Abstract
Background: Children diagnosed with type 1 diabetes mellitus (T1DM) are more prone to having celiac disease (CD) than the normal population. Moreover, patients with this dual diagnosis who are also on a diabetic and gluten-free diet (GFD) risk faltering growth and uncontrolled blood glucose levels. This review aims to assess the efficacy and effectiveness of managing patients with T1DM screened for CD with GFD to prevent complications associated with these chronic pathologies in childhood and adulthood. Materials and Methods: We abided by the PRISMA guidelines in this meta-analysis and used multiple databases and search engines. We included case–control studies. The primary outcomes were changes in the standard deviation score, body mass index (SDS BMI), and glycosylated hemoglobin (HA1C) after being on a GFD for at least twelve months. Results: The pooled data from the six studies included showed that there was neither a statistically significant difference in the mean SDS BMI (−0.28 (95% CI −0.75, 0.42)) (p = 0.24) nor in the mean of HA1C (mean −0.07 (95% CI −0.44, 0.30)) (p = 0.36) for the same group. HDL cholesterol improved significantly in patients on a strict GFD (p < 0.01). Conclusions: In children with T1DM and asymptomatic CD, being on a GFD had no significant effect on BMI or HA1C. However, it can have a protective effect on the other complications found in both chronic pathologies.
1. Introduction
The European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) [1] defines celiac disease (CD) as a chronic immune-mediated systemic disorder driven by gluten consumption from wheat, rye, and barley and their derivatives, which might also contaminate other grains, in the presence of specific genetic susceptibility. It presents a variable combination of clinical symptoms, specific serological markers, an HLA-DQ2/DQ8 haplotype, and enteropathy. Life-long strict adherence to a gluten-free diet leads to the disappearance of symptoms, negative titers of autoantibodies, histological recovery of the intestinal mucosa, and the elimination of long-term sequelae [2].
CD is diagnosed in pediatric patients diagnosed with type 1 diabetes mellitus (T1DM) more frequently than in the general population. In T1DM patients, CD prevalence can be up to twenty times higher than in the general population. The prevalence of CD in patients with T1DM is 3–12% [3,4].
Despite this increased risk, many health care providers struggle to reach an optimal approach for managing CD in T1DM to prevent the short- and long-term complications of these two life-long pathologies, especially in the absence of a consensus to guide their management suggestions [1].
Diagnosing patients with symptomatic CD, including malabsorption, and patients with subclinical disease, detected via osteopenia, growth failure, hepatic dysfunction, menstrual irregularity, unexplained epilepsy, or ataxia, remains uncomplicated. A gluten-free diet (GFD) in these symptomatic children improves their presenting signs and symptoms, making this regime strongly recommended.
Serological autoimmune markers such as the IgA anti-tissue transglutaminase (TTG) and IgA anti-endomysial (EMA) antibodies are highly sensitive and specific, and they are now used for routine CD screening to identify ‘silent’ and ‘atypical’ forms of CD. Many of the patients identified by screening are usually asymptomatic. Evidence is inconclusive concerning the advantages versus the disadvantages of screening and treating asymptomatic individuals who are children that are already burdened with an established chronic illness [5,6].
As the development of T1DM usually precedes symptomatic and asymptomatic CD, systematic CD screening should be performed periodically in all children with T1DM [3].
GFD has been proven to benefit growth and nutrient absorption in patients with CD. On the other hand, it is unclear whether the institution of GFD in T1DM and subclinical CD patients is beneficial. Despite many screening studies on this subject, only a few have tackled the effects of putting T1DM and subclinical CD patients on a GFD. Only a handful of small prospective and retrospective studies have addressed the glycemic benefits of a GFD. Given the limited information on the role of GFD in subclinical CD cases, we decided to perform a meta-analysis and systematic review of observational case–control studies conducted on children with T1DM and asymptomatic biopsy-proven CD [7]. Children were asked to follow a GFD for at least twelve months. The primary objectives were to document the pooled effects, regardless of whether they were positive or negative, of a GFD on anthropometric parameters, mainly body mass index (BMI) and glycosylated hemoglobin (HbA1c) levels, for this group of patients. Secondary objectives were to report any other effects of a GFD in these patients, such as albumin excretion in urine, insulin dose requirement, and bone mineral homeostasis.
To date, no review on the benefits of a GFD in children with a dual diagnosis of T1DM and asymptomatic CD has been reported before this one. We used Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methods to assess the overall quality of evidence, and this method was not used in past reviews, Table A1. This systematic review and meta-analysis were registered in PROSPERO.
2. Materials and Methods
The studies included in this review were prospective and retrospective case–control studies; randomized control studies could not be found, as confirmed cases of CD should always be managed by a GFD and cannot be randomized to placebo management. This meta-analysis is reported according to the Preferred Reporting Items for the Systematic Reviews and Meta-Analyses (PRISMA) [8] statement and checklist (Appendix A).
The study protocol was pre-specified and registered in PROSPERO (ID: CRD42020186088) and submitted on the 5 July 2020. The registration record was published exactly as submitted. The PROSPERO team did not check for eligibility based on an exclusive focus on COVID-19 registrations during the 2020 pandemic.
We applied population, intervention, comparison, and outcome (PICO) characterization to operationalize the research questions and to determine the eligibility criteria. The inclusion criteria were as follows: children aged between one and eighteen years, an established diagnosis of T1DM at least one year before the diagnosis of CD, CD diagnosed and proven by specific antibodies and small intestinal biopsy, and prescription of a GFD for at least one year before the last assessment and follow up. As the control group of each study did not have CD, they were not put on a GFD. Studies with multiple outcomes were included. In our study, two different primary results obtained before and after adherence to a GFD for at least twelve months were studied in this review: body mass index SDS (BMI SDS) [9] was studied as an anthropometric index, and glycosylated hemoglobin (HA1C) was studied as a metabolic index. BMI SDS was used as an anthropometric index, as growth influences body weight and adiposity measurements during childhood; it is more easily standardized into SD scores (SDS) with respect to reference populations. Standard glycemic parameters such as blood glucose and glycosylated hemoglobin are used to monitor control in diabetic patients. New methods, such as glycemic variability, are emerging to aid in better management and monitoring. Most of the research in this domain still uses glycosylated hemoglobin as a metabolic parameter to follow in diabetics [10].
This review’s secondary outcomes were renal function, lipid profile, bone density, and hypoglycemic events.
The authors confirmed adherence to the GFD in subjects in the selected studies for the meta-analysis by considering the lack of symptoms in the studied subgroups. T1DM was diagnosed, and insulin therapy was initiated at least one year before confirming CD diagnosis to eliminate the effects of insulin therapy initiation on the weight and BMI of the patients. These two inclusion criteria were limiting factors in finding eligible studies.
Studies were identified using electronic sources. The following databases were used for the electronic searches: PubMed, EBSCO, the Cochrane Library, the Cochrane Central Register for Controlled Trials, Google scholar, and Springer (Appendix A). The investigation was conducted between the following dates: 28 September 2019 and 31 January 2020. Literature search strategies were developed using medical subject headings (MeSH) and free text. The complete electronic search strategy for all of the databases is presented in detail in the Appendix A.
No restrictions based on outcomes or publication were applied to the searches. The only rules applied to the searches were studies on humans, language (English and French studies included), and age from one to eighteen years.
Three authors independently screened the titles/abstracts of potential studies for inclusion of the pre-specified inclusion/exclusion criteria. Following the initial screening, the studies’ full texts were reviewed using the same inclusion/exclusion criteria as the initial screening. A third reviewer was consulted to evaluate a study’s inclusion whenever there was a conflict between the two authors.
Studies meeting the following inclusion criteria were included in the study: study population of pediatric age group, a control group with a diagnosis of T1DM, CD confirmed by small intestinal biopsy, and patients followed for at least one year.
We assessed the quality of the overall evidence using the GRADE approach. This quality assessment method considers the study type, within-study risk of bias (methodological quality), heterogeneity, directness of evidence, precision of effect estimates, and publication bias risk. We rated the quality of the body of evidence for each key outcome as ‘high, moderate, low,’ or ‘very low’, Table A1 [11].
3. Statistical Analysis
The mean and standard deviation scores (SDSs) of the included studies were then input into Cochrane Review Manager Software, RevMan Version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark). Weighted mean differences between the T1DM with CD groups at diagnosis and follow-up after being put on a GFD were calculated for BMI and HbA1c with 95% CIs using the random effects. In the random-effects analysis, we presumed that the actual effect size varied from one study to another. The studies in our analysis represent a random sample of effect sizes that could have been observed. The summary effect estimates the mean of these effects and accounts for heterogeneity in participant populations. Tests to determine slope heterogeneity between studies were performed, and forest plots were assessed using the x2 test and the I2 statistic. I2 values below 25% were considered to have no heterogeneity, and values up to 40% were not considered to represent significant heterogeneity. Funnel plots were performed to graphically assess potential publication bias, which was statistically evaluated with Egger’s test. Moreover, a one-way t-test was used to calculate the significant difference in the standard deviation scores (SDSs) for BMI and HbA1c among both arms (CD and T1DM at diagnosis and follow-up). A p-value of <0.05 was considered statistically significant for testing the pooled results of the included studies and for univariate analysis.
4. Results
4.1. Characteristics of Included Studies
Our literature search yielded 552 titles, as shown in Figure 1. According to the PRISMA guidelines, studies were removed due to duplication, not meeting the inclusion criteria, or the statistical variables not being unified. We included six case–control studies [12,13,14,15,16,17] in the meta-analysis, as summarized in Table 1. There were five prospective [12,14,15,16,17] and one retrospective [13] case–control studies on the effects of a GFD on patients with T1DM and CD involving 578 participants. In the six studies, children who had T1DM and were found to have high titers of screening antibodies for CD and who underwent small bowel biopsies were put on a GFD and matched to children diagnosed with T1DM according to age and gender. The control groups were not put on a GFD, as the children in these groups did not have CD.
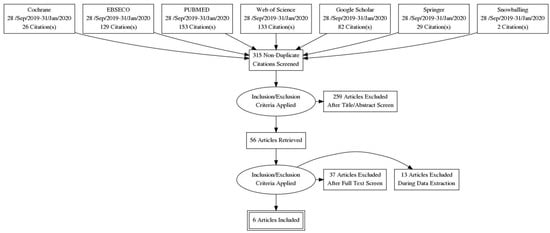
Figure 1.
PRISMA flow diagram.

Table 1.
Main characteristics of the studies included in the meta-analysis of the effects of GFD on BMI and HA1c in children with T1DM and CD.
Three studies were carried out in the United Kingdom [12,13,15]. The other three were carried out in Europe [14] (in 10 pediatric diabetic centers around Europe), Australia [16], and Italy [17].
The primary outcome results, including the basic demographics and inclusion/exclusion criteria from the six studies, were reported in peer-reviewed journals [12,13,14,15,16,17]. The number of patients varied between studies, as shown in Table 1. The study of Rami et al. [14] had the highest number of participants (n—293 at baseline and 269 at follow-up), while the study of Amin et al. [15] had the lowest number of participants (n—33) at baseline and at follow-up. However, all of the included studies had no significant loss to follow-up, except Rami et al. [14], which had 0.1% of the participants withdraw.
The patients’ age at CD diagnosis varied among the studies, ranging from 7.5 years in Saadah et al.’s study [16] to 13.8 years in Amin et al.’s study [15]. Regarding gender, the same percentage of females was distributed among the two groups, except in Rami et al. [14], where females accounted for 45% of the cases and 50% of the comparators. The follow-up time varied among the studies, but all of the studies had at least one year of follow-up, with Amin et al. having a longer follow-up period of 4 years [15]. According to GRADE guidelines, three studies [11,12,13] had a low risk of bias, and the other three [8,9,10] had a moderate risk of bias, Table A1.
Concerning the age of T1DM diagnosis in the group with a dual diagnosis of T1DM and CD, only one study, Saadah et al. [16], showed an earlier T1DM diagnosis in the groups of T1DM and CD patients in comparison to the control group with T1DM. The age range at T1DM diagnosis was between four and eight years, while the range of the diagnosis age for CD was between three and seventeen years. The average duration of T1DM diagnosis before CD diagnosis was 2.3 years (1–3.1 years).
4.2. Body Mass Index—SDS
Meta-analytical findings of the first condition are described in the forest plot, as shown in Figure 2. The pooled results of the overall effect of the six studies included in this research indicated that there was no statistically significant difference in the mean (0.00 (95% CI −0.03, 0.02)) of the SDS BMI of the T1DM patients between baseline and follow-up (p = 0.22). The pooled results indicated no significant heterogeneity between studies (p-value = 0.47). The index to quantify the dispersion of the effect (I2) statistic = zero, reflecting minimal heterogeneity. For the T1DM and CD patients, the pooled results of the overall effect studies indicated that there was no statistically significant difference in the mean (−0.28 (95% CI −0.75, 0.42)) of the SDS BMI for the T1DM group with CD between baseline and follow-up (p = 0.24). The pooled results indicate that there was significant heterogeneity between studies (p ≤ 0.001), and the index to quantify the dispersion of the effect (I2) statistic = 98%, which reflects significant amounts of heterogeneity, as shown in Figure 3.
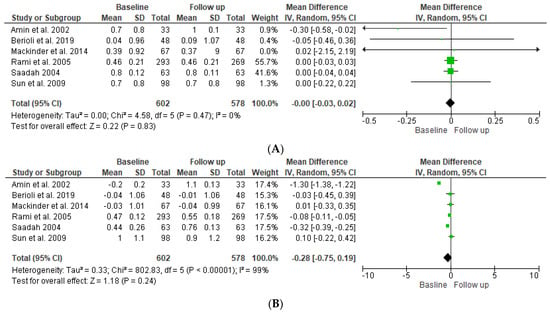
Figure 2.
Forest plot showing standard deviation scores (SDSs) of the BMI for DM patients (A) and DM and CD patients (B) at baseline compared to follow-up [12,13,14,15,16,17].
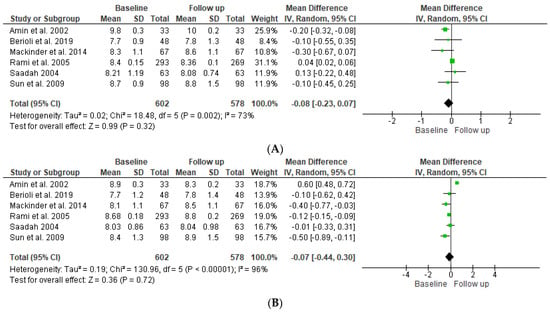
Figure 3.
Forest plot showing HbA1c for T1DM patients (A) and T1DM and CD patients (B) at baseline compared to follow-up [12,13,14,15,16,17].
4.3. Glycosylated Hemoglobin
Regarding HbA1c, as shown in the forest plot in Figure 3A, the pooled results of the overall effect of the six studies indicated that there was no statistically significant difference in the mean (mean difference −0.08 (95% −0.23, 0.07)) of the SDS BMI of T’DM patients between baseline and follow-up (p = 0.72). The pooled results indicate significant heterogeneity between studies (p ≤ 0.001), and the index to quantify the dispersion of effect (I2) statistic = 96%, which reflects significant heterogeneity. As shown in Figure 3B, the overall impact of both groups indicated that there was no statistically significant difference in the mean (mean difference −0.07 (95% CI −0.44, 0.30)) between baseline and follow-up ((p = 0.36). The pooled results indicate significant heterogeneity between studies (p ≤ 0.001), and the index to quantify the dispersion of effect (I2) statistic = 96%, which reflects significant heterogeneity.
4.4. Lipid Profile, Hemoglobin Levels, Diabetic Renopathy, and Retinopathy
As a secondary outcome, an improved lipid profile (HDL, total cholesterol, and triglyceride) after strict adherence to a GFD for at least six months was seen in three studies [18,19,20]. Hemoglobin and serum iron improved significantly after being on a GFD for at least six months [21,22]. Adherence to a GFD in patients with T1DM and CD might have a protective effect on the development of diabetic reno- and retinopathy [23,24,25,26], as seen in Table 2.

Table 2.
Effects of GFD in pediatric patients with a dual diagnosis of T1DM and asymptomatic CD.
5. Discussion
CD and T1DM are autoimmune pathologies where the body’s immune system produces autoantibodies that destroy its organs and tissues. These types of pathologies can affect any system in the body. Most of the time, more than one autoimmune disease can be present in the same patient concurrently, suggesting the necessity of a comprehensive management technique for multiple pathologies simultaneously. In this systematic review, a GFD did not significantly affect BMI-SDS and HA1c but showed the benefits of this specific diet on other health aspects, such as the lipid profile, diabetic retinopathy, and nephropathy.
CD and T1DM are highly associated with the HLA system, where they share common characteristics, such as the haplotypes A1, B8, DR3, and DQ2. The DQ2 locus, and mainly DQA1*0501/DQB1*, is found in over 90% of CD patients [40]. In patients with a dual diagnosis of CD and T1DM, the classic form of CD with typical gastrointestinal symptoms such as chronic diarrhea, body mass deficiency, abdominal pain, and flatulence may be observed in less than 25% of T1DM patients. However, extra-intestinal manifestations (short stature, iron deficiency anemia, or delayed puberty) and silent forms are more frequent. Recently published studies have shown that up to 71.4% of children with T1DM do not have gastrointestinal symptoms when CD-specific antibodies are detected [41,42]. International guidelines recommend CD screening at T1DM diagnosis and annually for five years [27]. In contrast, the Canadian Diabetes Association [28] recommends serologic testing based solely on clinical symptoms, including recurring gastrointestinal or extra-gastrointestinal symptoms and unexplained frequent low blood sugar levels. For adults, the recommendations are less specific [29].
In children, the most vital management technique for T1DM is dietary intervention, which can be challenging to follow and abide by in this age group [43]. In this particular group of patients, CD has a higher prevalence than in the normal population, and it is also managed by the annulment of gluten from the patient’s diet [44]. Combining these two strict dietary regimens (diabetic diet and GFD) in children can be very demanding, especially in the subgroup of patients with asymptomatic CD discovered by screening with specific antibodies and proven by small intestinal biopsy [30].
This meta-analysis and systematic review aimed to find an evidence-based approach to the effects of dietary management on these patients. The patients were diagnosed with T1DM for at least one year before CD diagnosis and starting a GFD to eliminate the effects of controlling blood sugar on weight at T1DM diagnosis that are associated with insulin therapy initiation.
The collected data show that children with T1DM and CD lost weight after being on a gluten-free diet for at least one year. This weight loss could be explained by the difficulty of following two restrictive diets, but these studies failed to report a significant change in BMI SDS for these patients despite ensuring adherence to GFD [7,20,31].
Saadeh et al. illustrated that all of the anthropometric parameters of their populations were above the mean for the reference population, but none of them reached statistical significance. On the other hand, Acerini and colleagues [40] concluded that the positive benefits of dietary therapy in CD patients without gastrointestinal symptoms were uncertain after studying seven children with T1DM and CD being managed with a GFD. Westman and colleagues [45] found that dietary compliance did not change growth parameters in twenty children with T1DM and CD. Height was another anthropometric parameter that was studied. It should be noted that there were no significant changes observed in any of the studies involved in this systematic review [13,14,15,16,17,40].
This meta-analysis did not show any changes in the HA1c levels of children with a dual diagnosis of T1DM and CD after being put on a GFD. In the years before CD diagnosis, HA1c levels were usually lower than the levels measured after the confirmed diagnosis in the same patients [12]; this could be attributed to malabsorption in the absence of serum antibodies. Studies show that the presence of antibodies in the duodenal mucosa before their presence in the serum could be responsible for some of the elements of malabsorption [46,47]. However, some studies have shown an increase in HA1C levels at the beginning of GFD, but this might be explained by restoring the small intestinal mucosa and increased absorption capabilities. Nevertheless, this did not prove significant after a follow-up of at least one year [40,45,48,49].
Rami et al. [14] further subdivided the group of patients with dual diagnoses of T1DM and CD according to adherence to a GFD. Comparing the adherent and non-adherent to GFD groups, a trend toward a lower BMI SDS but not a higher z-score was noted in the non-adherent group, raising the notion of a positive influence of a GFD on weight gain in patients who adhere to a GFD.
Kaur et al. [32] reported a significant decline in the mean HbA1c level of 0.73% in the GFD group; on the other hand, it increased by 0.99% in the non-GFD group at the end of the follow-up period. Additional prospective studies have reported similar findings [15,48]. Meanwhile, other studies did not find any significant improvement in the HbA1c levels after GFD initiation [16,33].
Kaur et al. [32] did not report any differences in the insulin dose. At the same time, Packer et al. showed improvement in the HbA1c levels after GFD initiation compared to the controls despite no changes in the daily insulin dose being noted in their longitudinal prospective study. GFD might positively affect insulin sensitivity, explaining the improvement in HA1c values. Although the glycemic index of gluten-free and gluten-containing foods is similar [34], data suggest that the carbohydrate type might influence insulin sensitivity [50]. Moreover, for hypoglycemic events experienced before the diagnosis of CD, one study, the one by Rami et al. [14], reported no differences in the frequency of severe hypoglycemic episodes (loss of consciousness or abnormal movements) in both of the studied groups. The required insulin dosage also was not significantly altered in the group with bothT1DM and CD.
During this search, some studies did not fully meet the inclusion/exclusion criteria of this meta-analysis but still provided a growing body of evidence on the beneficial effects of a GFD for patients with concomitant T1DM and CD and indicated that it may protect against the development of other T1DM-related complications, as seen Table 2.
Compared to T1DM patients without CD, Bakker et al. [26] showed that adult patients with TIDM and CD had a lower prevalence of retinopathy and lower total cholesterol than T1DM patients without CD. Warncke et al. [18] reported lower absolute systolic blood pressure in T1DM patients with CD than in those without CD. In the same cohort, patients with a dual diagnosis had significantly lower HDL cholesterol levels than individuals with T1DM alone at diagnosis; after the institution of a GFD, these levels increased significantly. The same effect was also noticed on bone density [51] as well as on hemoglobin and calcium levels [22]. A possible explanation for these improvements in cholesterol levels could be the normalization of the intestinal mucosa with the adoption of a GFD, demonstrating a beneficial effect of adhering to the diet.
Throughout our review, multiple papers have illustrated the possible reno-protective value of GFD on the progression of diabetic nephropathy. Malalasekera et al. [25] found that T1DM and CD patients had urinary albumin to creatinine ratios that were two-fold lower than those of patients with T1DM after adhering to a GFD for at least one year. Gopee et al. [24] reached similar findings of significantly lower urinary albumin to creatinine ratios in the same type of patients.
Although the pathophysiology of diabetic nephropathy is multifactorial, local inflammatory stress may result from both metabolic and hemodynamic derangements at a very early stage. Elmarakby et al. found significantly lower IL-1B, IL-4, and IL-5 levels in T1DM+CD patients who were adherent to a GFD than those with T1DM alone, suggesting a protective effect of a GFD on diabetic nephropathy progression [35,52].
Hypoglycemic episodes or a reduction in insulin requirements can be the presenting sign of CD in children with controlled T1DM. In contrast, a lack of hypoglycemic episodes at the clinical onset of CD could be the result of a higher mean blood glucose level [36]. In the six studies in this systematic review, only Rame et al. studied hypoglycemic episodes and insulin requirements. They found that no significant differences were documented after strict adherence to a GFD for at least one year of follow-up [14]. On the other hand, Mohn et al. reported a reduction in hypoglycemic events in children under a GFD [37].
Most of the studies in this systematic review, especially those with a large patient population, showed that a GFD normalizes the bowel mucosa and frequently leads to the disappearance of antibodies but may not necessarily lead to improved glycemic control, as seen in Table 2.
Some studies showed a positive effect on growth parameters after adhering to a GFD for at least one year [18,19,21,38,39], yet others report no significant effect on the anthropometric indices [7,31,37,45,48,49,53]. On the other hand, long-standing CD may be associated with an increased risk of retinopathy [24], while non-adherence to a GFD may increase microalbuminuria risk [21,23,25].
A recently published study [54] reported that no significant adverse outcomes were found in children with T1DM with positive celiac serology who delayed therapy with a GFD for two years. On the other hand, the ISPAD states that in asymptomatic children with proven CD, a GFD can be considered justified to reduce the long-term risk of gastrointestinal malignancy and conditions associated with subclinical malabsorption (i.e., osteoporosis and iron deficiency) [55].
Although there were more than 500 studies found during our search due to the different search methods implemented to cover as much of the online databases as possible, only six studies met our strict inclusion criteria to decrease confounders and to have more robust results. Studies not included in online databases were not explored, as this study was not funded. Three of the six studies included in the study had low-quality evidence, and adherence to a GFD was inconsistently documented.
6. Conclusions
This meta-analysis and systematic review could not establish any significant positive or negative effects of a GFD on anthropometric indices or glycemic control. Meanwhile, none of the studies could individually prove a significant negative effect of a GFD on these patients. On the other hand, many clinical studies demonstrated the positive impact of a strict GFD in these patients on different health aspects, such as the possible benefit of a GFD being reno-protective and establishing a favorable atherogenic profile. Screening for CD in patients with T1DM is recommended, even in asymptomatic children. Untreated CD can lead to long-term sequelae in children with CD and in those with dual diagnoses of T1DM and CD. Further studies with large cohorts and control trials are needed to establish substantial evidence of the positive effect of GFD in children with T1DM and CD.
Author Contributions
Conceptualization, S.B.; methodology, S.B.; software, A.F.; validation, S.B. and R.T.; formal analysis, N.E.; resources, A.F., O.A. and R.A.-N.; data curation, A.F., O.A. and R.A.-N.; writing—original draft preparation, S.B.; writing—review and editing, S.B, and R.T.; visualization, S.B; supervision, S.B. and R.T.; project administration, R.T. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was provided for this research. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available in Appendix A.
Conflicts of Interest
None of the authors declare any conflict of interest.
Appendix A
Search Strategy
We searched Pubmed (both Medline- and Non-Medline-indexed papers), Cochrane, EBSCO, Google Scholar, Springer, and Web of Science. Two or more search queries were used in some of the databases. Our main databases were Pubmed (both Medline- and Non-Medline-indexed papers), Cochrane, EBSCO, and Web of Science. We considered Springer and Google Scholar as ‘other’ sources. We also gathered some papers through snowballing. This document is divided into three sections: Main Databases, Other Databases, and Snowballing. Each section explains the strategy used.
Breakdown of the Strategy by Database
1. Main Databases
- A.
- Cochrane: Strategy #1
- Search Query:#1 (“coeliac disease”):ti,ab,kw OR (“celiac disease”):ti,ab,kw OR (“coeliac diseases”):ti,ab,kw OR (“gluten-sensitive enteropathies”):ti,ab,kw OR (“gluten-sensitive enteropathies”):ti,ab,kw#2 (“diabetes mellitus type 1”):ti,ab,kw OR (“DM 1”):ti,ab,kw OR (“DM-1”):ti,ab,kw OR (“juvenile diabetes”):ti,ab,kw OR (“juvenile diabetes mellitus”):ti,ab,kw#3 MeSH descriptor: [Celiac Disease] explode all trees#4 MeSH descriptor: [Diabetes Mellitus, Type 1] explode all trees#5 (“gluten free diet”):ti,ab,kw OR (“gluten free diets”):ti,ab,kw OR (“gluten-free”):ti,ab,kw OR (“gluten free”):ti,ab,kw#6 MeSH descriptor: [Diet, Gluten-Free] explode all trees#7 (#4 OR #2) AND (#6 OR #5)#8 ((#4 OR #2) AND (#1 OR #3)) AND (#5 OR #6)
- Time of Search:September 28, 2019, 12:54:31.
- Results:
9.
| BMC Gastroenterology (2015) 15:181 |
| BMJ Open 2015;5:e008097 |
| ClinicalTrials.gov Identifier: NCT02605564 |
| ClinicalTrials.gov Identifier: NCT02867436 |
| Diabetes Care. 1999 Oct;22(10):1747-8 |
| ClinicalTrials.gov Identifier: NCT03037190 |
| Diabetes Care 25:1111–1116, 2002 |
| ClinicalTrials.gov Identifier: NCT02605148 |
| ClinicalTrials.gov Identifier: NCT02680054 |
Strategy #2
- Search Query:#1 (“coeliac disease”):ti,ab,kw OR (“celiac disease”):ti,ab,kw OR (“coeliac diseases”):ti,ab,kw OR (“gluten-sensitive enteropathies”):ti,ab,kw OR (“gluten-sensitive enteropathies”):ti,ab,kw#2 (“diabetes mellitus type 1”):ti,ab,kw OR (“DM 1”):ti,ab,kw OR (“DM-1”):ti,ab,kw OR (“juvenile diabetes”):ti,ab,kw OR (“juvenile diabetes mellitus”):ti,ab,kw#3 MeSH descriptor: [Celiac Disease] explode all trees#4 MeSH descriptor: [Diabetes Mellitus, Type 1] explode all trees#5 (“gluten free diet”):ti,ab,kw OR (“gluten free diets”):ti,ab,kw OR (“gluten-free”):ti,ab,kw OR (“gluten free”):ti,ab,kw#6 MeSH descriptor: [Diet, Gluten-Free] explode all trees#7 (#4 OR #2) AND (#6 OR #5)#8 ((#4 OR #2) AND (#1 OR #3)) AND (#5 OR #6)
- Time of Search:September 28, 2019, 12:54:31.
- Results:
5.
| BMC Gastroenterology (2015) 15:181 |
| BMJ Open 2015;5:e008097 |
| ClinicalTrials.gov Identifier: NCT03037190 |
| Diabetes Care 1999 Oct;22(10):1747-8 |
| Diabetes Care 25:1111–1116, 2002 |
Strategy #3
- Search Query:#1 MeSH descriptor: [Celiac Disease] explode all trees 338#2 MeSH descriptor: [Diabetes Mellitus, Type 1] explode all trees 5369#3 MeSH descriptor: [Diet, Gluten-Free] explode all trees 84#4 MeSH descriptor: [Glycated Hemoglobin A] explode all trees 5489#5 MeSH descriptor: [Bone Density] explode all trees 4536#6 MeSH descriptor: [Quality of Life] explode all trees 22705#7 MeSH descriptor: [Growth] explode all trees 19573#8 MeSH descriptor: [Anemia, Iron-Deficiency] explode all trees 1269#9 (Celiac):ti,ab,kw OR (Coeliac):ti,ab,kw OR (Gluten sensitive Enteropathy):ti,ab,kw 1261#10 (T1DM):ti,ab,kw OR (Type 1 DM):ti,ab,kw OR (Type 1 Diabetes Mellitus):ti,ab,kw OR (Juvenile Diabetes):ti,ab,kw OR (Insulin Dependent Diabetes):ti,ab,kw 38096#11 (GFD):ti,ab,kw OR (Gluten Free):ti,ab,kw 550#12 (Glycemic control):ti,ab,kw OR (HbA1C):ti,ab,kw 25407#13 (BMD):ti,ab,kw OR (Bone Mineral Density):ti,ab,kw OR (Bone Density):ti,ab,kw 12701#14 (QOL):ti,ab,kw OR (Quality of Life):ti,ab,kw 112273#15 (Growth):ti,ab,kw 45637#16 (Iron Deficiency Anemia):ti,ab,kw 2988#17 (#1 OR #9) AND (#2 OR #10) AND (#3 OR #11) AND (#4 OR #5 OR #6 OR #7 OR #8 OR #12 OR #13 OR #14 OR #15 OR #16) 12
- Time of Search:February 7, 2020, 15:33:48.
- Results:
12.
| BMJ open, 2015, 5(5), e008097 |
| BMC gastroenterology, 2015, 15, 181 |
| BMJ open, 2015, 5(5), e008097 |
| Journal of pediatric gastroenterology and nutrition, 2017, 64(2), 175-179 |
| Pediatric diabetes 2018;19:49 |
| https://clinicaltrials.gov/show/NCT01566110, 2012 |
| BMC gastroenterology 2015;15(1): Article number: 181 |
| Pediatric diabetes 2014; 15: 114 |
| https://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/06/014508 |
| Diabetes care, 2011, 34(6), 1301-1305 |
| Pediatric Diabetes 2016;17(S24):33-34 |
| Diabetes care 1999;22(10):1747-1748 |
- B.
- EBSCO:
Strategy #1
- Search Query:S1: diabetes mellitus type 1 OR dm type 1 OR juvenile diabetes OR insulin dependent diabetes OR iddmS2: celiac disease OR gluten sensitive OR gluten enteropathy OR coeliac diseaseS3: gluten free diet OR special diets OR gluten free OR gfdS4: glycemic control OR hba1c OR glucosetolerance test OR blood glucose levels OR quality of lifeS5: S1 AND S3 AND S4S6: S1 AND S2 S3 AND S4*Limit for paper date: 1 January 2011–31 December 2020.*Databases Searched: Business Source Complete, Academic Search Ultimate, Art & Architecture Complete, MEDLINE Complete, Education Research Complete, Communication & Mass Media Complete, Environment Complete, Computers & Applied Sciences Complete, Legal Collection, Energy & Power Source, Research Starters—Education, Research Starters—Business.
- Time of Search:October 11, 2019, 5:13:41 AM.
- Results:
81.
| Food Reviews International. 2019, Vol. 35 Issue 6, p587-608 |
| SpringerPlus (2016) 5:994 |
| BMC Res Notes (2019) 12:50 |
| BMC Gastroenterology (2015) 15:181 |
| Indian J Med Res 149, January 2019, pp 18-25 |
| BMJ Open 2015;5:e008097 |
| BMC Gastroenterology 2014, 14:99 |
| Diabetes Ther (2019) 10:1151–1161 |
| British Journal of Nursing, 2019, Vol 28, No 15, 1015-1019 |
| Diabetes Therapy. Feb2019, Vol. 10 Issue 1, p119-134 |
| Acta Diabetol (2017) 54:889–894 |
| PLoS ONE. Nov2013, Vol. 8 Issue 11, p1-9 |
| Diabetic Medicine: A Journal Of The British Diabetic Association [Diabet Med] 2014 Feb; Vol. 31 (2), pp. 208-12 |
| Ethiop J Health Sci. Vol. 29, No. 4, 447-452 |
| Pediatric Diabetes [Pediatr Diabetes] 2011 Jun; Vol. 12 (4 Pt 1), pp. 322-5 |
| Journal of Diabetes Research. 12/20/2018, p1-11 |
| J Diabetes Investig 2019; 10: 104–107 |
| Turk J Endocrinol Metab 2017;21:127-130 |
| BMJ Case Reports 2012; doi:10.1136/bcr.02.2012.5878 |
| JURNALUL PEDIATRULUI – Year XVII, Vol. XVII, Supplement 1, 2014 |
| Pediatric Diabetes [Pediatr Diabetes] 2012 Dec; Vol. 13 (8), pp. 597-606 |
| Acta Diabetol (2013) 50:319–324 |
| Pediatric Diabetes [Pediatr Diabetes] 2012 Mar; Vol. 13 (2), pp. 163-9 |
| Scientific Reports volume7, Article number: 45286 (2017) |
| Diabetes Obes Metab. 2019;21:1769–1779 |
| Diabetes Care 34:2158-2163, 2011 |
| Journal of Diabetes 9 (2017), 865–873 |
| Diabetes Care 2015;38:801–807 |
| Diabetes Ther (2017) 8:875–886 |
| Diabetol Metab Syndr (2016) 8:51 |
| International Journal of Health Sciences Vol. 11, Issue 2 (April–June 2017), 65-71 |
| Nutrition & Metabolism (2019) 16:60 |
| ANNALS VOL 23, ISSUE 3, JULY–SEPT. 2017, 289-293 |
| Dig Dis Sci (2012) 57:1314–1320 |
| Nutrition & Metabolism (2017) 14:11 |
| JAMA August 15, 2017 Volume 318, Number 7, 637-646 |
| Aust N Z J Obstet Gynaecol 2019; 59: 208–214 |
| Indian Journal of Endocrinology and Metabolism (2016), 443-450 |
| Am J Clin Nutr 2019;109:288–296 |
| Nutrition Journal 2013, 12:29 |
| Pediatric Diabetes [Pediatr Diabetes] 2016 May; Vol. 17 (3), pp. 191-8 |
| Diabetic Medicine. Jul2013, Vol. 30 Issue 7, p835-839 |
| Lipids in Health and Disease (2016) 15:78 |
| Food & Nutrition Research 2016, 60: 32594 |
| Diabetic Medicine. May2018, Vol. 35 Issue 5, p541-547 |
| Nutrition Journal (2018) 17:42 |
| Asia Pac J Clin Nutr 2018;27(3):728-734 |
| Vojnosanit Pregl 2012; 69(10): 858–863 |
| J Community Health (2016) 41:584–592 |
| Diabet. Med. 36, 653–654 (2019) |
| Pediatric Diabetes. Sep2013, Vol. 14 Issue 6, p455-458 |
| Diabetes Care 2016;39:808–815 |
| J Nippon Med Sch 2013; 80 (3) |
| Journal of Nutrition & Metabolism. 2012, p1-21 |
| International Journal of Endocrinology. 2013, p1-7 |
| Eur J Nutr (2013) 52:813–824 |
| Journal of Jahrom University of Medical Sciences. 2012, Vol. 9 Issue 4, following p7-7 |
| Am J Clin Nutr 2017;106:1394–400 |
| Diabetes 2017;66:1373–1379 |
| Nutrition & Metabolism (2018) 15:80 |
| Diabetic Medicine. Nov2017, Vol. 34 Issue 11, p1554-1567 |
| Journal of Human Nutrition & Dietetics. Jun2017, Vol. 30 Issue 3, p385-393 |
| Diabetic Medicine. Sep2015, Vol. 32 Issue 9, p1149-1155 |
| Journal of Clinical Pharmacy and Therapeutics (2011) 36, 592–601 |
| BMC Public Health (2018) 18:525 |
| Diabetes 2017;66:627–639 |
| British Journal of Nutrition (2018), 119, 910–917 |
| Diabetes, Obesity & Metabolism. Jul2014, Vol. 16 Issue 7, p577-587 |
| Journal of Human Nutrition & Dietetics. Apr2014 Supplement, p21-27 |
| Diabetes 2017;66:75–86 |
| Ann Nutr Metab 2011;58:74–78 |
| British Journal of Nursing, 2017, Vol 26, No 10, 543-551 |
| Diabetes Care 2014;37:1824–1830 |
| Diabetes Care 2016;39:893–901 |
| South African Journal of Clinical Nutrition. 2016, Vol. 29 Issue 2, p90-94 |
| Australian and New Zealand Journal of Obstetrics and Gynaecology 2016; 56: 333–335 |
| Geriatr Gerontol Int 2012; 12 (Suppl. 1): 41–49 |
| Journal of Cystic Fibrosis 15 (2016) 261–262 |
| ACP Journal Club. 2/17/2015, Vol. 162 Issue 4, p1-1 |
| American Journal of Clinical Nutrition. Mar2013, Vol. 97 Issue 3, p453-454 |
| Diabetes Health. Oct/Nov2011, Vol. 20 Issue 5, p20-20 |
Strategy #2
- Search Query:S1: diabetes mellitus type 1 OR dm type 1 OR juvenile diabetes OR insulin dependent diabetes OR iddmS2: celiac disease OR gluten sensitive OR gluten enteropathy OR coeliac diseaseS3: gluten free diet OR special diets OR gluten free OR gfdS4: glycemic control OR hba1c OR glucosetolerance test OR blood glucose levels OR quality of lifeS5: S1 AND S3 AND S4S6: S1 AND S2 S3 AND S4*Limit for paper date: 1 January 2011–31 December 2020*Databases Searched: Business Source Complete, Academic Search Ultimate, Art & Architecture Complete, MEDLINE Complete, Education Research Complete, Communication & Mass Media Complete, Environment Complete, Computers & Applied Sciences Complete, Legal Collection, Energy & Power Source, Research Starters—Education, Research Starters—Business
- Time of Search:October 11, 2019, 5:13:41 AM.
- Results:
24.
| Food Reviews International 2019, Vol. 35 Issue 6, p587-608 |
| Indian J Med Res 149, January 2019, pp 18-25 |
| BMJ Open 2015;5:e008097 |
| BMC Gastroenterology (2015) 15:181 |
| BMC Gastroenterology 2014, 14:99 |
| Acta Diabetol (2017) 54:889–894 |
| Diabetic Medicine: A Journal Of The British Diabetic Association [Diabet Med] 2014 Feb;Vol. 31 (2), pp. 208-12 |
| Pediatric Diabetes [Pediatr Diabetes] 2011 Jun; Vol. 12 (4 Pt 1), pp. 322-5 |
| Scientific Reports volume7, Article number: 45286 (2017) |
| Pediatric Diabetes [Pediatr Diabetes] 2012 Dec; Vol. 13 (8), pp. 597-606 |
| Diabetes Care 34:2158-2163, 2011 |
| Pediatric Diabetes [Pediatr Diabetes] 2012 Mar; Vol. 13 (2), pp. 163-9 |
| Diabetes Care 2015;38:801–807 |
| Dig Dis Sci (2012) 57:1314–1320 |
| Pediatric Diabetes [Pediatr Diabetes] 2016 May; Vol. 17 (3), pp. 191-8 |
| Turk J Endocrinol Metab 2017;21:127-130 |
| Diabetol Metab Syndr (2016) 8:51 |
| JURNALUL PEDIATRULUI – Year XVII, Vol. XVII, Supplement 1, 2014 |
| Acta Diabetol (2013) 50:319–324 |
| ANNALS VOL 23, ISSUE 3, JULY–SEPT. 2017 |
| Diabetic Medicine. Jul2013, Vol. 30 Issue 7, p835-839 |
| BMJ Case Reports 2012; doi:10.1136/bcr.02.2012.5878 |
| Pediatric Diabetes. Sep2013, Vol. 14 Issue 6, p455-458 |
| Diabetes Health. Oct/Nov2011, Vol. 20 Issue 5, p20-20 |
Strategy #3
- Search Query:S1: Celiac OR Coeliac OR Gluten Sensitive EnteropathyS2: T1DM OR Type 1 DM OR Type 1 Diabetes Mellitus OR Juvenile Diabetes OR Insulin Dependent DiabetesS3: GFD OR Gluten FreeS4: Glycemic Control OR HBA1C OR BMD OR Bone Mineral Density OR Bone Density OR QOL OR Quality of Life OR Growth OR Iron Deficiency AnemiaS5: S1 AND S2 AND S3 AND S4*Databases Searched: MEDLINE Complete, eBook Collection (EBSCOhost), MEDLINE, eBook Academic Collection (EBSCOhost), CINAHL Plus with Full Text
- Time of Search:January 31, 2020. 3:00 PM.
- Results:
24.
| Diabetic Medicine Dec2009; 26(12): 1250-1254 |
| Journal of Pediatric Gastroenterology & Nutrition Feb2017; 64(2): 175-179 |
| BMC Gastroenterology 12/21/2015; 15: 181 |
| Pediatric Diabetes Dec2012; 13(8): 597-606 |
| Diabetic Medicine Jul2013; 30(7): 835-839 |
| Acta Paediatrica Apr 2017; 106(4): 639-646 |
| Diabetes Care Jul2002; 25(7): 1117-1122 |
| Pediatric Diabetes Jun2011; 12(4pt1): 322-325 |
| Pediatric Diabetes May2016; 17(3): 191-198 |
| Healy, Shavonne Access Nov2014; 28(9): 14-15 NLM UID: 9885733 |
| Diabetic Medicine Aug2005; 22(8): 1079-1082 |
| http://dx.doi.org/10.1136/bcr-2013-200472 |
| Archives of Disease in Childhood Sep2004; 89(9): 871-876 |
| Diabetes Care Oct2011; 34(10): 2158-2163. |
| Turkish Journal of Endocrinology & Metabolism 2017; 21(4): 127-130 |
| Digestive Diseases & Sciences May2012; 57(5): 1314-1320 |
| BMC Gastroenterology 2014; 14(1): 99 https://doi.org/10.1186/1471-230X-14-99 |
| Practical Diabetes International Apr2011; 28(3): 110-112 |
| http://dx.doi.org/10.1136/adc.87.6.495 |
| Gastroenterology & Hepatology from Bed to Bench Autumn2014; 7(4): 189-197 |
| Pediatric Diabetes Mar2012; 13(2): 163-169 |
| BioMed Research International 2013; 2013: 127589-127589 |
| Clinical Medicine & Research Oct2007; 5(3): 184-192 |
| British Journal of Diabetes & Vascular Disease Mar2008; 8(2): 67-71 |
- C.
- Pubmed:
Strategy #1
- Search Query:(((“Diabetes Mellitus, Type 1”[MeSH Terms] AND (“Diet, Gluten-Free”[MeSH Terms] OR “Foods, Specialized”[MeSH Terms] OR “specialized diet”[All Fields]OR “special diet”[All Fields] OR “modified diet”[All Fields] OR “selective diet”[All Fields] OR “restrictive diet”[All Fields] OR “restricted diet”[All Fields] OR “restricted food intake”[All Fields] OR “restricted dietary intake”[All Fields] OR (restricted[All Fields] AND (“diet”[MeSH Terms] OR “diet”[All Fields] OR “dietary”[All Fields]) AND practices[All Fields]))) AND (“Blood Glucose”[MeSH Terms] OR “Hyperglycemia”[MeSH Terms] OR “Glucose Tolerance Test”[MeSH Terms] OR “Glycated Hemoglobin A”[MeSH Terms] OR “Diabetes Complications”[MeSH Terms] OR “Quality of Life”[MeSH Terms] OR “Life Style”[MeSH Terms] OR “Hospitalization”[MeSH Terms] OR “glycemic control”[All Fields] OR “re admission”[All Fields] OR “blood sugar control”[All Fields] OR “blood glucose control”[All Fields] OR “hospital admission”[All Fields])) AND (“humans”[MeSH Terms] AND (“infant”[MeSH Terms] OR “child”[MeSH Terms] OR “adolescent”[MeSH Terms]))) OR (((((“celiac”[All Fields] OR “coeliac”[All Fields] OR “celiac sprue”[All Fields] OR “nontropical sprue”[All Fields] OR (gluten-sensitive[All Fields] AND enteropath[All Fields]) OR “gluten-induced enteropathy”[All Fields] OR “gluten induced enteropathy”[All Fields]) OR (“type 1 diabetes mellitus”[All Fields] OR “type 1 diabetes”[All Fields] OR “juvenile diabetes”[All Fields] OR “t1dm”[All Fields] OR “t1d”[All Fields] OR “type-1 diabetes”[All Fields] OR “insulin dependent diabetes”[All Fields] OR “insulin dependant diabetes”[All Fields] OR “insulin-dependent diabetes”[All Fields] OR “insulin-dependant diabetes”[All Fields])) AND (“gluten-free diet”[All Fields] OR “gfd”[All Fields] OR “gluten free diet”[All Fields] OR “specialized diet”[All Fields] OR “restrictive diet”[All Fields] OR “restricted diet”[All Fields] OR “special diet”[All Fields] OR “gf diet”[All Fields] AND “specialized diet”[All Fields] OR “special diet”[All Fields] OR “modified diet”[All Fields] OR “selective diet”[All Fields] OR “restrictive diet”[All Fields] OR “restricted diet”[All Fields] OR “restricted food intake”[All Fields] OR “restricted dietary intake”[All Fields] OR (restricted[All Fields] AND (“diet”[MeSH Terms] OR “diet”[All Fields] OR “dietary”[All Fields]) AND practices[All Fields]))) AND (“blood glucose”[All Fields] OR “hyperglycemia”[All Fields] OR “glucose tolerance test”[All Fields] OR “glycated hemoglobin A”[All Fields] OR “diabetes complications “[All Fields] OR “quality of life”[All Fields] OR “Life Style”[All Fields] OR “hospitalization”[All Fields] OR “glycemic control”[All Fields] OR “re admission”[All Fields] OR “blood sugar control”[All Fields] OR “blood glucose control”[All Fields] OR “hospital admission”[All Fields] OR “qol”[All Fields] OR “hba1c”[All Fields] OR “blood sugar”[All Fields] OR “re admission”[All Fields] OR (hemoglobyn[All Fields] AND a1c[All Fields]))) NOT medline[sb]) AND (“humans”[MeSH Terms] AND (“infant”[MeSH Terms] OR “child”[MeSH Terms] OR “adolescent”[MeSH Terms]))
- Time of Search:October 4, 2019.
- Results:
29.
| Scientific Reports | 7:45286, 2017, 1-7 |
| J Pediatr. 2016 Dec;179:131-138 |
| Kylökäs et al. BMC Gastroenterology (2016) 16:76 |
| Assor et al. BMC Gastroenterology (2015) 15:181, 1-10 |
| Diabet Med. 2016 Jul;33(7):947-55 |
| Nutr Hosp. 2015;32(2):634-637 |
| BMJ Open 2015;5:e008097, 1-8 |
| BMC Gastroenterology 2014, 14:99, 1-8 |
| J Obstet Gynecol Neonatal Nurs. 2013 Nov-Dec;42(6):619-28 |
| Diabet Med. 2014 Feb;31(2):208-12 |
| Pediatr Diabetes 2013 Sep;14(6):455-8 |
| BMJ Case Reports 2012, 1-3 |
| Pediatr Diabetes 2012 Dec;13(8):597-606 |
| Dig Dis Sci. 2012 May;57(5):1314-20 |
| DIABETES CARE, VOLUME 34, OCTOBER 2011, 2158-2163 |
| Pediatr Diabetes. 2012 Mar;13(2):163-9 |
| J Pediatr Endocrinol Metab. 2010 Nov;23(11):1169-73 |
| Dtsch Med Wochenschr. 2011 Feb;136(5):172-5 |
| Acta Biomed. 2010 Dec;81(3):165-70 |
| Diabet Med. 2009 Dec;26(12):1250-4 |
| Diabetologia (2009) 52:798–800 |
| Rev Latino-am Enfermagem 2005 janeiro-fevereiro; 14(1) |
| European Journal of Clinical Nutrition (2004) 58, 1429–1431 |
| Atherosclerosis. 1999 Aug;145(2):389-97 |
| J Am Diet Assoc. 1996 May;96(5):458-63 |
| Nurse Pract Forum. 1991 Sep;2(3):193-5 |
| Diabetes Care. 1987 Jan-Feb;10(1):33-8 |
| Zentralbl Gynakol. 1986;108(11):691-8 |
| Diabetes. 1975 Jul;24(7):672-9 |
Strategy #2
- Search Query”(“Celiac Disease”[Mesh] AND “Diabetes Mellitus, Type 1”[Mesh]) AND (“Diet Therapy”[Mesh] OR “diet therapy”[Subheading]) AND (“2009/12/03”[PDat]: “2019/11/30”[PDat] AND (“infant”[MeSH Terms] OR “child”[MeSH Terms] OR “adolescent”[MeSH Terms]))
- Time of Search:November 30, 2019.
- Results:
40.
| Indian J Med Res. 2019 Jan;149(1):18-25 |
| Pediatr Diabetes. 2019 May;20(3):293-303 |
| Can J Diet Pract Res. 2018 Sep 1;79(3):118-124 |
| Acta Diabetol. 2017 Oct;54(10):889-894 |
| Sci Rep. 2017 Mar 24;7:45286 |
| J Pediatr Gastroenterol Nutr. 2017 Aug;65(2):195-199 |
| J Pediatr. 2016 Dec;179:131-138 |
| BMC Gastroenterol. 2016 Jul 25;16(1):76 |
| Rev Gaucha Enferm. 2016 Mar;37(1):e53787 |
| J Diabetes Complications. 2016 Mar;30(2):295-9 |
| BMC Gastroenterol. 2015 Dec 21;15:181 |
| Lik Sprava. 2015 Jan-Mar;(1-2):167-9 |
| BMJ Open. 2015 May 11;5(5):e008097 |
| Diabetes Care. 2015 May;38(5):760-6 |
| Diabetes Care. 2014 Sep;37(9):e194-5 |
| J Paediatr Child Health. 2014 Oct;50(10):811-6 |
| BMC Gastroenterol. 2014 May 28;14:99 |
| Nutrients. 2013 Nov 18;5(11):4540-52 |
| Eur J Clin Invest. 2014 Jan;44(1):74-82 |
| Diabet Med. 2014 Feb;31(2):208-12 |
| Indian J Gastroenterol. 2013 Sep;32(5):330-4 |
| Pediatr Diabetes. 2013 Sep;14(6):455-8 |
| Indian J Gastroenterol. 2014 Mar;33(2):188-9 |
| J Gastroenterol Hepatol. 2013 Jan;28(1):99-105 |
| Indian J Gastroenterol. 2013 Mar;32(2):98-102 |
| Pediatr Diabetes. 2012 Dec;13(8):597-606 |
| Clin Exp Immunol. 2012 Feb;167(2):226-34 |
| Dig Dis Sci. 2012 May;57(5):1314-20 |
| Diabetes Care. 2011 Oct;34(10):2158-63 |
| Transl Res. 2011 Sep;158(3):140-5 |
| Pediatr Diabetes. 2012 Mar;13(2):163-9 |
| Pediatr Diabetes. 2011 Jun;12(4 Pt 1):322-5 |
| Diabetes Care. 2011 Jun;34(6):1301-5 |
| J Pediatr Endocrinol Metab. 2010 Nov;23(11):1169-73 |
| J Gastroenterol Hepatol. 2011 Feb;26(2):378-81 |
| Diabetes Res Clin Pract. 2011 Apr;92(1):53-6 |
| Acta Biomed. 2010 Dec;81(3):165-70 |
| J Pediatr. 2011 Feb;158(2):276-81.e1 |
| Gastroenterol Clin Biol. 2010 Apr-May;34(4-5):319-20. |
| Rheumatol Int. 2010 Apr;30(6):793-5 |
Strategy #3
- Search Query((((((“diabetes mellitus, type 1”[mesh]) and “celiac disease”[mesh]))) AND (((((“diet therapy”[mesh]) or “nutrition therapy”[mesh]) or “diet, gluten-free”[mesh]))))) AND (((((((readmission) OR hospital readmission*s) OR “Patient Readmission”[Mesh]) OR patient readmission)) OR ((((((blood glucose) OR “Blood Glucose”[Mesh]) OR hypoglycemic control) OR glycemic control) OR diabetes management)) OR ((((((quality of life) OR “Quality of Life”[Mesh]) OR life quality) OR health quality)))) OR ((“diabetes “ and “celiac “ and “gluten” and “diet” NOT medline[sb]))
- Time of Search:November 18, 2019.
- Results:
63.
| Diabetes Care. 2016 Aug;39(8):e119-20. |
| JAMA. 2016 Sep 20;316(11):1181-1192. |
| World J Diabetes. 2013 Aug 15;4(4):130-4. |
| doi: 10.2174/0929867326666190409120716. |
| Gastroenterol Hepatol Bed Bench. 2014 Fall;7(4):189-97. |
| World J Diabetes. 2015 Jun 10;6(5):707-14. |
| Horm Res Paediatr. 2019 Oct 8:1-8. |
| Diseases. 2015 Jun 19;3(2):111-121 |
| Clin Diabetes Endocrinol. 2018 Dec 19;4:24. |
| Exp Ther Med. 2014 Dec;8(6):1906-1908. Epub 2014 Oct 15. |
| Pak J Med Sci. 2014 Mar;30(2):287-90. |
| Case Rep Med. 2012;2012:813461. |
| Prz Gastroenterol. 2018;13(3):249-250 |
| J Clin Gastroenterol. 2019 Nov/Dec;53(10):e416-e423. |
| Clin Exp Gastroenterol. 2014 Dec 29;8:43-8 |
| Eat Weight Disord. 2018 Oct 27. |
| Case Rep Pediatr. 2012;2012:269689 |
| Middle East J Dig Dis. 2011 Mar;3(1):5-12. |
| Front Neurosci. 2017 Mar 27;11:155. |
| Case Rep Endocrinol. 2017;2017:2652403. |
| Ont Health Technol Assess Ser. 2011;11(3):1-63. Epub 2011 Jul 1. |
| J Community Hosp Intern Med Perspect. 2019 Feb 11;9(1):22-24 |
| Mayo Clin Proc. 2016 Dec 5. pii: S0025-6196(16)30634-6. |
| Clin Transl Gastroenterol. 2019 May 22;10(5):1-10. |
| United European Gastroenterol J. 2018 Aug;6(7):1022-1031 |
| Ann Hepatol. 2016 Jul–Aug;15(4):588-591. |
| Indian J Gastroenterol. 2019 Jun;38(3):263-267. |
| Nutrients. 2019 Aug 16;11(8). pii: E1925. |
| Indian J Med Res. 2019 Jan;149(1):18-25. |
| Hum Mol Genet. 2019 Sep 15;28(18):3037-3042 |
| Acta Paediatr. 2019 Apr;108(4):676-680 |
| J Clin Gastroenterol. 2019 Oct 31. |
| J Pediatr. 2016 Dec;179:131-138.e1 |
| BMC Gastroenterol. 2015 Dec 21;15:181 |
| Sci Rep. 2017 Mar 24;7:45286 |
| Can J Diet Pract Res. 2018 Sep 1;79(3):118-124. |
| Pediatr Diabetes. 2012 Mar;13(2):163-9 |
| Dig Dis Sci. 2012 May;57(5):1314-20. |
| Pediatr Diabetes. 2011 Jun;12(4 Pt 1):322-5 |
| Indian J Med Res. 2019 Jan;149(1):18-25. |
| Nutr Diabetes. 2017 Jan 9;7(1):e239 |
| Ann Hepatol. 2016 Jul-Aug;15(4):588-91 |
| BMJ Open. 2015 May 11;5(5):e008097. |
| Vnitr Lek. 2014 Jul-Aug;60(7-8):562-6 |
| J Paediatr Child Health. 2014 Oct;50(10):811-6. |
| BMC Gastroenterol. 2014 May 28;14:99. |
| Nutrients. 2013 Nov 27;5(12):4869-79. |
| Eur J Clin Invest. 2014 Jan;44(1):74-82 |
| Pediatr Diabetes. 2013 Sep;14(6):455-8 |
| Diabetes Educ. 2013 Jul-Aug;39(4):532-40 |
| Diabet Med. 2013 Jul;30(7):835-9 |
| Pediatr Diabetes. 2012 Dec;13(8):597-606 |
| Acta Diabetol. 2013 Jun;50(3):319-24 |
| Diabetes Care. 2011 Oct;34(10):2158-63 |
| Pediatr Diabetes. 2011 Jun;12(4 Pt 1):322-5 |
| Atherosclerosis. 2011 Aug;217(2):531-5. |
| J Pediatr Endocrinol Metab. 2010 Nov;23(11):1169-73. |
| Acta Biomed. 2010 Dec;81(3):165-70. |
| Tunis Med. 2010 Jan;88(1):18-22. |
| Przegl Lek. 2009;66(4):170-5. |
| Diabetologia. 2009 May;52(5):798-800 |
| Bone. 2008 Aug;43(2):322-6 |
| Diabetes Care. 2002 Jul;25(7):1117-22. |
Strategy #4
- Search Query:((“Celiac Disease”[Mesh] AND “Diabetes Mellitus, Type 1”[Mesh]) AND “Diet, Gluten-Free”[Mesh]) AND ((((“Glycated Hemoglobin A”[Mesh] OR “Bone Density”[Mesh]) OR “Quality of Life”[Mesh]) OR “Growth”[Mesh]) OR “Anemia, Iron-Deficiency”[Mesh])
- Time of Search:February 8, 2020. 11:54 AM.
- Results:
18.
| Nutr Diabetes. 2017 Jan 9;7(1):e239 |
| J Pediatr. 2016 Dec;179:131-138 |
| BMC Gastroenterol. 2015 Dec 21;15:181 |
| BMJ Open. 2015 May 11;5(5):e008097 |
| Diabetes Care. 2015 May;38(5):801-7 |
| BMC Gastroenterol. 2014 May 28;14:99 |
| Diabet Med. 2014 Feb;31(2):208-12 |
| Diabet Med. 2013 Jul;30(7):835-9 |
| Pediatr Diabetes. 2012 Dec;13(8):597-606 |
| Acta Diabetol. 2013 Jun;50(3):319-24 |
| Dig Dis Sci. 2012 May;57(5):1314-20 |
| Pediatr Diabetes. 2012 Mar;13(2):163-9 |
| Atherosclerosis. 2011 Aug;217(2):531-5 |
| J Pediatr Endocrinol Metab. 2010 Nov;23(11):1169-73 |
| J Gastroenterol Hepatol. 2011 Feb;26(2):378-81 |
| Acta Biomed. 2010 Dec;81(3):165-70 |
| Tunis Med. 2010 Jan;88(1):18-22 |
| Diabet Med. 2009 Dec;26(12):1250-4 |
Strategy #5
- Search Query:(“Celiac” OR “Coeliac” OR “Gluten Sensitive Enteropathy”) AND (“T1DM” OR “Type 1 DM” OR “Type 1 Diabetes Mellitus” OR “Juvenile Diabetes” OR “Insulin Dependent Diabetes”) AND (“GFD” OR “Gluten Free”) AND (“Glycemic Control” OR “HbA1C” OR “BMD” OR “Bone Mineral Density” OR “Bone Density” OR “QOL” OR “Quality of Life” OR “Growth” OR “Iron Deficiency Anemia”) NOT Medline[Sb]
- Time of Search:February 9, 2020. 6:59 PM.
- Results:
3.
| Diabetol Metab Syndr. 2016 Jul 29;8:51 |
| World J Diabetes. 2015 Jun 10;6(5):707-14 |
| World J Diabetes. 2013 Aug 15;4(4):130-4 |
- D.
- Web of Science:
Strategy #1
- Search Query:TS=(“type 1 diabetes mellitus” OR “dm type 1” OR “dmt1” OR “juvenile diabetes” OR “insulin-dependent diabetes mellitus” OR “diabetes mellitus type 1” OR “type 1 diabetes”) AND TS=(“gluten-free diet” OR “gluten free” OR “gfd” OR “special diet” OR “gluten free diet” OR “gluten-free”) AND ALL=(glycemic control OR blood glucose OR hba1c OR glucose tolerance test OR quality of life)Timespan: 2011-2019.Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI.
- Time of Search:October 18, 2019.
- Results:
53.
| International Journal of Endocrinology Volume 2019, 1-9 |
| Pediatric Diabetes. 2019;1–10 |
| European Journal of Inflammation,2019, Volume 17: 1–5 |
| Journal of Functional Foods Volume 56, May 2019, Pages 163-170 |
| PAEDIATRICS AND INTERNATIONAL CHILD HEALTH 2019, VOL. 39, NO. 1, 23–31 |
| John Wiley & Sons Ltd. 2018 107, pp. 1879–1887 |
| Velasco-Benítez CA/et al/Colombia Médica - Vol. 49 Nº4 2018 (Oct-Dec), 273-279 |
| Pediatric Diabetes October 2018; 19 (Suppl. 27); 275-286 |
| Diabetes Research and Clinical Practice Volume 143, September 2018, Pages 282-287 |
| United European Gastroenterology Journal 2018, Vol. 6(7) 1022–1031 |
| Journal of Child Psychology and Psychiatry, 60, 7, (803-812), (2018) |
| J Endocrinol Metab. 2018;8(2-3):37-42 |
| The Lancet Child & Adolescent Health Volume 2, Issue 2, February 2018, Pages 133-143 |
| Dig Dis 2018;36:399–408 |
| British Journal of Hospital Medicine,2017, Vol. 78, No. 10 |
| Acta Diabetologica October 2017, Volume 54, Issue 10, pp 889–894 |
| ANNALS OF KING EDWARD MEDICAL UNIVERSITY LAHORE PAKISTAN, 2017, Volume: 23 Issue: 3 Pages: 307-311 |
| Scientific Reports | 7:45286, 2017, 1-7 |
| Adv Nutr 2017;8:356–61 |
| JPGN Volume 64, Number 2, February 2017, 175-179 |
| Nutrition & Diabetes (2017) 7, e239, 1-6 |
| The Journal of Pediatrics Volume 179, December 2016, Pages 131-138 |
| Indian Journal of Gastroenterology September 2016, Volume 35, Issue 5, pp 372–378 |
| Diabetol Metab Syndr (2016) 8:51 |
| SpringerPlus (2016) 5:994 |
| ANNALS OF HEPATOLOGY,2016, Volume: 15 Issue: 4 Pages: 588-591 |
| Gastroenterology (2015) 15:181 |
| Canadian Journal of Diabetes Volume 39, Issue 6, December 2015, Pages 513-519 |
| Nature Reviews Gastroenterology & Hepatology volume 12, pages 580–591 (2015) |
| Nutrients 2015, 7, 8733–8751 |
| Nature Reviews Gastroenterology & Hepatology volume 12, pages 507–515 (2015) |
| WORLD JOURNAL OF DIABETES,2015, Volume: 6 Issue: 5 Pages: 707-714 |
| Diabetes Care 2015;38:760–766 |
| United European Gastroenterology Journal 2015, Vol. 3(2) 106–120 |
| Journal of Pediatric Nursing Volume 30, Issue 2, March–April 2015, Pages 353-363 |
| BMJ Open 2015;5:e008097, 1-8 |
| Diabetes, Obesity and Metabolism 17: 3–8, 2015 |
| Scandinavian Journal of Gastroenterology. 2014; 49: 1304–1310 |
| J Paediatr Child Health,2014, 50: 811-816 |
| Pediatr Diabetes,2014, 15: 270-278 |
| BMC Gastroenterology 2014, 14:99 |
| PLOS ONE, November 2013 | Volume 8 | Issue 11, 1-9 |
| Acta Diabetologica October 2013, Volume 50, Issue 5, pp 821–822 |
| Acta Diabetologica June 2013, Volume 50, Issue 3, pp 319–324 |
| Der Diabetologe March 2013, Volume 9, Issue 2, pp 128–134 |
| Pediatric Diabetes,2012, Volume 13, Issue 8, 597-606 |
| Italian Journal of Pediatrics 2012, 38:10 |
| Pediatric Diabetes,2012, 13: 163-169 |
| European Journal of Gastroenterology & Hepatology: December 2011 - Volume 23 - Issue 12 - p 1270–1272 |
| Diabetes Care 34:2158–2163, 2011 |
| Atherosclerosis Volume 217, Issue 2, August 2011, Pages 531-535 |
| Pediatric Diabetes,2011, 12: 322-325 |
| J Pediatr. 2011 February; 158(2): 276–281 |
Strategy #2
- Search Query:#1 (TS=(Celiac OR Coeliac OR Gluten Sensitive Enteropathy)) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article)Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCITimespan=All years#2 (TS=(T1DM OR Type 1 DM OR Type 1 Diabetes Mellitus OR Juvenile Diabetes OR Insulin Dependent Diabetes)) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article)Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCITimespan=All years#3 (TS=(GFD OR Gluten Free)) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article)Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCITimespan=All years#4 (TS=(Glycemic Control OR HbA1C OR BMD OR Bone Mineral Density OR Bone Density OR QOL OR Quality of Life OR Growth OR Iron Deficiency Anemia)) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article)Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCITimespan=All years#5 #4 AND #3 AND #2 AND #1Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCITimespan=All years
- Time of Search:January 31, 2020. 4:38 PM.
- Results:
80.
| PEDIATRICS DEC 2005;116(6):E754-E759 |
| DIABETES CARE NOV 2006;29(11):2452-2456 |
| CURRENT OPINION IN GASTROENTEROLOGY MAR 2010;26(2):116-122 |
| AMERICAN JOURNAL OF CLINICAL NUTRITION MAR 1998;67(3):477-481 |
| JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION OCT 2001;33(4):462-465 |
| CANADIAN JOURNAL OF GASTROENTEROLOGY AND HEPATOLOGY MAY 2001;15(5):297-301 |
| DIABETES CARE JUL 2002;25(7):1117-1122 |
| DIABETES CARE OCT 2011;34(10):2158-2163 |
| ARCHIVES OF DISEASE IN CHILDHOOD SEP 2004;89(9):871-876 |
| GASTROENTEROLOGY APR 2005;128(4)(suppl. 1):S52-S56 |
| JOURNAL OF PEDIATRIC ENDOCRINOLOGY & METABOLISM MAY-JUN 1999;12(3):433-442 |
| HEPATO-GASTROENTEROLOGY MAR-APR 2001;48(38):462-464 |
| DIABETIC MEDICINE AUG 2005;22(8):1079-1082 |
| POSTGRADUATE MEDICAL JOURNAL FEB 2007;83(976):132-136 |
| ALIMENTARY PHARMACOLOGY & THERAPEUTICS APR 15 2009;29(8):898-905 |
| JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION SEP 2005;41(3):317-321 |
| ACTA PAEDIATRICA 2002;91(3):297-302 |
| JOURNAL OF PEDIATRICS MAY 2007;150(5):461-466 |
| JOURNAL OF GASTROENTEROLOGY AND HEPATOLOGY FEB 2011;26(2):378-381 |
| PEDIATRIC DIABETES JUN 2011;12(4pt1):322-325 |
| ATHEROSCLEROSIS AUG 2011;217(2):531-535 |
| PEDIATRIC DIABETES JUN 2007;8(3):171-176 |
| WORLD JOURNAL OF GASTROENTEROLOGY AUG 28 2012;18(32):4399-4403 |
| JOURNAL OF PEDIATRICS FEB 2011;158(2):276-U141 |
| DIABETES CARE MAY 2015;38(5):760-766 |
| PEDIATRICS SEP 2009;124(3):E489-E495 |
| PEDIATRIC DIABETES DEC 2012;13(8):597-606 |
| JOURNAL OF PEDIATRICS APR 2011;158(4):589-U94 |
| DIABETIC MEDICINE DEC 2009;26(12):1250-1254 |
| JOURNAL OF PEDIATRIC HEMATOLOGY ONCOLOGY FEB 2003;25(2):169-172 |
| JOURNAL OF CLINICAL GASTROENTEROLOGY MAY-JUN 2008;42(5):460-465 |
| CLINICAL AND EXPERIMENTAL RHEUMATOLOGY JAN-FEB 2012;30(1):126-131 |
| DIABETOLOGIA JUL 2014;57(7):1339-1345 |
| PEDIATRIC DIABETES AUG 2008;9(4pt1):277-284 |
| DIABETIC MEDICINE JUL 2005;22(7):889-892 |
| ACTA DIABETOLOGICA JUN 2013;50(3):319-324 |
| HORMONE AND METABOLIC RESEARCH APR 2002;34(4):192-195 |
| BEST PRACTICE & RESEARCH CLINICAL GASTROENTEROLOGY JUN 2005;19(3):479-486 |
| PEDIATRIC DIABETES MAR 2012;13(2):163-169 |
| SCANDINAVIAN JOURNAL OF GASTROENTEROLOGY MAR 3 2016;51(3):288-294 |
| PEDIATRICS INTERNATIONAL FEB 2015;57(1):107-112 |
| BMJ Open. 2015 May 11;5(5):e008097 |
| JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION FEB 2017;64(2):175-179 |
| DIABETES EDUCATOR JUL 2013;39(4):532-540 |
| JOURNAL OF PEDIATRICS 2016 Dec;179:131-138 |
| PEDIATRICS INTERNATIONAL AUG 2010;52(4):579-583 |
| BONE AUG 2008;43(2):322-326 |
| JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION SEP 2015;61(3):297-302 |
| JOURNAL OF PEDIATRIC ENDOCRINOLOGY & METABOLISM NOV-DEC 2000;13(9):1629-1631 |
| Bratisl Lek Listy. 2009;110(4):258-62 |
| SAUDI MEDICAL JOURNAL DEC 2002;23(12):1514-1517 |
| BMC Gastroenterol. 2014 May 28;14:99 |
| ACTA DIABETOLOGICA OCT 2017;54(10):889-894 |
| ACTA DIABETOLOGICA DEC 2015;52(6):1167-1174 |
| JOURNAL OF PAEDIATRICS AND CHILD HEALTH OCT 2014;50(10):811-816 |
| EUROPEAN JOURNAL OF GASTROENTEROLOGY & HEPATOLOGY NOV 2011;23(12):1270-1272 |
| DIABETIC MEDICINE DEC 2014;31(12):E33-E36 |
| JOURNAL OF PEDIATRIC ENDOCRINOLOGY & METABOLISM NOV 2010;23(11):1169-1173 |
| ACTA PAEDIATRICA APR 2017;106(4):639-646 |
| JOURNAL OF PEDIATRIC NURSING-NURSING CARE OF CHILDREN & FAMILIES MAR-APR 2015;30(2):353-363 |
| PEDIATRIC DIABETES JUN 2018;19(4):741-748 |
| PEDIATRIC DIABETES JUN 2018;19(4):749-755 |
| Nutr Diabetes. 2017 Jan 9;7(1):e239 |
| CANADIAN JOURNAL OF DIABETES SEP 2011;35(4):334-339 |
| DIABETES RESEARCH AND CLINICAL PRACTICE SEP 2018;143():282-287 |
| JOURNAL OF ENDOCRINOLOGY AND METABOLISM MAY 2018;8(2-3):37-42 |
| INDIAN JOURNAL OF GASTROENTEROLOGY SEP 2016;35(5):372-378 |
| Book (Capter 10:Extraintestinal Manifestations of Celiac Disease and Associated Disorders) https://doi.org/10.3926/oms.258 |
| Book (Chapter: Celiac Disease in Adults) https://doi.org/10.3926/oms.233 |
| Int J Endocrinol. 2019 Sep 19;2019:7895207 |
| PEDIATRIC DIABETES DEC 2019;20(8):1100-1109 |
| EUROPEAN JOURNAL OF INFLAMMATION JUN 2019;17():- |
| JOURNAL OF PEDIATRIC ENDOCRINOLOGY & METABOLISM JAN 2019;32(1):89-93 |
| COLOMBIA MEDICA OCT-DEC 2018;49(4):273-279 |
| PEDIATRIC GASTROENTEROLOGY HEPATOLOGY & NUTRITION DEC 2017;20(4):222-226 |
| ANNALS OF KING EDWARD MEDICAL UNIVERSITY LAHORE PAKISTAN JUL-SEP 2017;23(3):307-311 |
| GAZZETTA MEDICA ITALIANA ARCHIVIO PER LE SCIENZE MEDICHE MAY 2017;176(5):308-313 |
| Book (Chapter 9.3: Coeliac Disease and diabetes) https://doi.org/10.1002/9781119121725.ch32 |
| Book (Chapter:Extraintestinal Manifestations and Associated Diseases) https://doi.org/10.3926/oms.215 |
| Przegląd Gastroenterologiczny 2011; 6 (4): 209–212 |
2. Other Databases
- A.
- Google Scholar:
Strategy #1
- Search Query:with all of the words:“diabetes mellitus type 1” “gluten free” “glycemic” “glucose” celiac childrenwith the exact phrase:gluten-free dietwith at least one of the words:without the words:where my words occur:Return articles authored by:Return articles published in:Return articles dated between:2011–2020
- Time of Search:October 18, 2019.
- Results:
40
| Nutrients 2015, 7, 7143-7162 |
| Pediatr Diabetes. 2018 October; 19(Suppl 27): 275–286 |
| Pediatr Diabetes. 2015 November; 16(7): 485–492 |
| Ann. Pak. Inst. Med. Sci. 2018, 47-51 |
| Human and Veterinary Medicine; Cluj-Napoca Vol. 9, Iss. 1, (Mar 2017): 11-15 |
| Clinical Biochemistry 54 (2018) 11–17 |
| European Journal of Endocrinology (2016) 174, R127–R138 |
| Nat. Rev. Gastroenterol. Hepatol. 8, 405–415 (2011) |
| Google E-book Autoimmune associated diseases in pediatric patients with type 1 diabetes mellitus according to hla-dq genetic polymorphism |
| Oman Medical Journal (2015), Vol. 30, No. 2: 83–89 |
| Autoimmunity Reviews 16 (2017) 712–721 |
| The Impact of Coexisting Coeliac Disease on Type 1 Diabetes By Anna Pham-Short |
| American Society for Nutrition. Adv. Nutr. 4: 277–286, 2013 |
| Diabetes Research and Clinical Practice Volume 123, January 2017, Pages 63-74 |
| book Diabetic bone disease: Basic and translational research and clinical applications (pp.3-24) |
| Bakker-van Waarde, W. M. (2006). Bile salt and cholesterol metabolism in diabetes mellitus type 1 |
| Google E-book Medical Management of Type 1 Diabetes Sixth Edition American Diabetes Association |
| Ontario Health Technology Assessment Series 2011; Vol. 11, No. 3, 1-63 |
| Children 2018, 5, 169, 1-13 |
| Book STUDY ON THE GLYCAEMIC CONTROL AND RELATED COMPLICATIONS IN TYPE 1 DIABETIC CHILDREN By Dr. HEMA G. R. MBBS |
| Google E-book Glucose Regulation, an issue of nursing clinics by Celia M. Levesque |
| Physiol. Res. 67 (Suppl. 3): S441-S454, 2018 |
| Current Diabetes Reports October 2017, 17:83 |
| Journal of Pediatric Endocrinology and Metabolism Volume 20, Issue 12 |
| Feingold KR, Anawalt B, Boyce A et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000- |
| Feingold KR, Anawalt B, Boyce A et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000- |
| The Diabetes Textbook,2019, pp 941-966 |
| Hindawi Publishing Corporation Journal of Diabetes Research Volume 2015, 1-20 |
| A Practical Approach to Adolescent Bone Health,2018, pp 179-218 |
| Book Wheat Syndromes How Wheat, Gluten and ATI Cause Inflammation, IBS and Autoimmune Diseases |
| Journal of Pediatric Endocrinology and Metabolism Volume 22, Issue 12 |
| Critical Reviews™ in Immunology Volume 33, 2013 Issue 3, 245-281 |
| Book MUDr. Michal Huml Dizertační práce: Vliv Gastrointestinálního traktu na kompenzaci diabetes mellitus typu 1 v dětském věku Gastrointestinální hormony |
| Diabetol Metab Syndr 2018, 10(Suppl 1):27 |
| Current Opinion in Pediatrics: August 2010 - Volume 22 - Issue 4 - p 545–558 |
| Zsu, Hma, Pzheng - pdfs.semantischolar.org (Link not found) |
| Book MA Weinberg, SL Segelnick, JS Insler, S Kramer -2015 Wiley online library (File not found) |
| Book Endocrinology A methodological approach towards integrative understanding BOLK’S COMPANIONS ON THE PRACTICE OF MEDICINE |
| Book COMPARATIVE ANALYSIS OF THE SOLUBLE WHEAT PROTEINS AND HUMAN HEALTH Nhu Tuyen Vu The University of Sydney March 2014 |
| Book Analyza wybranych czynikow u dzieci z nowo rozpoznana cukryca typu 1 i ich wplyw na przebieg kliniczny choroby |
Strategy #2
- Search Query:with all of the words → celiac diabeteswith the exact phrase → gluten free dietwith at least one of the words → pediatrics children infants adolescent “diabetes type 1” “type 1 diabetes” “gluten free” diet regimen2011-2020*Results listed below (42 references) include results of this search strategy plus papers retrieved through snowballing.
- Time of Search:
November 18, 2019.
- Results:
42.
| Diabetes Care 2011 Oct; 34(10): 2158-2163 |
| World J Diabetes. Aug 15, 2013; 4(4): 130-134 |
| Acta Diabetologica; Heidelberg Vol. 50, Iss. 5, (Oct 2013): 821-2. |
| Nutrients 2015, 7(9), 7143-7162 |
| J Pediatr. 2011 February; 158(2): 276–281 |
| The Journal of Pediatrics. April 2011, 158, (4):Pages 589-593.e2 |
| Pediatr Diabetes. 2011 Jun;12(4 Pt 1):322-5 |
| Pediatr Diabetes. 2012 Dec;13(8):597-606. |
| Diabetes Care 2015 May; 38(5): 760-766. |
| BMC Gastroenterology volume 14, Article number: 99 (2014) |
| BMC Gastroenterol. 2015 Dec 21;15:181 |
| Diabetes Care 2016 Aug; 39(8): e119-e120 |
| Acta Bio Med 2011, 81, 165-170 |
| Journal of Pediatric Gastroenterology and Nutrition. 41(3):317-321, SEPTEMBER 2005 |
| Pediatric Diabetes. 10():92–93, SEPTEMBER 2009 |
| J ASEAN Fed Endocr Soc, 2016, 31(1): 5-9 |
| Berioli, M. G., Mancini, G., Principi, N., Santi, E., Ascenzi, M., Rogari, F., … Esposito, S. (2019). Growth and glycemic control in children with type 1 diabetes and asymptomatic celiac disease treated with a gluten -free diet for 1 year. European Journal of Inflammation. https://doi.org/10.1177/2058739219855574 |
| Gastroenterology Rev 2018; 13 (3): 249–250 |
| AGA.May 2009,136, (5)(Suppl 1):A-473, |
| Diabetes Research and Clinical Practice,June 2009,84,(3), 332-334 |
| The Journal of Clinical Endocrinology & Metabolism, Volume 88, Issue 1, 1 January 2003, Pages 162–165, |
| Gastroenterol Clin Biol. 2010 Apr-May;34(4-5):319-20 |
| Clin Nutr. 2004 Apr;23(2):281-2. |
| La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics, 01 Mar 2007, 29(2):99-104 |
| Pediatric Diabetes, 2(3), 95–97 |
| Journal of Pediatric Endocrinology & Metabolism, 9, 101-111 (1996) |
| pediatric diabetes Volume12, Issue4pt1 June 2011 |
| Clin Pediatr Endocrinol 1998; 7(2), 125-129 |
| Hormone Research. February 2002; 57,(Suppl. 1):97-100 |
| Diabetic Medicne.December 2009, 26;(12): 1250-1254 |
| Diabetes Care 1998 Aug; 21(8): 1379-1380. |
| Diabetes Care 2006 Nov; 29(11): 2452-2456. |
| Diabetic medicine.1998;15,(1):38-44 |
| Diabetic Medicine. 2005;22(8),1079-1082 |
| acta paediatrica. March 2002;91(3):297-302 |
| Journal of Pediatric Endocrinology & Metabolism, 12,433-442 (1999) |
| World J Diabetes. 2013 Aug 15; 4(4): 130–134. |
| Acta Biomed. 2010 Dec;81(3):165-70 |
| Acta Diabetol 2013;50:319–324 |
| BMC Gastroenterology,December 2014, 14:99 |
| Prz Gastroenterol. 2018;13(3):249-250 |
| J Pediatr Endocrinol Metab. 2010 Nov;23(11):1169-73 |
- B.
- Springer:
- Search Query:(diabetes mellitus type 1 OR juvenile diabetes OR dmt1 OR dm-1 OR insulin dependent diabetes) AND (Celiac OR coeliac OR gluten sensitive OR gluten enteropathy) AND (gluten free OR gfd OR special diet) AND (glycemic control OR hba1c OR blood glucose OR glucose tolerance test OR blood glucose control)Limiters used: ‘Article Type’ ‘English Language’ ‘2011–2020′
- Time of Search:October 12, 2019.
- Results:
29.
| Diabetologia September 2019, Volume 62, Supplement 1, pp 1–600 |
| Diabetologia (2014) 57:[Suppl1]S1–S566 |
| Internal and Emergency Medicine December 2012, Volume 7, Supplement 4, pp 361–568 |
| Journal of General Internal Medicine May 2019, Volume 34, Supplement 2, pp 99–867 |
| Internal and Emergency Medicine December 2011, Volume 6, Supplement 2, pp 191–392 |
| Journal of General Internal Medicine,2017, Vol. 32, Issue 2, S83-S808 |
| European Journal of Pediatrics November 2016, Volume 175, Issue 11, pp 1393–1880 |
| Journal of General Internal Medicine,2014, Vol. 29, Issue 1, S1- S545 |
| Journal of General Internal Medicine, 2018, Vol. 33, Issue. 2, S1- S758 |
| Journal of General Internal Medicine, 2015, Vol. 30, Issue. 2, S45-S551 |
| Hepatology International June 2013, Volume 7, Supplement 1, pp 1–754 |
| Journal of General Internal Medicine, 2016, Vol. 31, Issue. 2, S85-S922 |
| Obesity Surgery August 2011, Volume 21, Issue 8, pp 956–1156 |
| Journal of General Internal Medicine June 2013, Volume 28, Supplement 1, pp 1–489 |
| Osteoporosis International April 2018, Volume 29, Supplement 1, pp 149–565 |
| Indian Journal of Gastroenterology October 2018, Volume 37, Supplement 1, pp 1–137 |
| Internal and Emergency Medicine December 2011, Volume 6, Supplement 2, pp 141–190 |
| Inflammation Research June 2011, Volume 60, Supplement 1, pp 1–321 |
| Hepatology International March 2015, Volume 9, Supplement 1, pp 1–391 |
| Journal of Inherited Metabolic Disease September 2015, Volume 38, Supplement 1, pp 35–378 |
| Journal of Neurology June 2013, Volume 260, Supplement 1, pp 1–280 |
| Journal of Inherited Metabolic Disease September 2016, Volume 39, Supplement 1, pp 35–284 |
| Internal and Emergency Medicine December 2012, Volume 7, Supplement 4, pp 309–359 |
| Journal of Neurology June 2012, Volume 259, Supplement 1, pp 1–236 |
| Indian Journal of Gastroenterology November 2013, Volume 32, Supplement 1, pp 1–132 |
| Italian Journal of Pediatrics 2017, 43(Suppl 2):110, 1-42 |
| Journal of Inherited Metabolic Disease September 2018, Volume 41, Supplement 1, pp 37–219 |
| Irish Journal of Medical Science (1971 -) March 2018, Volume 187, Supplement 3, pp 17–113 |
| Advances in Rheumatology 2018, 58(Suppl 1):23, 1-115 |
3. Snowballing
- Results:
2.
| J Pediatr Gastroenterol Nutr. 2001 Jan;32(1):37-40. |
| Endocrine Abstracts (2011);27:58 |

Table A1.
Table of Quality Assessment GRADE.
Table A1.
Table of Quality Assessment GRADE.
| Reference | Sun et al., 2009 [12] | Mackinder et al., 2014 [13] | Rami et al., 2005 [14] | Amin et al., 2002 [15] | Berioli et al., 2019 [17] | Saadah et al., 2004 [16] |
|---|---|---|---|---|---|---|
| Study Design | Observational | Observational | Observational | Observational | Observational | Observational |
| Risk of Bias | None Found | None Found | None found | Not serious | Serious | Serious |
| Inconsistency | None | None | None | Serious | Not serious | Not serious |
| Indirectness | None | None | None | |||
| Imprecision | None | None | None | Serious | Serious | Serious |
| Publication Bias | None | None | None | None | None | None |
| Large Effect | No | No | No | No | No | No |
| Dose Response (Gradient) | Yes (A 5-year gradient is shown) | Yes (A 4-year gradient is shown) | Yes | Yes | Yes | Yes |
| All Plausible Confounding | - | - | - | - | - | - |
| Strength | Moderate (3) | Moderate (3) | Moderate (3) | Low (2) | Low (2) | Low (2) |
References
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2019. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Riechmann, E.R.; de Villasante, G.C.; Pascual, M.L.C.; Aliaga, E.D.; Allué, I.P.; Sánchez-Valverde, F.; Koninckx, C.R. Rational application of the new European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) 2020 criteria for the diagnosis of coeliac disease. An. Pediatría 2020, 92, 110.e1–110.e9. [Google Scholar] [CrossRef]
- Picarelli, A.; Di Tola, M.; Sabbatella, L.; Mercuri, V.; Pietrobono, D.; Bassotti, G.; D’Amico, T.; Donato, G.; Picarelli, G.; Marino, M.; et al. Type 1 diabetes mellitus and celiac disease: Endothelial dysfunction. Acta Diabetol. 2013, 50, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Elfström, P.; Sundström, J.; Ludvigsson, J.F. Systematic review with meta-analysis: Associations between coeliac disease and type 1 diabetes. Aliment. Pharmacol. Ther. 2014, 40, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Assor, E.; Marcon, M.A.; Hamilton, N.; Fry, M.; Cooper, T.; Mahmud, F.H. Design of a dietary intervention to assess the impact of a gluten-free diet in a population with type 1 Diabetes and Celiac Disease. BMC Gastroenterol. 2015, 15, 181. [Google Scholar] [CrossRef][Green Version]
- Al, H.E.T. Cölyak Tanı Guideline I. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 1–19. [Google Scholar]
- Taler, I.; Phillip, M.; Lebenthal, Y.; de Vries, L.; Shamir, R.; Shalitin, S. Growth and metabolic control in patients with type 1 diabetes and celiac disease: A longitudinal observational case-control study. Pediatr. Diabetes 2012, 13, 597–606. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 372, n71. [Google Scholar] [CrossRef]
- Brannsether, B.; Eide, G.E.; Roelants, M.; Bjerknes, R.; Júlíusson, P.B. BMI and BMI SDS in childhood: Annual increments and conditional change. Ann. Hum. Biol. 2017, 44, 28–33. [Google Scholar] [CrossRef]
- Satya Krishna, S.; Kota, S.; Modi, K. Glycemic variability: Clinical implications. Indian J. Endocrinol. Metab. 2013, 17, 611. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; Debeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Puttha, R.; Ghezaiel, S.; Skae, M.; Cooper, C.; Amin, R. The effect of biopsy-positive silent coeliac disease and treatment with a gluten-free diet on growth and glycaemic control in children with Type 1 diabetes. Diabet. Med. 2009, 26, 1250–1254. [Google Scholar] [CrossRef]
- Mackinder, M.; Allison, G.; Svolos, V.; Buchanan, E.; Johnston, A.; Cardigan, T.; Laird, N.; Duncan, H.; Fraser, K.; Edwards, C.A.; et al. Nutritional status, growth and disease management in children with single and dual diagnosis of type 1 diabetes mellitus and coeliac disease. BMC Gastroenterol. 2014, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Rami, B.; Sumnik, Z.; Schober, E.; Waldhör, T.; Battelino, T.; Bratanic, N.; Kürti, K.; Lebl, J.; Limbert, C.; Madacsy, L.; et al. Screening detected celiac disease in children with type 1 diabetes mellitus: Effect on the clinical course (a case control study). J. Pediatr. Gastroenterol. Nutr. 2005, 41, 317–321. [Google Scholar] [CrossRef]
- Amin, R.; Murphy, N.; Edge, J.; Ahmed, M.L.; Acerini, C.L.; Dunger, D.B. A longitudinal study of the effects of a gluten-free diet on glycemic control and weight gain in subjects with type 1 diabetes and celiac disease. Diabetes Care 2002, 25, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Saadah, O.I.; Zacharin, M.; O’Callaghan, A.; Oliver, M.R.; Catto-Smith, A.G. Effect of gluten-free diet and adherence on growth and diabetic control in diabetics with coeliac disease. Arch. Dis. Child. 2004, 89, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Berioli, M.G.; Mancini, G.; Principi, N.; Santi, E.; Ascenzi, M.; Rogari, F.; Ceccarini, G.; Grohmann, U.; Esposito, S. Growth and glycemic control in children with type 1 diabetes and asymptomatic celiac disease treated with a gluten -free diet for 1 year. Eur. J. Inflamm. 2019, 17, 1–5. [Google Scholar] [CrossRef]
- Warncke, K.; Liptay, S.; Fröhlich-Reiterer, E.; Scheuing, N.; Schebek, M.; Wolf, J.; Rohrer, T.R.; Meissner, T.; Holl, R.W. Vascular risk factors in children, adolescents, and young adults with type 1 diabetes complicated by celiac disease: Results from the DPV initiative. Pediatr. Diabetes 2016, 17, 191–198. [Google Scholar] [CrossRef]
- Nagl, K.; Bollow, E.; Liptay, S.; Rosenbauer, J.; Koletzko, S.; Pappa, A.; Näke, A.; Fröhlich-Reiterer, E.; Döring, C.; Wolf, J.; et al. Lower HbA1c in patients with type 1 diabetes and celiac disease who reached celiac-specific antibody-negativity—A multicenter DPV analysis. Pediatr. Diabetes 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- Salardi, S.; Maltoni, G.; Zucchini, S.; Iafusco, D.; Zanfardino, A.; Confetto, S.; Toni, S.; Zioutas, M.; Marigliano, M.; Cauvin, V.; et al. Whole lipid profile and not only HDL cholesterol is impaired in children with coexisting type 1 diabetes and untreated celiac disease. Acta Diabetol. 2017, 54, 889–894. [Google Scholar] [CrossRef]
- Hansen, D.; Brock-Jacobsen, B.; Lund, E.; Bjørn, C.; Hansen, L.P.; Nielsen, C.; Fenger, C.; Lillevang, S.T.; Husby, S. Clinical benefit of a gluten-free diet in type 1 diabetic children with screening-detected celiac disease: A population-based screening study with 2 years’ follow-up. Diabetes Care 2006, 29, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Avinash, A.; Sukriti, K.; Syed, R.M.; Keshav, G.K.; Abhinav, G. Impact of a gluten-free diet on several growth parameters in children with type 1 diabetes mellitus and celiac disease in western Uttar Pradesh, India. J. ASEAN Fed. Endocr. Soc. 2016, 31, 5–9. [Google Scholar] [CrossRef][Green Version]
- Pham-Short, A.; Donaghue, K.C.; Ambler, G.; Chan, A.K.; Hing, S.; Cusumano, J.; Craig, M.E. Early elevation of albumin excretion rate is associated with poor gluten-free diet adherence in young people with coeliac disease and diabetes. Diabet. Med. 2014, 31, 208–212. [Google Scholar] [CrossRef]
- Gopee, E.; van den Oever, E.L.; Cameron, F.; Thomas, M.C. Coeliac disease, gluten-free diet and the development and progression of albuminuria in children with type 1 diabetes. Pediatr. Diabetes 2013, 14, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Malalasekera, V.; Cameron, F.; Grixti, E.; Thomas, M.C. Potential reno-protective effects of a gluten-free diet in type 1 diabetes. Diabetologia 2009, 52, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Bakker, S.F.; Tushuizen, M.E.; Von Blomberg, M.E.; Mulder, C.J.; Simsek, S. Type 1 diabetes and celiac disease in adults: Glycemic control and diabetic complications. Acta Diabetol. 2013, 50, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Uchigata, Y. Diabetes in childhood and adolescence. J. Jpn. Diabetes Soc. 2004, 47, 895–897. [Google Scholar]
- Wherrett, D.; Huot, C.; Mitchell, B.; Pacaud, D. Type 1 Diabetes in Children and Adolescents. Can. J. Diabetes 2013, 37 (Suppl. S1), S153–S162. [Google Scholar] [CrossRef]
- DeMelo, E.N.; McDonald, C.; Saibil, F.; Marcon, M.A.; Mahmud, F.H. Celiac Disease and Type 1 Diabetes in Adults: Is This a High-Risk Group for Screening? Can. J. Diabetes 2015, 39, 513–519. [Google Scholar] [CrossRef]
- Kaur, N.; Bhadada, K.; Minz, R.W. Interplay between Type 1 Diabetes Mellitus and Celiac Disease: Implications in Treatment. Dig. Dis. 2018, 160012, 399–408. [Google Scholar] [CrossRef]
- Goh, V.L.; Elizabeth Estrada, D.; Lerer, T.; Balarezo, F.; Sylvester, F.A. Effect of gluten-free diet on growth and glycemic control in children with type 1 diabetes and asymptomatic celiac disease. J. Pediatr. Endocrinol. Metab. 2010, 23, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Agarwala, A.; Makharia, G.; Bhatnagar, S.; Tandon, N. Effect of gluten-free diet on metabolic control and anthropometric parameters in type 1 diabetes with subclinical celiac disease: A randomized controlled trial. Endocr. Pract. 2020, 26, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Arató, A.; Körner, A.; Verès, G.; Dezsöfi, A.; Ujpál, I.; Madácsy, L. Frequency of coeliac disease in Hungarian children with type 1 diabetes mellitus. Eur. J. Pediatr. 2003, 162, 1–5. [Google Scholar] [CrossRef]
- Packer, S.C.; Dornhorst, A.; Frost, G.S. The glycaemic index of a range of gluten-free foods. Diabet. Med. 2000, 17, 657–660. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Russell, G.B.; Miller, M.E.; Rich, S.S.; Mauer, M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care 2013, 36, 3234–3239. [Google Scholar] [CrossRef] [PubMed]
- Kiayias, J.A.; Vlachou, E.D.; Lakka-Papadodima, E. Venlafaxine HCl in the Treatmant of Painful Peripheral Diabetic Neuropathy. Diabetes Care 2000, 23, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Mohn, A.; Cerruto, M.; Iafusco, D.; Prisco, F.; Tumini, S.; Stoppoloni, O.; Chiarelli, F. Celiac disease in children and adolescents with type I diabetes: Importance of hypoglycemia. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Reiterer, E.E.; Kaspers, S.; Hofer, S.; Schober, E.; Kordonouri, O.; Pozza, S.B.D.; Holl, R.W. Anthropometry, metabolic control, and follow-up in children and adolescents with type 1 diabetes mellitus and biopsy-proven celiac disease. J. Pediatr. 2011, 158, 589–593.e2. [Google Scholar] [CrossRef]
- Narula, P.; Porter, L.; Langton, J.; Rao, V.; Davies, P.; Cummins, C.; Kirk, J.; Barrett, T.; Protheroe, S. Gastrointestinal symptoms in children with type 1 diabetes screened for celiac disease. Pediatrics 2009, 124, e489–e495. [Google Scholar] [CrossRef]
- Acerini, C.L.; Ahmed, M.L.; Ross, K.M.; Sullivan, P.B.; Bird, G.; Dunger, D.B. Coeliac diseaese in children and adolescents with IDDM: Clinical characteristics and response to Gluten-free diet. Diabet. Med. 1998, 15, 38–44. [Google Scholar] [CrossRef]
- Telega, G.; Bennet, T.R.; Werlin, S. Emerging new clinical patterns in the presentation of celiac disease. Arch. Pediatr. Adolesc. Med. 2008, 162, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Forde, L.; McGrath, N.; Devaney, D.; Awadalla, S.; McDonnell, C.M.; Murphy, N.P. Coeliac screening in a high-risk population: Paediatric type 1 diabetes—A review of current guidelines and practice. Ir. J. Med. Sci. 2019, 188, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Patton, S.R. Adherence to Diet in Youth with Type 1 Diabetes. J. Am. Diet. Assoc. 2011, 111, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Glissen Brown, J.R.; Singh, P. Coeliac disease. Paediatr. Int. Child Health 2019, 39, 23–31. [Google Scholar] [CrossRef]
- Westman, E.; Ambler, G.R.; Royle, M.; Peat, J.; Chan, A. Children with coeliac disease and insulin dependent diabetes mellitus—Growth, diabetes control and dietary intake. J. Pediatr. Endocrinol. Metab. 1999, 12, 433–442. [Google Scholar] [CrossRef]
- Kaukinen, K.; Peräaho, M.; Collin, P.; Partanen, J.; Woolley, N.; Kaartinen, T.; Nuutinen, T.; Halttunen, T.; Mäki, M.; Korponay-Szabo, I. Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: A prospective and randomized clinical study. Scand. J. Gastroenterol. 2005, 40, 564–572. [Google Scholar] [CrossRef]
- Fuchs, V.; Kurppa, K.; Huhtala, H.; Mäki, M.; Kekkonen, L.; Kaukinen, K. Delayed celiac disease diagnosis predisposes to reduced quality of life and incremental use of health care services and medicines: A prospective nationwide study. United Eur. Gastroenterol. J. 2018, 6, 567–575. [Google Scholar] [CrossRef]
- Sanchez-Albisua, I.; Wolf, J.; Neu, A.; Geiger, H.; Wäscher, I.; Stern, M. Coeliac disease in children with Type 1 diabetes mellitus: The effect of the gluten-free diet. Diabet. Med. 2005, 22, 1079–1082. [Google Scholar] [CrossRef]
- Sponzilli, I.; Chiari, G.; Iovane, B.; Scarabello, C.; Gkliati, D.; Monti, G.; Fanciullo, L.; de’ Angelis, G.L.; Vanelli, M. Celiac disease in children with type 1 diabetes: Impact of gluten free diet on diabetes management. Acta Biomed. 2010, 81, 165–170. [Google Scholar]
- Hu, F.B.; Van Dam, R.M.; Liu, S. Diet and risk of Type II diabetes: The role of types of fat and carbohydrate. Diabetologia 2001, 44, 805–817. [Google Scholar] [CrossRef]
- Valerio, G.; Spadaro, R.; Iafusco, D.; Lombardi, F.; Del Puente, A.; Esposito, A.; De Terlizzi, F.; Prisco, F.; Troncone, R.; Franzese, A. The influence of gluten free diet on quantitative ultrasound of proximal phalanxes in children and adolescents with type 1 diabetes mellitus and celiac disease. Bone 2008, 43, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Abdelsayed, R.; Liu, J.Y.; Mozaffari, M.S. Inflammatory cytokines as predictive markers for early detection and progression of diabetic nephropathy. EPMA J. 2010, 1, 117–129. [Google Scholar] [CrossRef]
- Abid, N.; Mcglone, O.; Cardwell, C.; Mccallion, W.; Carson, D. Clinical and metabolic effects of gluten free diet in children with type 1 diabetes and coeliac disease. Pediatr. Diabetes 2011, 12, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.H.; Klingensmith, G.J.; McFann, K.; Rewers, M.; Ide, L.M.; Taki, I.; Liu, E.; Hoffenberg, E.J. Celiac autoimmunity in children with type 1 diabetes: A two-year follow-up. J. Pediatr. 2011, 158, 276–281.e1. [Google Scholar] [CrossRef]
- Kordonouri, O.; Klingensmith, G.; Knip, M.; Holl, R.W.; Aanstoot, H.J.; Menon, P.S.N.; Craig, M.E. Other complications and diabetes-associated conditions in children and adolescents. Pediatr. Diabetes 2014, 15 (Suppl. S20), 270–278. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).