Sensory Profile of Children and Adolescents with Autism Spectrum Disorder and Tip-Toe Behavior: Results of an Observational Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Data Collection
2.2. Measures
2.2.1. Short Sensory Profile
2.2.2. Tip-Toe Behavior Assessment
- (1)
- The presence or absence of TTB during standing, walking, and running was noted by an expert physical therapist observing the spontaneous behavior of the person during daily life activities.
- (2)
- The same therapist asked the main caregiver about the presence or absence of TTB during standing, walking, and running in daily life activities, without informing about the results of the previous observation.
- (3)
- For the final decision about the presence or not of TTB while standing, persons were invited to play while standing in front of a table once a day for 3 days. For the final decision about the presence or not of TTB during walking, persons were invited to walk three times for a distance of 10 meters on 3 separate days. For the final decision about the presence or not of TTB during running, persons were asked to run 10 meters three times on 3 separate days.
2.3. Statistical Analysis
3. Results
3.1. Preliminary Statistical Analysis
3.2. Differences between the Two Groups
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; ISBN 9780890425541. [Google Scholar]
- Ausderau, K.; Sideris, J.; Furlong, M.; Little, L.M.; Bulluck, J.; Baranek, G.T. National survey of sensory features in children with ASD: Factor structure of the sensory experience questionnaire (3.0). J. Autism Dev. Disord 2014, 44, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, A.; Gal, E.; Fluss, R.; Katz-Zetler, N.; Cermak, S.A. Update of a Meta-analysis of Sensory Symptoms in ASD: A New Decade of Research. J. Autism Dev. Disord. 2019, 49, 4974–4996. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef]
- Dunn, W.; Little, L.; Dean, E.; Robertson, S.; Evans, B. The State of the Science on Sensory Factors and Their Impact on Daily Life for Children: A Scoping Review. OTJR 2016, 36 (Suppl. 2), 3S–26S. [Google Scholar] [CrossRef]
- Simpson, K.; Adams, D.; Alston-Knox, C.; Heussler, H.S.; Keen, D. Exploring the Sensory Profiles of Children on the Autism Spectrum Using the Short Sensory Profile-2 (SSP-2). J. Autism Dev. Disord. 2019, 49, 2069–2079. [Google Scholar] [CrossRef]
- Lane, A.E.; Molloy, C.A.; Bishop, S.L. Classification of children with autism spectrum disorder by sensory subtype: A case for sensory-based phenotypes. Autism Res. 2014, 7, 322–333. [Google Scholar] [CrossRef]
- Lane, A.E.; Simpson, K.; Masi, A.; Grove, R.; Moni, M.A.; Montgomery, A.; Roberts, J.; Silove, N.; Whalen, O.; Whitehouse, A.; et al. Patterns of sensory modulation by age and sex in young people on the autism spectrum. Aut. Res. 2022; advance online publication. [Google Scholar] [CrossRef]
- Burns, C.O.; Dixon, D.R.; Novack, M.; Granpeesheh, D. A Systematic Review of Assessments for Sensory Processing Abnormalities in Autism Spectrum Disorder. Rev. J. Autism Dev. Disord. 2017, 4, 209–224. [Google Scholar] [CrossRef]
- Dunn, W. Sensory Profile: User’s Manual; The Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Baum, S.H.; Stevenson, R.A.; Wallace, M.T. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol. 2015, 134, 140–160. [Google Scholar] [CrossRef] [Green Version]
- Fournier, K.A.; Kimberg, C.I.; Radonovich, K.J.; Tillman, M.D.; Chow, J.W.; Lewis, M.H.; Bodfish, J.W.; Chriss, J.H. Decreased static and dynamic postural control in children with autism spectrum disorders. Gait Posture 2010, 32, 6–9. [Google Scholar] [CrossRef]
- Perin, C.; Valagussa, G.; Mazzucchelli, M.; Gariboldi, V.; Cerri, C.G.; Meroni, R.; Grossi, E.; Cornaggia, C.M.; Menant, J.; Piscitelli, D. Physiological Profile Assessment of Posture in Children and Adolescents with Autism Spectrum Disorder and Typically Developing Peers. Brain Sci. 2020, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Lum, J.A.G.; Shandley, K.; Albein-Urios, N.; Kirkovski, M.; Papadopoulos, N.; Wilson, R.B.; Enticott, P.G.; Rinehart, N.J. Meta-Analysis Reveals Gait Anomalies in Autism. Autism Res. 2021, 14, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Venuti, P.; Apicella, F.; Muratori, F. Analysis of unsupported gait in toddlers with autism. Brain Dev. 2011, 33, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Shetreat-Klein, M.; Shinnar, S.; Rapin, I. Abnormalities of joint mobility and gait in children with autism spectrum disorders. Brain Dev. 2014, 36, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Accardo, P.J.; Monasterio, E.; Oswald, D. Toe Walking in Autism. In Comprehensive Guide to Autism; Patel, V.B., Preedy, V.R., Martin, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 519–532. [Google Scholar] [CrossRef]
- Weber, D. Toe-walking in children with early childhood autism. Acta Paedopsychiatr. Int. J. Child Adolesc. Psychiatry 1978, 43, 73–83. [Google Scholar]
- Robert, M. Toe walking in childhood. Prospective study of 130 toe walking children among which 19 affected of disorders of the autism spectrum [La démarche en équin chez l’enfant. Étude prospective de 130 enfants marchant en équin dont 19 atteints de troubles du spectre autistique]. Rev. Chir. Orthop. Traumatol. 2011, 97S, S62–S66. [Google Scholar]
- Valagussa, G.; Trentin, L.; Balatti, V.; Grossi, E. Assessment of presentation patterns, clinical severity, and sensorial mechanism of tip-toe behavior in severe ASD subjects with intellectual disability: A cohort observational study. Autism Res. 2017, 10, 1547–1557. [Google Scholar] [CrossRef]
- Williams, C.M.; Tinley, P.; Curtin, M. Idiopathic toe walking and sensory processing dysfunction. J. Foot Ankle Res. 2010, 3, 16. [Google Scholar] [CrossRef]
- Oetgen, M.E.; Peden, S. Idiopathic toe walking. J. Am. Acad. Orthop. Surg. 2012, 20, 292–300. [Google Scholar] [CrossRef]
- Accardo, P.J.; Barrow, W. Toe walking in autism: Further observations. J. Child Neurol. 2015, 30, 606–609. [Google Scholar] [CrossRef]
- Valagussa, G.; Balatti, V.; Trentin, L.; Piscitelli, D.; Yamagata, M.; Grossi, E. Relationship between tip-toe behavior and soleus—Gastrocnemius muscle lengths in individuals with autism spectrum disorders. J. Orthop. 2020, 21, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Caselli, M.A.; Rzonca, E.C.; Lue, B.Y. Habitual toe-walking: Evaluation and approach to treatment. Clin. Podiatr. Med. Surg. 1988, 5, 547–559. [Google Scholar] [PubMed]
- Calhoun, M.; Longworth, M.; Chester, V.L. Gait patterns in children with autism. Clin. Biomech. 2011, 26, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.; Soto-Garcia, M. Chiropractic management of toe-walking in an eight-year-old male diagnosed with autism spectrum disorder utilizing a functional approach: A case study. J. Bodyw. Mov. Ther. 2021, 26, 538–541. [Google Scholar] [CrossRef]
- Wilder, D.A.; Ingram, G.; Hodges, A.C. Evaluation of shoe inserts to reduce toe walking in young children with autism. Behav. Interv. 2021, 37, 754–765. [Google Scholar] [CrossRef]
- Fanchiang, H.D.; Geil, M.D.; Wu, J.; Ajisafe, T.; Chen, Y.P. The Effects of Walking Surface on the Gait Pattern of Children with Idiopathic Toe Walking. J. Child Neurol. 2016, 31, 858–863. [Google Scholar] [CrossRef]
- Liu, T. Sensory processing and motor skill performance in elementary school children with autism spectrum disorder. Percept. Mot. Skills 2013, 116, 197–209. [Google Scholar] [CrossRef]
- Mikami, M.; Hirota, T.; Takahashi, M.; Adachi, M.; Saito, M.; Koeda, S.; Yoshida, K.; Sakamoto, Y.; Kato, S.; Nakamura, K.; et al. Atypical Sensory Processing Profiles and Their Associations With Motor Problems In Preschoolers With Developmental Coordination Disorder. Child Psychiatry Hum. Dev. 2021, 52, 311–320. [Google Scholar] [CrossRef]
- Purpura, G.; Cerroni, F.; Carotenuto, M.; Nacinovich, R.; Tagliabue, L. Behavioural Differences in Sensorimotor Profiles: A Comparison of Preschool-Aged Children with Sensory Processing Disorder and Autism Spectrum Disorders. Children 2022, 9, 408. [Google Scholar] [CrossRef]
- McIntosh, D.N.; Miller, L.J.; Shyu, V. Development and validation of the short sensory profile. In Sensory Profile: User’s Manual; Dunn, W., Ed.; The Pscyhological Corporation: San Antonio, TX, USA, 1999; pp. 59–73. [Google Scholar]
- NCS Pearson Inc. Research Translation License Agreement; IP Licensing Clinical Assessment North America: San Antonio, TX, USA, 2017. [Google Scholar]
- Davis, L.L. Instrument review: Getting the most from a panel of experts. Appl. Nurs. Res. 1992, 5, 194–197. [Google Scholar] [CrossRef]
- Lynn, M.R. Determination and quantification of content validity. Nurs. Res. 1986, 35, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Topf. Three estimates of interrater reliability for nominal data. Nurs. Res. 1986, 35, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Polit, D.F.; Beck, C.T. The content validity index: Are you sure you know what’s being reported? Crit. Recomm. 2006, 29, 489–497. [Google Scholar]
- Beck, C.T.; Gable, R. Ensuring content validity: An illustration of the process. J. Nurs. Meas. 2001, 9, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Waltz, C.F.; Strickland, O.L.; Lenz, E.R. Measurement in Nursing and Health Research; Springer Publishing Company: New York, NY, USA, 2005. [Google Scholar]
- Neil, L.; Green, D.; Pellicano, E. The Psychometric Properties of a New Measure of Sensory Behaviors in Autistic Children. J. Autism Dev. Disord. 2017, 47, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.A.; van Balkom, I.; Landlust, A.M.; Priolo, M.; Menke, L.A.; Acero, I.H.; Alkuraya, F.S.; Arias, P.; Bernardini, L.; Bijlsma, E.K.; et al. Development, behaviour and sensory processing in Marshall-Smith syndrome and Malan syndrome: Phenotype comparison in two related syndromes. J. Int. Dis. Res. 2020, 64, 956–969. [Google Scholar] [CrossRef]
- Scheerer, N.E.; Curcin, K.; Stojanoski, B.; Anagnostou, E.; Nicolson, R.; Kelley, E.; Georgiades, S.; Liu, X.; Stevenson, R.A. Exploring sensory phenotypes in autism spectrum disorder. Mol. Autism 2021, 12, 67. [Google Scholar] [CrossRef]
- Fetta, A.; Soliani, L.; Trevisan, A.; Pugliano, R.; Ricci, E.; Di Pisa, V.; Pignataro, V.; Angotti, M.; Rocca, A.; Salce, B.; et al. Cognitive, Behavioral, and Sensory Profile of Pallister-Killian Syndrome: A Prospective Study of 22 Individuals. Genes 2022, 13, 356. [Google Scholar] [CrossRef]
- Lyons-Warren, A.M.; McCormack, M.C.; Holder, J.L., Jr. Sensory Processing Phenotypes in Phelan-McDermid Syndrome and SYNGAP1-Related Intellectual Disability. Brain Sci. 2022, 12, 137. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; PAFS consensus group. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 2, 64. [Google Scholar] [CrossRef]
- Tomchek, S.D.; Dunn, W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. Am. J. Occup. Ther. 2007, 61, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuang, Y.P.; Huang, C.L.; Tsai, H.Y. Sensory Integration and Perceptual-Motor Profiles in School-Aged Children with Autistic Spectrum Disorder. Neuropsychiatr. Dis. Treat. 2020, 16, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Uljarević, M.; Hedley, D.; Alvares, G.A.; Varcin, K.J.; Whitehouse, A. Relationship between early motor milestones and severity of restricted and repetitive behaviors in children and adolescents with autism spectrum disorder. Autism Res. 2017, 10, 1163–1168. [Google Scholar] [CrossRef]
- Williams, C.M.; Tinley, P.; Curtin, M.; Wakefield, S.; Nielsen, S. Is idiopathic toe walking really idiopathic? The motor skills and sensory processing abilities associated with idiopathic toe walking gait. J. Child Neurol. 2014, 29, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.; Girolami, G.L.; Grant-Beuttler, M. Assessing sensory processing differences in children with idiopathic toe walking: A pilot study. Physiother. Theory Pract. 2022; ahead of print. [Google Scholar] [CrossRef]
- Riquelme, I.; Hatem, S.M.; Montoya, P. Abnormal Pressure Pain, Touch Sensitivity, Proprioception, and Manual Dexterity in Children with Autism Spectrum Disorders. Neural Plast. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Tavassoli, T.; Bellesheim, K.; Tommerdahl, M.; Holden, J.M.; Kolevzon, A.; Buxbaum, J.D. Altered tactile processing in children with autism spectrum disorder. Autism Res. 2016, 9, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Tinley, P.; Curtin, M.; Nielsen, S. Vibration perception thresholds in children with idiopathic toe walking gait. J. Child Neurol. 2012, 27, 1017–1021. [Google Scholar] [CrossRef]
- Ganley, K.J.; Behnke, C. Distal Vibration Perception Threshold in Children Who Toe Walk. Pediatr. Phys. Ther. 2016, 28, 187–191. [Google Scholar] [CrossRef]
- Wozniak, R.H.; Leezenbaum, N.B.; Northrup, J.B.; West, K.L.; Iverson, J.M. The development of autism spectrum disorders: Variability and causal complexity. Wiley Interdiscip. Rev. Cogn. Sci. 2016, 8, e1426. [Google Scholar] [CrossRef]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part I: The physiologic basis of functional joint stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar]
- Muratori, F.; Calderoni, S.; Bizzari, V. George Frankl: An undervalued voice in the history of autism. Eur. Child Adolesc. Psychiatry 2021, 30, 1273–1280. [Google Scholar] [CrossRef]
- Cardillo, R.; Erbì, C.; Mammarella, I.C. Spatial Perspective-Taking in Children with Autism Spectrum Disorders: The Predictive Role of Visuospatial and Motor Abilities. Front. Hum. Neurosci. 2020, 14, 208. [Google Scholar] [CrossRef]
- Valagussa, G.; Piscitelli, D.; Baruffini, S.; Panzeri, V.; Perin, C.; Mazzucchelli, M.; Cornaggia, C.M.; Pellicciari, L.; Grossi, E. Little Evidence for Conservative Toe Walking Interventions in Autism Spectrum Disorders: A Systematic Review. Rev. J. Autism Dev. Disord. 2022, 1–14. [Google Scholar] [CrossRef]
- Leyden, J.; Fung, L.; Frick, S. Autism and toe-walking: Are they related? Trends and treatment patterns between 2005 and 2016. J. Child Orthop. 2019, 13, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Kaznica, S. Commentary on The Effectiveness of Serial Casting and Ankle Foot Orthoses in Treating Toe Walking in Children With Autism Spectrum Disorder. Pediatr. Phys. Ther. 2021, 33, 91. [Google Scholar] [CrossRef] [PubMed]
- Valagussa, G.; Trentin, L.; Signori, A.; Grossi, E. Toe Walking Assessment in Autism Spectrum Disorder Subjects: A Systematic Review. Autism Res. 2018, 11, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Clinical Findings | Total ASD Sample (N. 32) | ASD NO-TTB Group (n. 16) | ASD TTB Group (n. 16) |
|---|---|---|---|
| Mean Age (SD) (yrs); Age median (yrs) | 12.93 (2.92); 13.16 | 13.02 (2.89); 13.42 | 12.84 (3.04) 12.91 |
| Age range (yrs) | 7.31–17.88 | 7.44–17.51 | 7.31–17.88 |
| Gender (M/F) | 28/4 | 14/2 | 14/2 |
| Mean ADOS-2 CSS (SD); median ADOS-2 CSS range | 7.56 (1.63); 7 4–10 | 6.75 (1); 7 5–9 | 8.38 (1.75); 8.5 4–10 |
| ID Mild n. (%) | 1 (3.125%) | 0 | 1 (6.25%) |
| ID Moderate n. (%) | 1 (3.125%) | 0 | 1 (6.25%) |
| ID Severe n. (%) | 12 (37.50%) | 11 (48.49%) | 1 (6.25%) |
| ID Profound n. (%) | 18 (56.25%) | 5 (45.46%) | 13 (81.25%) |
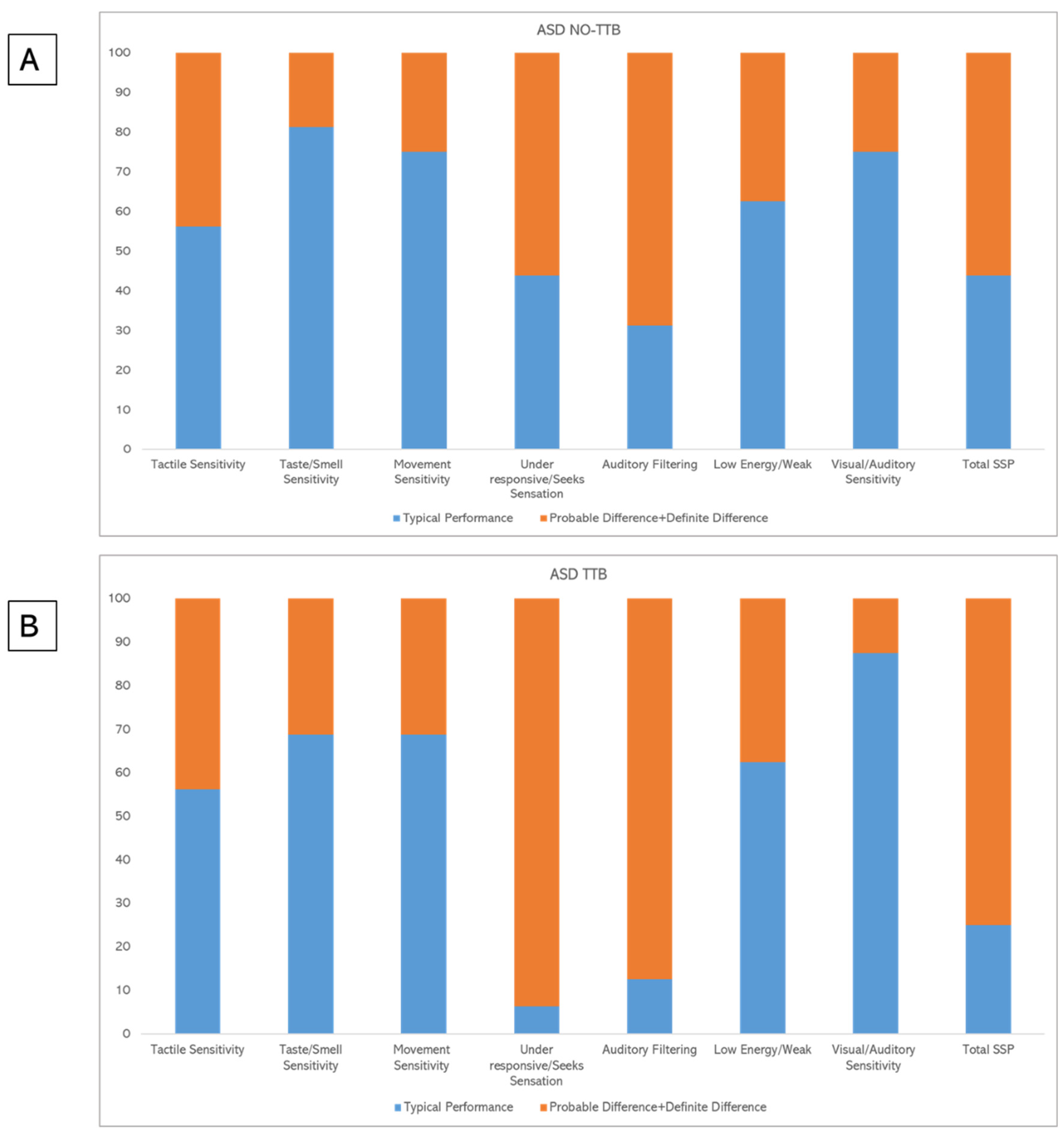
| Section | Class | NO-TTB n° 16 | TTB n° 16 |
|---|---|---|---|
| Tactile Sensitivity—n° (%) | Typical performance | 9 (56.2%) | 9 (56.2%) |
| Probable difference | 1 (6.3%) | 2 (12.5%) | |
| Definite difference | 6 (37.5%) | 5 (31.3%) | |
| Taste/Smell Sensitivity—n° (%) | Typical performance | 13 (81.3%) | 11 (68.8%) |
| Probable difference | 1 (6.3%) | 2 (12.5%) | |
| Definite difference | 2 (12.5%) | 3 (18.8%) | |
| Movement Sensitivity—n° (%) | Typical performance | 12 (75%) | 11 (68.8%) |
| Probable difference | 2 (12.5%) | 1 (6.3%) | |
| Definite difference | 2 (12.5%) | 4 (25%) | |
| Under responsive/Seeks Sensation—n° (%) | Typical performance | 7 (43.8%) | 1 (6.3%) |
| Probable difference | 2 (12.5%) | 2 (12.5%) | |
| Definite difference | 7 (43.8%) | 13 (81.3%) | |
| Auditory Filtering—n° (%) | Typical performance | 5 (31.3%) | 2 (12.5%) |
| Probable difference | 5 (31.3%) | 5 (31.3%) | |
| Definite difference | 6 (37.5%) | 9 (56.3%) | |
| Low Energy/Weak—n° (%) | Typical performance | 10 (62.5%) | 10 (62.5%) |
| Probable difference | 0 | 0 | |
| Definite difference | 6 (37.5%) | 6 (37.5%) | |
| Visual/Auditory Sensitivity—n° (%) | Typical performance | 12 (75%) | 14 (87.5%) |
| Probable difference | 4 (25%) | 2 (12.5%) | |
| Definite difference | 0 | 0 | |
| Total SSP—n° (%) | Typical performance | 7 (43.8%) | 4 (25%) |
| Probable difference | 5 (31.3%) | 4 (25%) | |
| Definite difference | 4 (25%) | 8 (50%) |
| SSP Total Score | Tactile Sensitivity | Taste/Smell Sensitivity | Movement Sensitivity | Under-Responsive/Seek Sensation | Auditory Filtering | Low Energy/Week | Visual/Auditory Sensitivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTB | NO TTB | TTB | NO TTB | TTB | NO TTB | TTB | NO TTB | TTB | NO TTB | TTB | NO TTB | TTB | NO TTB | TTB | NO TTB | |
| TP n° subjects | 4 | 7 | 9 | 9 | 11 | 13 | 11 | 12 | 1 | 7 | 2 | 5 | 10 | 10 | 14 | 12 |
| PD + DD n° subjects | 12 | 9 | 7 | 7 | 5 | 3 | 5 | 4 | 15 | 9 | 14 | 11 | 6 | 6 | 2 | 4 |
| Fisher’s exact test (two-tailed) | p = 0.458 | p = 1 | p = 0.685 | p = 1 | p = 0.037 * | p = 0.394 | p = 1 | p = 0.654 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valagussa, G.; Purpura, G.; Nale, A.; Pirovano, R.; Mazzucchelli, M.; Grossi, E.; Perin, C. Sensory Profile of Children and Adolescents with Autism Spectrum Disorder and Tip-Toe Behavior: Results of an Observational Pilot Study. Children 2022, 9, 1336. https://doi.org/10.3390/children9091336
Valagussa G, Purpura G, Nale A, Pirovano R, Mazzucchelli M, Grossi E, Perin C. Sensory Profile of Children and Adolescents with Autism Spectrum Disorder and Tip-Toe Behavior: Results of an Observational Pilot Study. Children. 2022; 9(9):1336. https://doi.org/10.3390/children9091336
Chicago/Turabian StyleValagussa, Giulio, Giulia Purpura, Alessandra Nale, Rita Pirovano, Miryam Mazzucchelli, Enzo Grossi, and Cecilia Perin. 2022. "Sensory Profile of Children and Adolescents with Autism Spectrum Disorder and Tip-Toe Behavior: Results of an Observational Pilot Study" Children 9, no. 9: 1336. https://doi.org/10.3390/children9091336
APA StyleValagussa, G., Purpura, G., Nale, A., Pirovano, R., Mazzucchelli, M., Grossi, E., & Perin, C. (2022). Sensory Profile of Children and Adolescents with Autism Spectrum Disorder and Tip-Toe Behavior: Results of an Observational Pilot Study. Children, 9(9), 1336. https://doi.org/10.3390/children9091336






