case Series in a Tertiary University Hospital. Children 2022, 9, 1335. https://doi.org/10.3390/children9091335
Alhasan KA, Alshuaibi W, Hamad MH, Salim S, Jamjoom DZ, Alhashim AH, AlGhamdi MA, Kentab AY, Bashiri FA. Hypermanganesemia with Dystonia Type 2: A Potentially Treatable Neurodegenerative Disorder: A Case Series in a Tertiary University Hospital. Children. 2022; 9(9):1335. https://doi.org/10.3390/children9091335
Chicago/Turabian StyleAlhasan, Khalid A., Walaa Alshuaibi, Muddathir H. Hamad, Suha Salim, Dima Z. Jamjoom, Aqeela H. Alhashim, Malak Ali AlGhamdi, Amal Y. Kentab, and Fahad A. Bashiri. 2022. "Hypermanganesemia with Dystonia Type 2: A Potentially Treatable Neurodegenerative Disorder: A Case Series in a Tertiary University Hospital" Children 9, no. 9: 1335. https://doi.org/10.3390/children9091335
APA StyleAlhasan, K. A., Alshuaibi, W., Hamad, M. H., Salim, S., Jamjoom, D. Z., Alhashim, A. H., AlGhamdi, M. A., Kentab, A. Y., & Bashiri, F. A. (2022). Hypermanganesemia with Dystonia Type 2: A Potentially Treatable Neurodegenerative Disorder: A Case Series in a Tertiary University Hospital. Children, 9(9), 1335. https://doi.org/10.3390/children9091335






