Evaluating the Arterial Stiffness as a Useful Tool in the Management of Obese Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Exam and Blood Parameters
2.2. The Evaluation of Arterial Stiffness Using the Mobil-O-Graph
2.3. Statistical Analysis
2.4. Ethics Approvals
3. Results
3.1. General Description
3.2. The BMI and Arterial Stiffness Parameters
3.3. Sex and Arterial Stiffness Parameters
3.4. Age and Arterial Stiffness Parameters
3.5. Tanner Stages and Arterial Stiffness Parameters
3.6. Waist Circumference and Arterial Stiffness Parameters
3.7. Blood Parameters and Arterial Stiffness Parameters
3.8. Multilinear Regression Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIx | index of augmentation |
| BMI | body mass index |
| BP | blood pressure |
| cDBP | central diastolic blood pressure |
| CI | interval of confidence |
| CIMT | carotid intima-media thickness |
| cPP | central pulse pressure |
| cSBP | central systolic blood pressure |
| DBP | diastolic blood pressure |
| GOT | aspartate aminotransferase |
| GPT | alanine aminotransferase |
| H | height |
| HDL-c | high-density lipoprotein cholesterol |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| HR | heart rate |
| iCa | ionized Calcium |
| IFG | impaired fasting glucose |
| LDL-c | low-density lipoprotein cholesterol |
| MAP | mean arterial pressure |
| MetS | metabolic syndrome |
| n | number of subjects |
| NAFLD | non-alcoholic fatty liver disease |
| N-weight | normal-weight |
| PCOS | polycystic ovary syndrome |
| PWV | pulse wave velocity |
| PP | pulse pressure |
| SBP | systolic blood pressure |
| TC | total cholesterol |
| TG | triglycerides |
| W | weight |
| WC | waist circumference |
References
- Stavridou, A.; Kapsali, E.; Panagouli, E.; Thirios, A.; Polychronis, K.; Bacopoulou, F.; Psaltopoulou, T.; Tsolia, M.; Sergentanis, T.N.; Tsitsika, A. Obesity in Children and Adolescents during COVID-19 Pandemic. Children 2021, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Gebreab, S.Z.; Vandeleur, C.L.; Rudaz, D.; Strippoli, M.F.; Gholam-Rezaee, M.; Castelao, E.; Lasserre, A.M.; Glaus, J.; Pistis, G.; Kuehner, C.; et al. Psychosocial stress over the lifespan, psychological factors, and cardiometabolic risk in the community. Psychosom. Med. 2018, 80, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.; Griebler, U.; Mahlknecht, P.; Thaler, K.; Bouskill, K.; Gartlehner, G.; Mendis, S. Socioeconomic inequalities in non-communicable diseases and their risk factors: An overview of systematic reviews. BMC Public Health 2015, 15, 914. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Newsroom. Fact Sheets. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 June 2022).
- United Nations. United Nations in Western Europe. Europe: One in Three Children Overweight or Obese. 2022. Available online: https://unric.org/en/europe-one-in-three-children-overweight-or-obese/ (accessed on 7 June 2022).
- Jastreboff, A.M.; Kotz, C.M.; Kahan, S.; Kelly, A.S.; Heymsfield, S.B. Obesity as a disease: The Obesity Society 2018 position statement. Obesity 2019, 27, 7–9. [Google Scholar] [CrossRef]
- Daniels, S.R.; Arnett, D.K.; Eckel, R.H.; Gidding, S.S.; Hayman, L.L.; Kumanyika, S.; Robinson, T.N.; Scott, B.J.; St Jeor, S.; Williams, C.L. Overweight in children and adolescents: Pathophysiology, consequences, prevention, and treatment. Circulation 2005, 111, 1999–2012. [Google Scholar] [PubMed]
- World Health Organization. Newsroom. Fact Sheets. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 7 June 2022).
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018, 3, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Zieske, A.W.; Malcom, G.T.; Strong, J.P. Natural history and risk factors of atherosclerosis in children and youth: The PDAY study. Pediatr. Pathol. Mol. Med. 2002, 21, 213–237. [Google Scholar] [CrossRef]
- Abraham, T.M.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 2015, 132, 1639–1647. [Google Scholar] [CrossRef]
- Mihuta, M.-S.; Paul, C.; Ciulpan, A.; Dacca, F.; Velea, I.P.; Mozos, I.; Stoian, D. Subclinical Atherosclerosis Progression in Obese Children with Relevant Cardiometabolic Risk Factors Can Be Assessed through Carotid Intima Media Thickness. Appl. Sci. 2021, 11, 10721. [Google Scholar] [CrossRef]
- Shirwany, N.A.; Zou, M.H. Arterial stiffness: A brief review. Acta Pharmacol. Sin. 2010, 31, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, H.; Glagov, S. Structural basis for the static mechanical properties of the aortic media. Circ. Res. 1964, 14, 400–413. [Google Scholar] [CrossRef]
- Tounian, P.; Aggoun, Y.; Dubern, B.; Varille, V.; Guy-Grand, B.; Sidi, D.; Girardet, J.P.; Bonnet, D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: A prospective study. Lancet 2001, 358, 1400–1404. [Google Scholar] [CrossRef]
- Townsend, R.R.; Black, H.R.; Chirinos, J.A.; Feig, P.U.; Ferdinand, K.C.; Germain, M.; Rosendorff, C.; Steigerwalt, S.P.; Stepanek, J.A. Clinical use of pulse wave analysis: Proceedings from a symposium sponsored by North American Artery. J. Clin. Hypertens. 2015, 17, 503–513. [Google Scholar] [CrossRef]
- Savant, J.D.; Furth, S.L.; Meyers, K.E.C. Arterial stiffness in children: Pediatric measurement and considerations. Pulse 2015, 2, 69–80. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Sakuragi, S.; Abhayaratna, K.; Gravenmaker, K.J.; O’Reilly, C.; Srikusalanukul, W.; Budge, M.M.; Telford, R.D.; Abhayaratna, W.P. Influence of adiposity and physical activity on arterial stiffness in healthy children: The lifestyle of our kids study. Hypertension 2009, 53, 611–616. [Google Scholar] [CrossRef]
- Kulsum-Mecci, N.; Goss, C.; Kozel, B.A.; Garbutt, J.M.; Schechtman, K.B.; Dharnidharka, V.R. Effects of Obesity and Hypertension on Pulse Wave Velocity in Children. J. Clin. Hypertens. 2017, 19, 221–226. [Google Scholar] [CrossRef]
- Herouvi, D.; Karanasios, E.; Karayianni, C.; Karavanaki, K. Cardiovascular disease in childhood: The role of obesity. Eur. J. Pediatr. 2013, 172, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Desamericq, G.; Tissot, C.M.; Akakpo, S.; Tropeano, A.I.; Millasseau, S.; Macquin-Mavier, I. Carotid–Femoral Pulse Wave Velocity Is Not Increased in Obesity. Am. J. Hypertens. 2015, 18, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Dangardt, F.; Osika, W.; Volkmann, R.; Gan, L.M.; Friberg, P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin. Physiol. Funct. Imaging 2008, 28, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef]
- Reusz, G.S.; Cseprekal, O.; Temmar, M.; Kis, E.; Cherif, A.B.; Thaleb, A.; Fekete, A.; Szabó, A.J.; Benetos, A.; Salvi, P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 2010, 56, 217–224. [Google Scholar] [CrossRef]
- Mozos, I.; Maidana, J.P.; Stoian, D.; Stehlik, M. Gender Differences of Arterial Stiffness and Arterial Age in Smokers. Int. J. Environ. Res. Public Health 2017, 14, 565. [Google Scholar] [CrossRef]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P., 3rd; Tracy, R.E.; Wattigney, W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef]
- Wilenius, M.; Tikkakoski, A.J.; Tahvanainen, A.M.; Haring, A.; Koskela, J.; Huhtala, H.; Kähönen, M.; Kööbi, T.; Mustonen, J.T.; Pörsti, I.H. Central wave reflection is associated with peripheral arterial resistance in addition to arterial stiffness in subjects without antihypertensive medication. BMC Cardiovasc. Disord. 2016, 16, 131. [Google Scholar] [CrossRef]
- Shimizu, M.; Kario, K. Review: Role of the augmentation index in hypertension. Ther. Adv. Cardiovasc. Dis. 2008, 2, 25–35. [Google Scholar] [CrossRef]
- Qi, Z.; Ding, S. Obesity-associated sympathetic overactivity in children and adolescents: The role of catecholamine resistance in lipid metabolism. J. Pediatr. Endocrinol. Metab. 2016, 29, 113–125. [Google Scholar] [CrossRef]
- Hughes, A.D.; Park, C.; Davies, J.; Francis, D.; Thom, S.A.M.; Mayet, J.; Parker, K.H. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS ONE 2013, 8, e59371. [Google Scholar] [CrossRef] [PubMed]
- Jakab, A.E.; Hidvégi, E.V.; Illyés, M.; Cziráki, A.; Kalmár, T.; Maróti1, Z.; Bereczki, C. Childhood Obesity: Does it Have Any Effect on Young Arteries? Front. Pediatr. 2020, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Močnik, M.; Nikolić, S.; Varda, N.M. Arterial Compliance Measurement in Overweight and Hypertensive Children. Indian J. Pediatr. 2016, 83, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef]
- Coutinho, T.; Bailey, K.R.; Turner, S.T.; Kullo, I.J. Arterial stiffness is associated with increase in blood pressure over time in treated hypertensives. J. Am. Soc. Hypertens. 2014, 6, 414–421. [Google Scholar] [CrossRef]
- Urbina, E.M.; Khoury, P.R.; McCoy, C.; Daniels, S.R.; Kimball, T.R.; Dolan, L.M. Cardiac and vascular consequences of pre-hypertension in youth. J. Clin. Hypertens. 2011, 13, 332–342. [Google Scholar] [CrossRef]
- Urbina, E.M.; Kimball, T.R.; Khoury, P.R.; Daniels, S.R.; Dolan, L.M. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J. Hypertens. 2010, 28, 1692–1698. [Google Scholar] [CrossRef]
- Tomiyama, H.; Yamashina, A. Ankle-Brachial Pressure Index and Pulse Wave Velocity in Cardiovascular Risk Assessment. In Encyclopedia of Cardiovascular Research and Medicine; Vasan, R.S., Sawyer, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-805154-2. [Google Scholar]
- Antsiperov, V.E.; Mansurov, G.K.; Bugaev, A. Methods of the Pulse Wave Velocity Estimation based on Pneumatic Blood Pressure Sensor Data and Synchronous ECG Records. In Proceedings of the BIOSTEC 2020-13th International Joint Conference on Biomedical Engineering Systems and Technologies, Valetta, Malta, 24–26 February 2020. [Google Scholar] [CrossRef]
- Benas, D.; Kornelakis, M.; Triantafyllidi, H.; Kostelli, G.; Pavlidis, G.; Varoudi, M.; Vlastos, D.; Lambadiari, V.; Parissis, J.; Ikonomidis, I. Pulse wave analysis using the Mobil-O-Graph, Arteriograph and Complior device: A comparative study. Blood Press. 2019, 28, 107–113. [Google Scholar] [CrossRef]
- Velea, I.P.; Albulescu, R.; Arghirescu, S.T. Obezitatea la copil. In Pediatrie-Curs Pentru Studentii Facultătii de Medicină: Obezitatea la Copil; Velea, I., Ed.; Editura Victor Babes: Timisoara, Romania, 2016; pp. 289–297. [Google Scholar]
- Cheung, Y.F. Arterial stiffness in the young: Assessment, determinants, and implications. Korean Circ. J. 2010, 40, 153–162. [Google Scholar] [CrossRef]
- Mihuta, M.S.; Paul, C.; Borlea, A.; Cepeha, C.M.; Velea, I.P.; Mozos, I.; Stoian, D. The Oscillometric Pulse Wave Analysis Is Useful in Evaluating the Arterial Stiffness of Obese Children with Relevant Cardiometabolic Risks. J. Clin. Med. 2022, 11, 5078. [Google Scholar] [CrossRef]
- Weiss, W.; Gohlisch, C.; Harsch-Gladisch, C.; Tölle, M.; Zidek, W.; van der Giet, M. Oscillometric estimation of central blood pressure: Validation of the Mobil-O-Graph in comparison with the SphygmoCor device. Blood Press. Monit. 2012, 17, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Mynard, J.P.; Goldsmith, G.; Springall, G.; Eastaugh, L.; Lane, G.K.; Zannino, D.; Smolich, J.J.; Avolio, A.; Cheung, M.M.H. Central aortic blood pressure estimation in children and adolescents: Results of the KidCoreBP study. J. Hypertens. 2020, 38, 821–828. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, S.H. Blood pressure measurements and hypertension in infants, children, and adolescents: From the postmercury to mobile devices. Clin. Exp. Pediatr. 2022, 65, 73–80. [Google Scholar] [CrossRef]
- Ek, A.; Delisle Nyström, C.; Chirita-Emandi, A.; Tur, J.A.; Nordin, K.; Bouzas, C.; Argelich, E.; Martínez, J.A.; Frost, G.; Garcia Perez, I.; et al. A randomized controlled trial for overweight and obesity in preschoolers: The More and Less Europe study-An intervention within the STOP project. BMC Public Health 2019, 19, 945. [Google Scholar] [CrossRef]
- Shiraishi, M.; Murakami, T.; Higashi, K. The accuracy of central blood pressure obtained by oscillometric noninvasive method using Mobil-O-Graph in children and adolescents. J. Hypertens. 2020, 38, 813–820. [Google Scholar] [CrossRef]
- Yano, S. Does body height affect vascular function? Hypertens. Res. 2022, 45, 369–371. [Google Scholar] [CrossRef]
- Qiu, Q.; Meng, X.; Li, Y.; Liu, X.; Teng, F.; Wang, Y.; Zang, X.; Wang, Y.; Liang, J. Evaluation of the associations of body height with blood pressure and early-stage atherosclerosis in Chinese adults. J. Clin. Hypertens. 2020, 22, 1018–1024. [Google Scholar] [CrossRef]
- Moon, J.; Hwang, I.C.; Han, S.H. Short stature is associated with higher pulse wave velocity in subjects without overt cardiovascular disease. Medicine 2020, 99, e22219. [Google Scholar] [CrossRef]
- Harbin, M.M.; Hultgren, N.E.; Kelly, A.S.; Dengel, D.R.; Evanoff, N.G.; Ryder, J.R. Measurement of central aortic blood pressure in youth: Role of obesity and sex. Am. J. Hypertens. 2018, 31, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.Y.; Qasem, A.; Ayer, J.G.; Butlin, M.; O’Meagher, S.; Melki, C.; Marks, G.B.; Avolio, A.; Celermajer, D.S.; Skilton, M.R. Central blood pressure in children and adolescents: Non-invasive development and testing of novel transfer functions. J. Hum. Hypertens. 2017, 31, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Ahimastos, A.A.; Formosa, M.; Dart, A.M.; Kingwell, B.A. Gender differences in large artery stiffness pre- and post-puberty. J. Clin. Endocrinol. Metab. 2003, 88, 5375–5380. [Google Scholar] [CrossRef] [PubMed]
- Karas, R.H.; Patterson, B.L.; Mendelsohn, M.E. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation 1994, 89, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef] [PubMed]
- Regnault, V.; Thomas, F.; Safar, M.E.; Osborne-Pellegrin, M.; Khalil, R.A.; Pannier, B.; Lacolley, P. Sex difference in cardiovascular risk: Role of pulse pressure amplification. J. Am. Coll. Cardiol. 2012, 59, 1771–1777. [Google Scholar] [CrossRef]
- Webb, C.M.; Elkington, A.G.; Kraidly, M.M.; Keenan, N.; Pennell, D.J.; Collins, P. Effects of oral testosterone treatment on myocardial perfusion and vascular function in men with low plasma testosterone and coronary heart disease. Am. J. Card. 2008, 101, 618–624. [Google Scholar] [CrossRef]
- Dockery, F.; Bulpitt, C.J.; Donaldson, M.; Fernandez, S.; Rajkumar, C. The relationship between androgens and arterial stiffness in older men. J. Am. Geriatr. Soc. 2003, 51, 1627–1632. [Google Scholar] [CrossRef]
- Hougaku, H.; Fleg, J.L.; Najjar, S.S.; Lakatta, E.G.; Harman, S.M.; Blackman, M.R.; Metter, E.J. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E234–E242. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, Y.; Sun, Z.; Trzeciakowski, J.P.; Gansner, M.; Depre, C.; Vatner, S.F. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 2010, 107, 615–619. [Google Scholar] [CrossRef]
- Díaz, A.; Galli, C.; Tringler, M.; Ramírez, A.; Fischer, E.I.C. Reference Values of Pulse Wave Velocity in Healthy People from an Urban and Rural Argentinean Population. Int. J. Hypertens. 2014, 2014, 653239. [Google Scholar] [CrossRef]
- Li, Y.; Staessen, J.; Sheng, C.S.; Huang, Q.F.; O’Rourke, M.; Wang, J.G. Age dependency of peripheral and central systolic blood pressures: Cross-sectional and longitudinal observations in a Chinese population. Hypertens. Res. 2012, 35, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lentferink, Y.E.; Kromwijk, L.A.J.; van der Aa, M.P.; Knibbe, C.A.J.; van der Vorst, M.M.J. Increased Arterial Stiffness in Adolescents with Obesity. Glob. Pediatr. Health 2019, 25, 6. [Google Scholar] [CrossRef]
- Solorzano, C.M.B.; Helm, K.D.; Patrie, J.T.; Shayya, R.F.; Cook-Andersen, H.L.; Chang, R.J.; McCartney, C.R.; Marshall, J.C. Increased adrenal androgens in overweight peripubertal girls. J. Endocr. Soc. 2017, 1, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Kahn, H.S.; Mei, Z.; Grummer-Strawn, L.M.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: The Bogalusa Heart Study. Am. J. Clin. Nutr. 2007, 86, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Bassali, R.; Waller, J.L.; Gower, B.; Allison, J.; Davis, C.L. Utility of waist circumference percentile for risk evaluation in obese children. Int. J. Pediatric. Obes. 2010, 5, 97–101. [Google Scholar] [CrossRef]
- Kamon, T.; Kaneko, H.; Itoh, H.; Kiriyama, H.; Mizuno, Y.; Morita, H.; Yamamichi, N.; Komuro, I. Association Between Waist Circumference and Carotid Intima-Media Thickness in the General Population. Int. Heart J. 2020, 61, 103–108. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context. 2018, 7, 212525. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Tohidi, M.; Mohebi, R.; Khalili, D.; Azizi, F.; Hadaegh, F. Adolescent lipoprotein classifications according to National Health and Nutrition Examination Survey (NHANES) vs. National Cholesterol Education Program (NCEP) for predicting abnormal lipid levels in adulthood in a Middle East population. Lipids Health Dis. 2012, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Kavey, R.E. Dyslipidemia and pediatric obesity. Pediatr. Clin. N. Am. 2011, 58, 1363–1373. [Google Scholar] [CrossRef]
- Boullart, A.C.; de Graaf, J.; Stalenhoef, A.F. Serum triglycerides and risk of cardiovascular disease. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 867–875. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, F.; Gao, B.; Ni, J.; Liu, Y.; Mo, X.; Huang, J. Association between lipid profiles and arterial stiffness: A secondary analysis based on a cross-sectional study. J. Int. Med. Res. 2020, 48, 300060520938188. [Google Scholar] [CrossRef]
- Krawczyk, M.; Rumińska, M.; Witkowska-Sędek, E.; Majcher, A.; Pyrżak, B. Usefulness of the triglycerides to high-density lipoprotein cholesterol ratio (TG/HDL-C) in prediction of metabolic syndrome in polish obese children and adolescents. ActaBiochim. Pol. 2018, 65, 605–611. [Google Scholar] [CrossRef]
- Iwani, N.A.K.Z.; Jalaludin, M.Y.; Zin, R.M.W.M.; Fuziah, M.Z.; Hong, J.Y.H.; Abqariyah, Y.; Mokhtar, A.H.; Nazaimoon, W.M.W. TG: HDL-C Ratio Is a Good Marker to Identify Children Affected by Obesity with Increased Cardiometabolic Risk and Insulin Resistance. Int. J. Endocrinol. 2019, 2019, 8586167. [Google Scholar] [CrossRef]
- Frontini, M.G.; Srinivasan, S.R.; Xu, J.; Tang, R.; Bond, M.G.; Berenson, G.S. Usefulness of childhood non-high density lipoprotein cholesterol levels versus other lipoprotein measures in predicting adult subclinical atherosclerosis: The Bogalusa Heart Study. Pediatrics 2008, 121, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Lamarche, B.; Couillard, C.; Pascot, A.; Cantin, B.; Bergeron, J.; Dagenais, G.R.; Després, J.P. Total Cholesterol/HDL Cholesterol Ratio vs LDL Cholesterol/HDL Cholesterol Ratio as Indices of Ischemic Heart Disease Risk in Men: The Quebec Cardiovascular Study. Arch. Intern. Med. 2001, 161, 2685–2692. [Google Scholar] [CrossRef]
- Hagman, E.; Reinehr, T.; Kowalski, J.; Ekbom, A.; Marcus, C.; Holl, R.W. Impaired fasting glucose prevalence in two nationwide cohorts of obese children and adolescents. Int. J. Obes. 2014, 38, 40–45. [Google Scholar] [CrossRef]
- Chakarova, N.; Dimova, R.; Grozeva, G.; Tankova, T. Assessment of glucose variability in subjects with prediabetes. Diabetes Res. Clin. Pract. 2019, 151, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hagman, E.; Rinehr, T.; Kowalski, J.; Ekbom, A.; Marcus, C.; Holl, R.W. Major differences of impaired fasting glucose prevalence in two nationwide cohorts of obese children. Int. J. Obes. 2014, 38, 40–45. [Google Scholar] [CrossRef]
- Hagman, E.; Ighani Arani, P.; Fischer, M.; Danielsson, P.; Marcinkiewicz, K.; Petriczko, E.; Marcus, C. Blood sugar levels are higher in young obese children in Sweden than in Poland. Acta Paediatr. 2014, 103, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Barseem, N.F.; Helwa, M.A. Homeostatic model assessment of insulin resistance as a predictor of metabolic syndrome: Consequences of obesity in children and adolescents. Gaz Egypt Paediatr. Assoc. 2015, 63, 19–24. [Google Scholar] [CrossRef]
- Peplies, J.; Börnhorst, C.; Günther, K.; Fraterman, A.; Russo, P.; Veidebaum, T.; Tornaritis, M.; De Henauw, S.; Marild, S.; Molnar, D.; et al. Longitudinal associations of lifestyle factors and weight status with insulin resistance (HOMA-IR) in preadolescent children: The large prospective cohort study IDEFICS. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 97. [Google Scholar] [CrossRef]
- Sabin, M.A.; Hunt, L.P.; Ford, A.L.; Werther, G.A.; Crowne, E.C.; Shield, J.P.H. Elevated glucose concentrations during an oral glucose tolerance test are associated with the presence of metabolic syndrome in childhood obesity. Diab. Med. 2008, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.; Zeitler, P.S. Oral Glucose Tolerance Testing in Asymptomatic Obese Children: More Questions than Answers. J. Clin. Endocrinol. Metab. 2008, 93, 4228–4230. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Pardee, P.E.; Lavine, J.E.; Blumkin, A.K.; Cook, S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 2008, 118, 277–283. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Manesis, E.; Baou, K.; Papatheodoridis, G.; Koskinas, K.; Tiniakos, D.; Aznaouridis, K.; Archimandritis, A.; Stefanadis, C. Increased Arterial Stiffness and Impaired Endothelial Function in Nonalcoholic Fatty Liver Disease: A Pilot Study. Am. J. Hypertens. 2010, 23, 1183–1189. [Google Scholar] [CrossRef]
- Man, E.; Cheung, P.T.; Cheung, Y.F. Associations between arterial structure and function and serum levels of liver enzymes in obese adolescents. J. Paediatr. Child. Health 2017, 53, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk. Manag. 2005, 1, 291–299. [Google Scholar] [CrossRef]
- Fraser, R.; Ingram, M.C.; Anderson, N.H.; Morrison, C.; Davies, E.; Connell, J.M. Cortisol effects on body mass, blood pressure and cholesterol in the general population. Hypertension 1999, 33, 1364–1368. [Google Scholar] [CrossRef]
- Mousa, S.; Hemeda, A.; Ghorab, H.; Abdelhamid, A.; Saif, A. Arterial Wall Stiffness and the Risk of Atherosclerosis in Egyptian Patients with Overt and Subclinical Hypothyroidism. AACE Endocr. Pract. 2020, 26, 161–166. [Google Scholar] [CrossRef]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef]
- Ghergherehchi, R.; Hazhir, N. Thyroid hormonal status among children with obesity. Ther. Adv. Endocrinol. Metab. 2015, 6, 51–55. [Google Scholar] [CrossRef]
- Al Mheid, I.; Patel, R.; Murrow, J.; Morris, A.; Rahman, A.; Fike, L.; Kavtaradze, N.; Uphoff, I.; Hooper, C.; Tangpricha, V.; et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J. Am. Coll. Cardiol. 2011, 58, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.A.; Desormeaux, A.; Labelle, A.; Soulez, M.; Soulez, G.; Langelier, Y.; Pshezhetsky, A.V.; Hébert, M.J. Endothelial stress induces the release of vitamin D-binding protein, a novel growth factor. Biochem. Biophys. Res. Commun. 2005, 338, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Peterson, C.A.; Belenchia, A.M. Vitamin D deficiency & childhood obesity: A tale of two epidemics. Mo. Med. 2014, 111, 49–53. [Google Scholar]
- Censani, M.; Hammad, H.T.; Christos, P.J.; Schumaker, T. Vitamin D Deficiency Associated With Markers of Cardiovascular Disease in Children With Obesity. Glob. Pediatr. Health 2018, 5, 2333794X17751773. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, K.; Moore, C.G.; Khalid, A.T.; Vallejo, A.N.; Virji, M.A.; Holick, M.F.; Greenspan, S.L.; Arslanian, S.; Reis, S.E. Effect of vitamin D3 supplementation on vascular and metabolic health of vitamin D-deficient overweight and obese children: A randomized clinical trial. Am. J. Clin. Nutr. 2020, 111, 757–768. [Google Scholar] [CrossRef]
- Underland, L.; Markowitz, M.; Gensure, R. Calcium and phosphate hormones: Vitamin d, parathyroid hormone, and fibroblast growth factor 23. Pediatr. Rev. 2020, 41, 3–11. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Wu, H. A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1078–1093. [Google Scholar] [CrossRef]
- Pamuk, N.; Akkan, T.; Dağdeviren, M.; Koca, A.O.; Beyan, E.; Derun Taner Ertuğrul, E.T.; Altay, M. Central and peripheral blood pressures and arterial stiffness increase in hypoparathyroidism. Arch. Endocrinol. Metab. 2020, 64, 4. [Google Scholar] [CrossRef]
- Moor, M.B.; Kruse, A.; Uehlinger, D.E.; Eisenberger, U. Arterial stiffness depends on serum ionized calcium levels during dialysis with regional citrate anticoagulation. Artif. Organs. 2013, 37, 467–474. [Google Scholar] [CrossRef]
- Shroff, R.C.; Donald, A.E.; Hiorns, M.P.; Watson, A.; Feather, S.; Milford, D.; Ellins, E.A.; Storry, C.; Ridout, D.; Deanfield, J.; et al. Mineral Metabolism and Vascular Damage in Children on Dialysis. J. Am. Soc. Nephrol. 2007, 18, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Ultrasound assessment of flow-mediated dilation. Hypertension 2010, 55, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.C.M.; de Oliveira, J.T.; Tochetto, S.; de Oliveira, C.P.M.S.; Sigrist, R.; Chammas, M.C. Ultrasound elastography in patients with fatty liver disease. Radiol. Bras. 2020, 53, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.; Sandulescu, L.; Saftoiu, A. The Role of Elastography in Non-Alcoholic Fatty Liver Disease. Curr. Health Sci. J. 2020, 46, 255–269. [Google Scholar] [CrossRef]
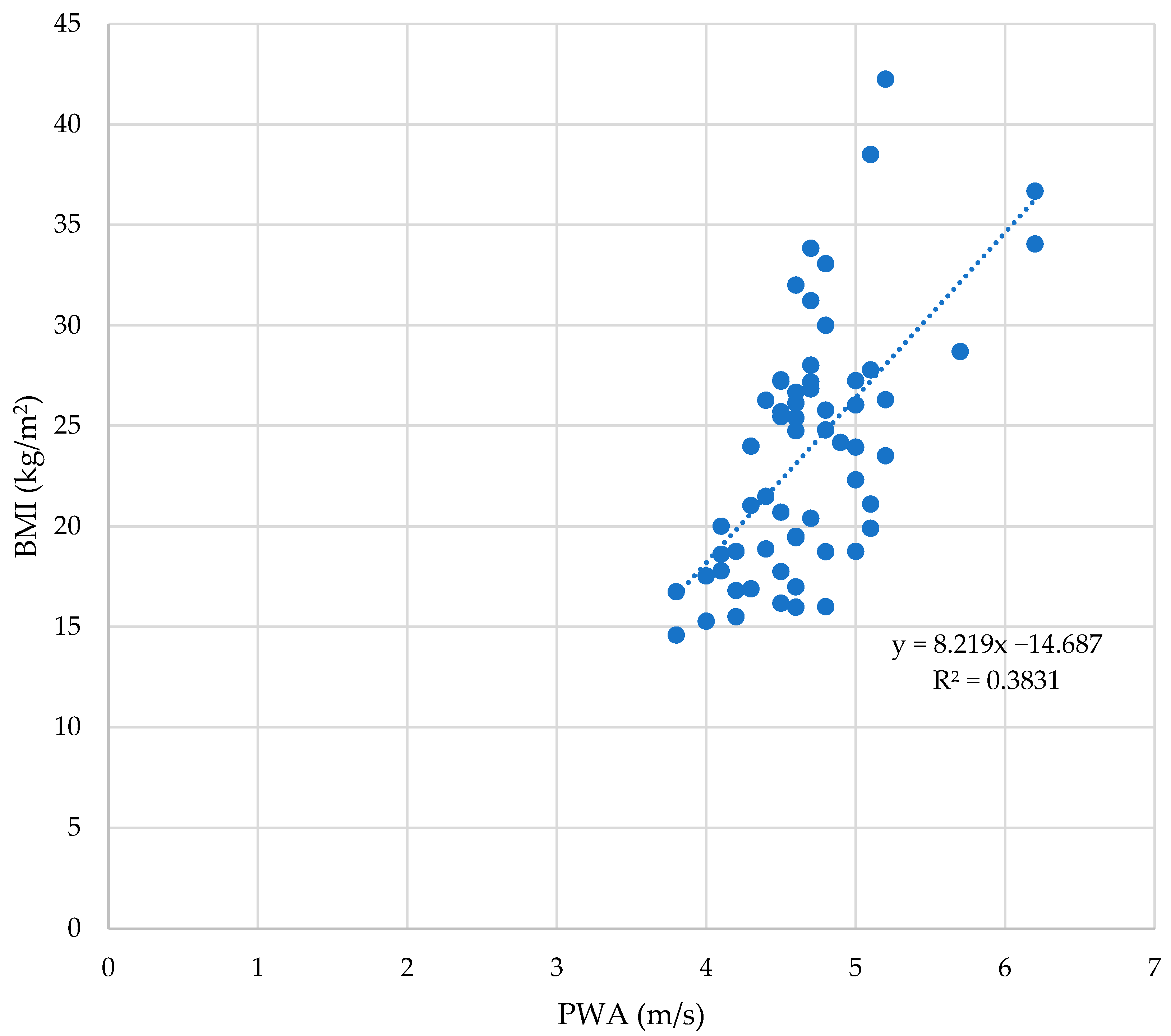
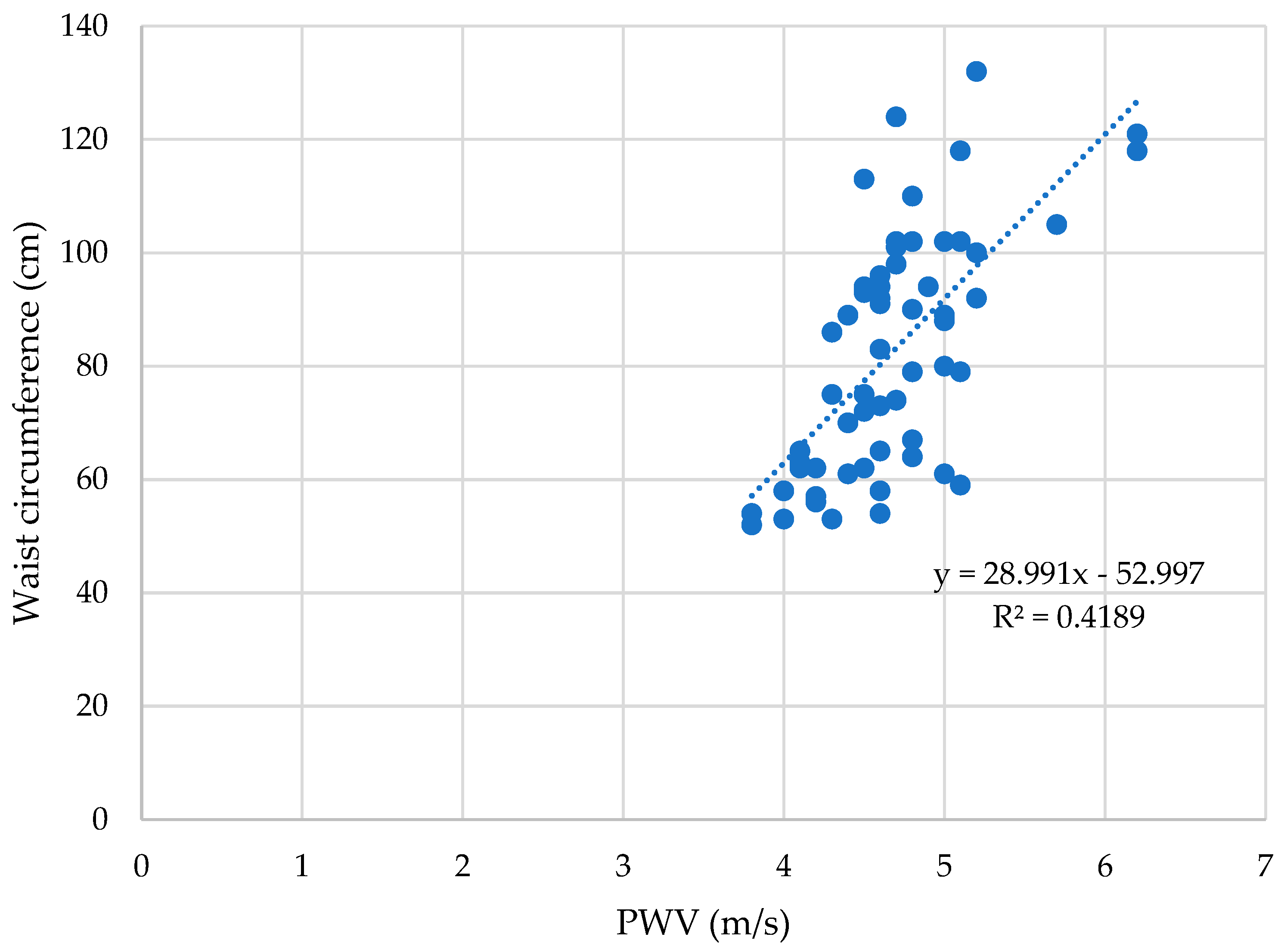

| Obese Group | Control Group | ||||
|---|---|---|---|---|---|
| Mean/Median | SD | Mean/Median | SD | p-Value | |
| BMI | 26.8 | 4.61 | 18.21 | 2.04 | <0.01 |
| WC | 98.32 | 13.82 | 63.37 | 8.1 | <0.01 |
| PWV | 4.7 | 0.45 | 4.4 | 0.3 | <0.01 |
| AIx | 24.34 | 10.92 | 22.94 | 10.42 | 0.61 |
| SBP | 122.3 | 11.38 | 115 | 8.6 | <0.01 |
| DBP | 75.91 | 10.21 | 78 | 7.95 | 0.88 |
| MAP | 98 | 9.55 | 96.5 | 7.82 | 0.08 |
| cSBP | 113 | 11.02 | 100.71 | 10.03 | <0.01 |
| cDBP | 77.42 | 9.91 | 69.11 | 8.62 | <0.01 |
| cPP | 37.89 | 10.53 | 32 | 5.7 | 0.01 |
| HR | 88.72 | 11.09 | 83.72 | 6.32 | 0.04 |
| Fasting glucose | 88.68 | 14.47 | 80.49 | 10.78 | 0.01 |
| HDL-c | 41 | 8.42 | 39 | 10.6 | 0.34 |
| LDL-c | 131.81 | 36.02 | 93.31 | 34.55 | <0.01 |
| Total cholesterol | 187.22 | 32 | 160.21 | 27.89 | <0.01 |
| Triglycerides | 112 | 53.71 | 80 | 49.1 | 0.14 |
| non-HDL-c | 146.5 | 35.32 | 117.18 | 29.2 | <0.01 |
| Triglycerides/HDL-c ratio | 2.9 | 1.78 | 2 | 1.3 | 0.09 |
| Total cholesterol/HDL-c ratio | 4.3 | 1.55 | 4 | 1.05 | <0.01 |
| Cortisol | 18.4 | 4.1.1 | 17 | 2.2 | 0.17 |
| TSH | 4.2 | 1.72 | 3.5 | 0.9 | 0.06 |
| GOT | 34.4 | 14.03 | 21 | 7.8 | <0.01 |
| GPT | 32 | 18.2 | 24 | 9.3 | <0.01 |
| 25-OH-Vitamin D | 28.5 | 14.11 | 24 | 8.9 | 0.1 |
| Ionized Calcium | 4 | 0.3 | 4.1 | 0.22 | 0.83 |
| PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP | |
|---|---|---|---|---|---|---|---|---|---|
| BMI | ρ = 0.59 | ρ = 0.18 | ρ = 0.53 | ρ = 0.14 | ρ = 0.36 | ρ = 0.18 | ρ = 0.65 | ρ = 0.45 | ρ = 0.36 |
| p-value | <0.01 | 0.15 | <0.01 | 0.26 | <0.01 | 0.16 | <0.01 | <0.01 | <0.01 |
| W | ρ = 0.68 | ρ = 0.29 | ρ = 0.64 | ρ = 0.36 | ρ = 0.48 | ρ = 0.16 | ρ = 0.64 | ρ = 0.50 | ρ = 0.38 |
| p-value | <0.01 | 0.03 | <0.01 | 0.006 | <0.01 | 0.21 | <0.01 | <0.01 | <0.01 |
| H | ρ = 0.50 | ρ = 0.29 | ρ = 0.49 | ρ = 0.42 | ρ = 0.52 | ρ = −0.02 | ρ = 0.46 | ρ = 0.52 | ρ = 0.15 |
| p-value | <0.01 | 0.024 | <0.01 | <0.01 | <0.01 | 0.87 | <0.01 | <0.01 | 0.24 |
| Sex | PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Obese | Boys | 4.8 | 19.5 | 120.5 | 75.5 | 98.05 | 85.5 | 113.7 | 76.3 | 41 |
| Girls | 4.79 | 38.6 | 118 | 75 | 97 | 93 | 113.8 | 78 | 36 | |
| p-value | 0.56 | 0.04 | 0.94 | 0.97 | 0.66 | 0.04 | 0.67 | 0.77 | 0.35 | |
| N-weight | Boys | 4.4 | 23 | 114.4 | 77.2 | 95.8 | 83.7 | 100.6 | 68.8 | 31 |
| Girls | 4.41 | 22.7 | 114.5 | 77 | 96.5 | 83.7 | 100.5 | 69.4 | 30.5 | |
| p-value | 0.67 | 0.94 | 0.66 | 0.39 | 0.64 | 0.97 | 0.27 | 0.86 | 0.17 |
| PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP | |
|---|---|---|---|---|---|---|---|---|---|
| Obese girls | 4.79 | 38.6 | 118 | 75 | 97 | 93 | 113.8 | 78 | 36 |
| N-weight girls | 4.41 | 22.7 | 114.5 | 77 | 96.5 | 83.7 | 100.5 | 69.4 | 30.5 |
| p-value | <0.01 | 0.01 | 0.03 | 0.63 | 0.41 | 0.02 | <0.01 | 0.02 | 0.11 |
| Obese boys | 4.8 | 19.5 | 120.5 | 75.5 | 98.05 | 85.5 | 113.7 | 76.3 | 41 |
| N-weight boys | 4.4 | 23 | 114.4 | 77.2 | 95.8 | 83.7 | 100.6 | 68.8 | 31 |
| p-value | 0.01 | 0.34 | 0.07 | 0.63 | 0.49 | 0.49 | <0.01 | 0.04 | 0.059 |
| n | PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP | |
|---|---|---|---|---|---|---|---|---|---|---|
| <12 years | 12 | 4.6 | 24.8 | 116 | 70.7 | 94.7 | 92 | 108 | 72.8 | 38.4 |
| 12–15 years | 15 | 4.75 | 23.5 | 121.6 | 77.9 | 99.7 | 87.9 | 113.9 | 79 | 36.5 |
| ≥16 years | 6 | 5.1 | 25.1 | 128.7 | 80.7 | 104.7 | 84.5 | 122 | 82.1 | 39.8 |
| n | PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <12 years | Obese | 12 | 4.6 | 24.8 | 116 | 68 | 94.7 | 92 | 108 | 72.8 | 41 |
| N-weight | 13 | 4.2 | 22.7 | 110 | 75 | 92 | 84 | 102 | 67.6 | 30 | |
| p-value | 0.03 | 0.64 | 0.07 | 0.17 | 0.91 | 0.03 | <0.01 | 0.2 | 0.01 | ||
| 12–15 years | Obese | 15 | 4.75 | 23.5 | 121.6 | 78 | 99.7 | 87.9 | 113.9 | 79 | 40 |
| N-weight | 10 | 4.5 | 21.6 | 115.8 | 78 | 96.8 | 82.1 | 103 | 68.9 | 31 | |
| p-value | <0.01 | 0.68 | 0.02 | 0.91 | 0.24 | 0.07 | <0.01 | <0.01 | 0.27 |
| Puberty Stage | n | PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 8 | 4.5 | 22.3 | 111.5 | 69.5 | 93.2 | 91.7 | 106.5 | 75.1 | 40 |
| II, III | 13 | 4.8 | 23.5 | 121.7 | 73 | 97.3 | 88.7 | 114.3 | 74.6 | 39.2 |
| IV, V | 12 | 4.85 | 26.5 | 122 | 80 | 99 | 86.6 | 118.4 | 82 | 37 |
| n | PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tanner I | Obese | 8 | 4.5 | 22.3 | 111.5 | 69.5 | 93.2 | 91.7 | 106.5 | 75.1 | 40 |
| N-weight | 10 | 4.15 | 20.1 | 109.5 | 75 | 92 | 84.1 | 92 | 65.1 | 27.5 | |
| p-value | 0.01 | 0.65 | 0.15 | 0.53 | 0.59 | 0.12 | <0.01 | 0.05 | 0.08 | ||
| Tanner II, III | Obese | 13 | 4.8 | 23.5 | 121.7 | 75 | 97.3 | 88.7 | 116 | 74.6 | 39.2 |
| N-weight | 11 | 4.5 | 24.5 | 116.5 | 81 | 97.5 | 82.7 | 106 | 72.8 | 34.9 | |
| p-value | 0.03 | 0.83 | 0.06 | 0.14 | 0.96 | 0.05 | 0.02 | 0.6 | 0.21 | ||
| Tanner IV, V | Obese | 12 | 4.85 | 26.5 | 122 | 80 | 99 | 86.6 | 118.4 | 82 | 37 |
| N-weight | 6 | 4.65 | 24.6 | 117 | 76 | 96.2 | 85 | 103.1 | 69.1 | 30 | |
| p-value | 0.18 | 0.73 | 0.23 | 0.74 | 0.26 | 0.77 | 0.01 | <0.01 | 0.45 |
| PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP | |
|---|---|---|---|---|---|---|---|---|---|
| Obese | ρ = 0.53 | r = 0.24 | ρ = 0.49 | r = 0.46 | r = 0.53 | r = −0.08 | ρ = 0.51 | r = 0.42 | r = 0.18 |
| p-value | <0.01 | 0.17 | <0.01 | <0.01 | <0.01 | 0.64 | <0.01 | 0.015 | 0.31 |
| Controls | r = 0.57 | r = 0.13 | r = 0.57 | ρ = 0.31 | ρ = 0.42 | r = −0.17 | r = 0.43 | r = 0.21 | ρ = 0.28 |
| p-value | <0.01 | 0.49 | <0.01 | 0.10 | 0.02 | 0.36 | <0.01 | 0.28 | 0.15 |
| Correlations for Obese Patients | PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP |
|---|---|---|---|---|---|---|---|---|---|
| HDL-c | ρ = −0.32 | ρ = −0.08 | ρ = −0.28 | ρ = −0.33 | ρ = −0.34 | ρ = 0.1 | ρ = −0.31 | ρ = −0.33 | ρ = 0.05 |
| p-value | 0.08 | 0.64 | 0.13 | 0.06 | 0.06 | 0.57 | 0.08 | 0.07 | 0.77 |
| LDL-c | ρ = 0.4 | r = 0.09 | ρ = 0.97 | r = 0.27 | ρ = 0.3 | r = 0.008 | ρ = 0.36 | r = 0.24 | r = 0.19 |
| p-value | 0.03 | 0.62 | <0.01 | 0.13 | 0.07 | 0.96 | 0.05 | 0.19 | 0.3 |
| Total cholesterol | r = 0.33 | r = 0.03 | ρ = 0.33 | r = 0.18 | ρ = 0.26 | r = 0.02 | ρ = 0.29 | r = 0.14 | r = 0.23 |
| p-value | 0.07 | 0.83 | 0.07 | 0.33 | 0.15 | 0.91 | 0.11 | 0.43 | 0.21 |
| Triglycerides | ρ = 0.48 | ρ = 0.08 | ρ = 0.5 | ρ = 0.29 | ρ = 0.3 | ρ = 0.05 | ρ = 0.47 | ρ = 0.26 | ρ = 0.19 |
| p-value | <0.01 | 0.65 | <0.01 | 0.11 | 0.04 | 0.75 | <0.01 | 0.16 | 0.29 |
| non-HDL-c | ρ = 0.38 | r = 0.06 | ρ = 0.38 | r = 0.24 | ρ = 0.3 | r = −0.005 | ρ = 0.38 | r = 0.2 | r = 0.22 |
| p-value | 0.03 | 0.71 | 0.03 | 0.19 | 0.1 | 0.97 | 0.04 | 0.26 | 0.23 |
| TG/HDL-c ratio | ρ = 0.46 | ρ = 0.14 | ρ = 0.46 | ρ = 0.31 | ρ = 0.38 | ρ = 0.002 | ρ = 0.45 | ρ = 0.31 | ρ = 0.13 |
| p-value | 0.01 | 0.43 | <0.01 | 0.08 | 0.03 | 0.98 | 0.01 | 0.08 | 0.47 |
| TC/HDL-c ratio | ρ = 0.39 | ρ = 0.08 | ρ = 0.37 | ρ = 0.33 | ρ = 0.34 | ρ = −0.05 | ρ = 0.36 | ρ = 0.30 | ρ = 0.07 |
| p-value | 0.03 | 0.64 | 0.049 | 0.06 | 0.059 | 0.78 | 0.04 | 0.1 | 0.67 |
| Fasting glucose | ρ = 0.26 | r = 0.13 | ρ = 0.25 | r = 0.26 | ρ = 0.37 | r = 0.009 | ρ = 0.28 | r = 0.26 | r = 0.19 |
| p-value | 0.16 | 0.46 | 0.17 | 0.16 | 0.04 | 0.95 | 0.12 | 0.16 | 0.31 |
| GPT | ρ = 0.44 | ρ = 0.35 | ρ = 0.41 | ρ = 0.5 | ρ = 0.46 | ρ = 0.15 | ρ = 0.35 | ρ = 0.49 | ρ = 0.03 |
| p-value | 0.01 | 0.056 | 0.02 | 0.04 | 0.01 | 0.41 | 0.054 | <0.01 | 0.85 |
| GOT | ρ = 0.5 | r = 0.23 | ρ = 0.48 | r = 0.38 | ρ = 0.46 | r = −0.07 | ρ = 0.44 | r = 0.37 | r = 0.26 |
| p-value | <0.01 | 0.2 | <0.01 | 0.03 | <0.01 | 0.7 | 0.01 | 0.04 | 0.16 |
| Cortisol 8 am | ρ = 0.32 | r = 0.031 | ρ = 0.28 | r = 0.1 | ρ = 0.16 | r = −0.002 | ρ = 0.23 | r = 0.07 | r = 0.09 |
| p-value | 0.08 | 0.86 | 0.12 | 0.59 | 0.39 | 0.98 | 0.22 | 0.68 | 0.6 |
| TSH | ρ = 0.06 | r = 0.1 | ρ = 0.06 | r = 0.03 | ρ = 0.09 | r = −0.03 | ρ = 0.21 | r = 0.04 | r = 0.21 |
| p-value | 0.74 | 0.58 | 0.73 | 0.87 | 0.61 | 0.86 | 0.25 | 0.83 | 0.25 |
| 25-OH- Vitamin D | ρ = −0.45 | ρ = 0.02 | ρ = −0.42 | ρ = −0.3 | ρ = −0.36 | ρ = −0.05 | ρ = −0.31 | ρ = −0.29 | ρ = −0.07 |
| p-value | 0.01 | 0.89 | 0.01 | 0.09 | 0.05 | 0.77 | 0.09 | 0.11 | 0.71 |
| Ionized Calcium | ρ = −0.59 | r = 0.04 | ρ = −0.44 | r = −0.52 | ρ = −0.49 | r = −0.17 | ρ = −0.38 | r = −0.44 | r = 0.006 |
| p-value | <0.01 | 0.8 | 0.01 | <0.01 | <0.01 | 0.36 | 0.03 | 0.01 | 0.97 |
| Correlations for Controls | PWV | AIx | SBP | DBP | MAP | HR | cSBP | cDBP | cPP |
|---|---|---|---|---|---|---|---|---|---|
| HDL-c | r = −0.42 | r = −0.47 | r = −0.35 | ρ = −0.25 | ρ = −0.25 | r = −0.34 | r = −0.40 | r = −0.43 | ρ = −0.22 |
| p-value | 0.03 | 0.01 | 0.08 | 0.21 | 0.22 | 0.09 | 0.04 | 0.02 | 0.27 |
| LDL-c | r = 0.05 | r = 0.07 | r = 0.31 | ρ = 0.05 | ρ = 0.13 | r = 0.03 | r = 0.34 | r = 0.29 | ρ = −0.01 |
| p-value | 0.79 | 0.71 | 0.13 | 0.78 | 0.53 | 0.89 | 0.08 | 0.14 | 0.95 |
| Totalcholesterol | r = −0.04 | r = −0.05 | r = 0.29 | ρ = 0.03 | ρ = 0.12 | r = −0.002 | r = 0.28 | r = 0.23 | ρ = −0.08 |
| p-value | 0.84 | 0.79 | 0.14 | 0.87 | 0.56 | 0.98 | 0.16 | 0.25 | 0.67 |
| Triglycerides | ρ = 0.28 | ρ = 0.37 | ρ = 0.4 | ρ = 0.3 | ρ = 0.36 | ρ = 0.15 | ρ = 0.4 | ρ = 0.29 | ρ = 0.04 |
| p-value | 0.16 | 0.06 | 0.04 | 0.14 | 0.07 | 0.46 | 0.05 | 0.15 | 0.85 |
| non-HDL-c | r = 0.11 | r = 0.11 | r = 0.41 | ρ = 0.13 | ρ = 0.24 | r = 0.12 | r = 0.41 | r = 0.37 | ρ = 0.02 |
| p-value | 0.58 | 0.57 | 0.04 | 0.51 | 0.24 | 0.56 | 0.03 | 0.06 | 0.91 |
| TG/HDL-c ratio | ρ = 0.3 | ρ = 0.4 | ρ = 0.4 | ρ = 0.36 | ρ = 0.39 | ρ = 0.17 | ρ = 0.38 | ρ = 0.39 | ρ = 0.08 |
| p-value | 0.14 | 0.04 | 0.04 | 0.07 | 0.05 | 0.4 | 0.06 | 0.05 | 0.68 |
| TC/HDL-c ratio | ρ = 0.38 | ρ = 0.36 | ρ = 0.52 | ρ = 0.41 | ρ = 0.49 | ρ = 0.31 | ρ = 0.5 | ρ = 0.54 | ρ = 0.11 |
| p-value | 0.06 | 0.07 | <0.01 | 0.04 | 0.01 | 0.12 | <0.01 | <0.01 | 0.57 |
| Fasting glucose | r = 0.04 | r = −0.09 | r = 0.02 | ρ = 0.002 | ρ = −0.06 | r = −0.03 | r = 0.02 | r = −0.12 | ρ = 0.1 |
| p-value | 0.85 | 0.64 | 0.9 | 0.99 | 0.76 | 0.87 | 0.91 | 0.55 | 0.61 |
| GPT | ρ = 0.48 | ρ = 0.57 | ρ = 0.37 | ρ = 0.39 | ρ = 0.36 | ρ = 0.42 | ρ = 0.24 | ρ = 0.24 | ρ = 0.36 |
| p-value | 0.01 | <0.01 | 0.06 | 0.05 | 0.07 | 0.03 | 0.24 | 0.24 | 0.07 |
| GOT | ρ = 0.37 | ρ = 0.3 | ρ = 0.27 | ρ = 0.15 | ρ = 0.19 | ρ = 0.13 | ρ = 0.23 | ρ = 0.09 | ρ = 0.44 |
| p-value | 0.06 | 0.14 | 0.18 | 0.44 | 0.36 | 0.52 | 0.26 | 0.66 | 0.02 |
| Cortisol 8 am | ρ = 0.23 | ρ = 0.38 | ρ = 0.24 | ρ = 0.34 | ρ = 0.29 | ρ = 0.23 | ρ = 0.25 | ρ = 0.27 | ρ = 0.007 |
| p-value | 0.25 | 0.056 | 0.24 | 0.09 | 0.15 | 0.25 | 0.23 | 0.18 | 0.97 |
| TSH | r = −0.06 | r = 0.1 | r = −0.16 | ρ = 0.1 | ρ = −0.008 | r = 0.17 | r = 0.38 | r = −0.23 | ρ = −0.02 |
| p-value | 0.74 | 0.62 | 0.41 | 0.62 | 0.96 | 0.41 | 0.057 | 0.24 | 0.9 |
| 25-OH- Vitamin D | ρ = −0.12 | ρ = −0.28 | ρ = 0.11 | ρ = 0.08 | ρ = 0.09 | ρ = −0.03 | ρ = 0.1 | ρ = 0.09 | ρ = 0.02 |
| p-value | 0.56 | 0.16 | 0.58 | 0.69 | 0.63 | 0.86 | 0.61 | 0.65 | 0.89 |
| Ionized Calcium | ρ = 0.33 | ρ = 0.05 | ρ = 0.5 | ρ = 0.39 | ρ = 0.51 | ρ = 0.18 | ρ = 0.54 | ρ = 0.53 | ρ = 0.39 |
| p-value | 0.1 | 0.79 | 0.01 | 0.053 | 0.008 | 0.38 | <0.01 | <0.01 | 0.048 |
| Independent Variables | Dependent Variable (Significant) | Coefficient of Determination R2 | Coefficient | Std. Error | t | p-Value |
|---|---|---|---|---|---|---|
| HDL-c | AIx | 0.09 | −0.33 | 0.14 | −2.30 | 0.02 |
| DBP | 0.26 | −0.27 | 0.11 | −2.34 | 0.02 | |
| LDL-c | PWV | 0.19 | 0.006 | 0.001 | 3.55 | <0.01 |
| SBP | 0.22 | 0.15 | 0.03 | 3.90 | <0.01 | |
| cSBP | 0.24 | 0.18 | 0.04 | 4.19 | <0.01 | |
| cDBP | 0.32 | 0.08 | 0.03 | 2.52 | 0.01 | |
| Triglycerides | cPP | 0.24 | 0.06 | 0.02 | 3.06 | <0.01 |
| TG/HDL-c ratio | DBP | 0.26 | 2.17 | 0.71 | 3.07 | <0.01 |
| HR | 0.14 | 2.29 | 0.75 | 3.03 | <0.01 | |
| cPP | 0.24 | −2.49 | 0.7 | −3.54 | <0.01 | |
| TC/HDL-c ratio | MAP | 0.25 | 3.50 | 0.81 | 4.28 | <0.01 |
| cDBP | 0.32 | 3.20 | 0.91 | 3.49 | <0.01 | |
| GOT | AIx | 0.34 | −0.81 | 0.24 | −3.27 | <0.01 |
| MAP | 0.17 | 0.21 | 0.09 | 2.38 | 0.02 | |
| cSBP | 0.35 | 0.57 | 0.1 | 5.45 | <0.01 | |
| cPP | 0.15 | 0.25 | 0.08 | 3.09 | <0.01 | |
| GPT | PWV | 0.42 | 0.01 | 0.003 | 6.19 | <0.01 |
| AIx | 0.34 | 0.89 | 0.19 | 4.51 | <0.01 | |
| SBP | 0.35 | 0.4 | 0.07 | 5.44 | <0.01 | |
| DBP | 0.16 | 0.22 | 0.07 | 3.22 | <0.01 | |
| cDBP | 0.30 | 0.34 | 0.07 | 4.85 | <0.01 | |
| 25-OH-Vitamin D | MAP | 0.17 | 0.27 | 0.09 | 2.88 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihuta, M.S.; Stoian, D.; Borlea, A.; Roi, C.M.; Velea-Barta, O.-A.; Mozos, I.; Paul, C. Evaluating the Arterial Stiffness as a Useful Tool in the Management of Obese Children. Children 2023, 10, 183. https://doi.org/10.3390/children10020183
Mihuta MS, Stoian D, Borlea A, Roi CM, Velea-Barta O-A, Mozos I, Paul C. Evaluating the Arterial Stiffness as a Useful Tool in the Management of Obese Children. Children. 2023; 10(2):183. https://doi.org/10.3390/children10020183
Chicago/Turabian StyleMihuta, Monica Simina, Dana Stoian, Andreea Borlea, Cristina Mihaela Roi, Oana-Alexandra Velea-Barta, Ioana Mozos, and Corina Paul. 2023. "Evaluating the Arterial Stiffness as a Useful Tool in the Management of Obese Children" Children 10, no. 2: 183. https://doi.org/10.3390/children10020183
APA StyleMihuta, M. S., Stoian, D., Borlea, A., Roi, C. M., Velea-Barta, O.-A., Mozos, I., & Paul, C. (2023). Evaluating the Arterial Stiffness as a Useful Tool in the Management of Obese Children. Children, 10(2), 183. https://doi.org/10.3390/children10020183











