Interventions for Promoting Meconium Passage in Very Preterm Infants—A Survey of Current Practice at Tertiary Neonatal Centers in Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Questionnaire and Study Population
2.3. Statistical Analysis
3. Results
3.1. Demographics
3.2. Enteral Nutrition in Preterm Infants with a Birthweight <1000 g
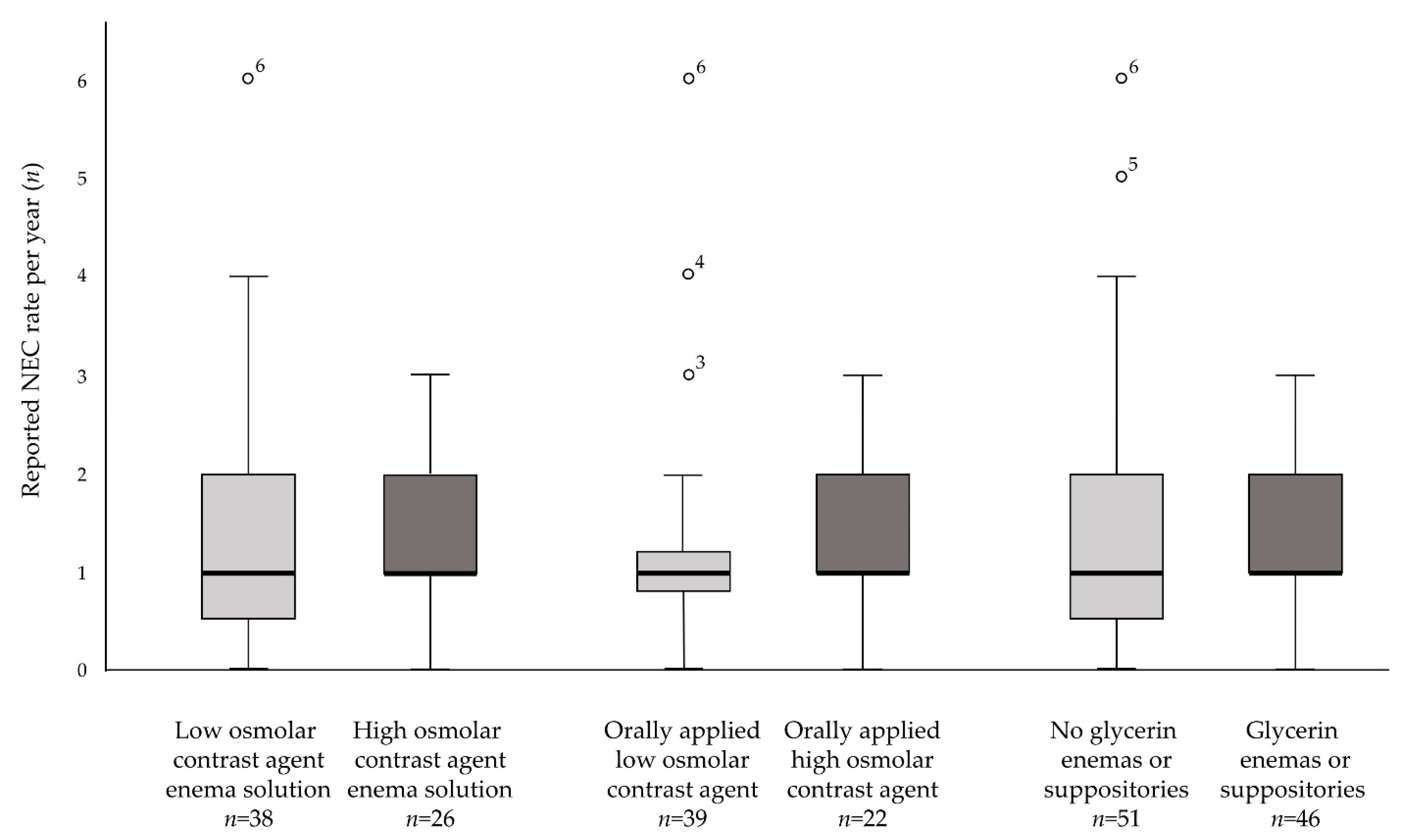
3.3. Gastrointestinal Complications
3.4. Use of Steroids during the First Two Weeks and Pharmacological Treatment of Patent Ductus Arteriosus
3.5. Interventions to Promote Meconium Evacuation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bekkali, N.; Hamers, S.L.; Schipperus, M.R.; Reitsma, J.B.; Valerio, P.G.; Van Toledo, L.; Benninga, M.A. Duration of meconium passage in preterm and term infants. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F376–F379. [Google Scholar] [CrossRef]
- Mihatsch, W.A.; Franz, A.R.; Lindner, W.; Pohlandt, F. Meconium passage in extremely low birthweight infants and its relation to very early enteral nutrition. Acta Paediatr. 2001, 90, 409–411. [Google Scholar] [CrossRef]
- Shim, S.Y.; Kim, H.S.; Kim, D.H.; Kim, E.K.; Son, D.W.; Kim, B.I.; Choi, J.H. Induction of early meconium evacuation promotes feeding tolerance in very low birth weight infants. Neonatology 2007, 92, 67–72. [Google Scholar] [CrossRef]
- de Pipaon Marcos, M.S.; Montes Bueno, M.T.; SanJose, B.; Torralba, E.; Gil, M.; Parada, I.; Amo, P. Acquisition of full enteral feeds may depend on stooling pattern in very premature infants. J. Perinat. Med. 2012, 40, 427–431. [Google Scholar] [CrossRef]
- Haiden, N.; Norooz, F.; Klebermass-Schrehof, K.; Horak, A.S.; Jilma, B.; Berger, A.; Repa, A. The effect of an osmotic contrast agent on complete meconium evacuation in preterm infants. Pediatrics 2012, 130, e1600–e1606. [Google Scholar] [CrossRef]
- Deshmukh, M.; Balasubramanian, H.; Patole, S. Meconium Evacuation for Facilitating Feed Tolerance in Preterm Neonates: A Systematic Review and Meta-Analysis. Neonatology 2016, 110, 55–65. [Google Scholar] [CrossRef]
- Solaz-García, A.; Segovia-Navarro, L.; De Dios-Benlloch, J.R.; Benavent-Taengua, L.; Castilla-Rodríguez, D.; Company-Morenza, M. Prevention of meconium obstruction in very low birth weight preterm infants. Enfermia Intensiva 2019, 30, 72–77. [Google Scholar] [CrossRef]
- Gross, M.; Poets, C.F. Lipid enemas for meconium evacuation in preterm infants—A retrospective cohort study. BMC Pediatr. 2021, 21, 454. [Google Scholar] [CrossRef]
- Kamphorst, K.; Sietsma, Y.; Brouwer, A.J.; Rood, P.J.; van den Hoogen, A. Enemas, suppositories and rectal stimulation are not effective in accelerating enteral feeding or meconium evacuation in low-birthweight infants: A systematic review. Acta Paediatr. 2016, 105, 1280–1287. [Google Scholar] [CrossRef]
- Ibrahim, T.; Wei, C.L.; Bautista, D.; Sriram, B.; Fay, L.X.; Rajadurai, V.S. Saline Enemas versus Glycerin Suppositories to Promote Enteral Feeding in Premature Infants: A Pilot Randomized Controlled Trial. Neonatology 2017, 112, 347–353. [Google Scholar] [CrossRef]
- Haiden, N.; Jilma, B.; Gerhold, B.; Klebermass, K.; Prusa, A.R.; Kuhle, S.; Rohrmeister, K.; Kohlhauser-Vollmuth, C.; Pollak, A. Small volume enemas do not accelerate meconium evacuation in very low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 270–273. [Google Scholar] [CrossRef]
- Burchard, P.R.; Lay, R.; Ruffolo, L.I.; Ramazani, S.N.; Walton, J.M.; Livingston, M.H. Glycerin Suppositories and Enemas in Premature Infants: A Meta-analysis. Pediatrics 2022, 149, e2021053413. [Google Scholar] [CrossRef]
- Sharma, A.; Duc, N.T.M.; Thang, T.L.L.; Nam, N.H.; Ng, S.J.; Abbas, K.S.; Huy, N.T.; Marušić, A.; Paul, C.L.; Kwok, J.; et al. A Consensus-Based Checklist for Reporting of Survey Studies (CROSS). J. Gen. Intern. Med. 2021, 36, 3179–3187. [Google Scholar] [CrossRef]
- Gemeinsamer Bundesausschuss, I. Perinatalzentren 2018. Available online: https://perinatalzentren.org/startseite/ (accessed on 28 February 2022).
- Cho, H.-H.; Cheon, J.-E.; Choi, Y.H.; Lee, S.M.; Kim, W.S.; Kim, I.-O.; Shin, S.-M.; Kim, E.-K.; Kim, H.-S.; Choi, J.-H.; et al. Ultrasound-guided contrast enema for meconium obstruction in very low birth weight infants: Factors that affect treatment success. Eur. J. Radiol. 2015, 84, 2024–2031. [Google Scholar] [CrossRef]
- Livingston, M.H.; Shawyer, A.C.; Rosenbaum, P.L.; Williams, C.; Jones, S.A.; Walton, J.M. Glycerin enemas and suppositories in premature infants: A meta-analysis. Pediatrics 2015, 135, 1093–1106. [Google Scholar] [CrossRef]
- Burke, M.S.; Ragi, J.M.; Karamanoukian, H.L.; Kotter, M.; Brisseau, G.F.; Borowitz, D.S.; Ryan, M.E.; Irish, M.S.; Glick, P.L. New strategies in nonoperative management of meconium ileus. J. Pediatr. Surg. 2002, 37, 760–764. [Google Scholar] [CrossRef]
- Mitani, Y.; Kubota, A.; Goda, T.; Kato, H.; Watanabe, T.; Riko, M.; Tsuno, Y.; Kumagai, T.; Yamaue, H. Optimum therapeutic strategy for meconium-related ileus in very-low-birth-weight infants. J. Pediatr. Surg. 2021, 56, 1117–1120. [Google Scholar] [CrossRef]
- Leung, A.K.; Hon, K.L. Paediatrics: How to manage functional constipation. Drugs Context 2021, 10, 2020-11-2. [Google Scholar] [CrossRef]
- Ahmed, M.; Pai, B.; Reynolds, T. Use of polyethylene glycol in children less than 3 years of age. J. Coll. Physicians Surg. Pak. 2012, 22, 267–268. [Google Scholar]
- Michail, S.; Gendy, E.; Preud’Homme, D.; Mezoff, A. Polyethylene glycol for constipation in children younger than eighteen months old. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 197–199. [Google Scholar] [CrossRef]
- Shaw, A. Safety of N-acetylcysteine in treatment of meconium obstruction of the newborn. J. Pediatr. Surg. 1969, 4, 119–125. [Google Scholar] [CrossRef]
- Schauble, A.L.; Bisaccia, E.K.; Lee, G.; Nasr, S.Z. N-acetylcysteine for Management of Distal Intestinal Obstruction Syndrome. J. Pediatr. Pharmacol. Ther. 2019, 24, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Yarkin, Y.; Maas, C.; Franz, A.R.; Kirschner, H.J.; Poets, C.F. Epidemiological study on intestinal volvulus without malrotation in VLBW infants. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F415–F418. [Google Scholar] [CrossRef]
- Saenz de Pipaon Marcos, M.; Teresa Montes Bueno, M.; Sanjose, B.; Gil, M.; Parada, I.; Amo, P. Randomized controlled trial of prophylactic rectal stimulation and enemas on stooling patterns in extremely low birth weight infants. J. Perinatol. 2013, 33, 858–860. [Google Scholar] [CrossRef][Green Version]
- Wood, B.P.; Katzberg, R.W. Tween 80/diatrizoate enemas in bowel obstruction. AJR Am. J. Roentgenol. 1978, 130, 747–750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burns, K.E.A.; Kho, M.E. How to assess a survey report: A guide for readers and peer reviewers. Can. Med. Assoc. J. 2015, 187, E198–E205. [Google Scholar] [CrossRef] [PubMed]
- Rina, P.; Zeng, Y.; Ying, J.; Qu, Y.; Mu, D. Association of initial empirical antibiotic therapy with increased risk of necrotizing enterocolitis. Eur. J. Pediatr. 2020, 179, 1047–1056. [Google Scholar] [CrossRef]
- Kelley, K.; Clark, B.; Brown, V.; Sitzia, J. Good practice in the conduct and reporting of survey research. Int. J. Qual. Health Care 2003, 15, 261–266. [Google Scholar] [CrossRef]
- Awolaran, O.; Sheth, J. Management strategies for functional intestinal obstruction of prematurity. J. Neonatal Surg. 2021, 10, 12. [Google Scholar] [CrossRef]

| Total (n = 102) | Enemas n (%) | Orally Applied Contrast Agent n (%) |
|---|---|---|
| Use | ||
| Yes | 100 (98.0%) | 63 (61.8%) |
| No | 2 (2.0%) | 39 (38.2%) |
| Intended use | n = 100 # | n = 61 # |
| Prophylactic | 29 (29.0%) | 5 (8.2%) |
| Therapeutic | 71 (71.0%) | 56 (91.8%) |
| Target population | n = 100 # | n = 63 |
| All preterm infants | 27 (27.0%) | 18 (28.6%) |
| <32 weeks of gestation | 19 (19.0%) | 6 (9.5%) |
| <28 weeks of gestation | 27 (27.0%) | 16 (25.4%) |
| <1500 g birth weight | 32 (32.0%) | 7 (11.1%) |
| <1000 g birth weight | 26 (26.0%) | 15 (23.8%) |
| Other criteria | 5 (5.0%) “Absent passage of meconium” | 7 (11.1%) “If other interventions failed” |
| 3 (3.0%) “No meconium until day three” | 3 (4.8%) “Only in rare cases” | |
| 2 (2.0%) “Small for gestational age” | 3 (4.8%) “Small for gestational age” | |
| 2 (2.0%) “Special cases” | 3 (4.8%) “Ileus or mechanical obstruction” | |
| 2 (3.2%) “Birth weight >1000–1500 g” | ||
| Initiation | n = 100 # | n = 63 |
| First postnatal day | 18 (18.0%) | 3 (4.8%) |
| Given postnatal day | 36 (36.0%) Postnatal day 2.0 (2.0–4.0) | 10 (15.9%) Postnatal day 3.5 (3.0–5.0) |
| No or little meconium passed until | 52 (52.0%) Postnatal day 3.0 (2.0–6.0) | 46 (73.0%) Postnatal day 5.0 (3.0–10.0) |
| Frequency | n = 100 # | n = 59 § |
| Once a day | 23 (23.0%) | 45 (71.4%) |
| Multiple times a day | 41 (41.0%) | 10 (15.9%) |
| Only once or twice in total | 34 (34.0%) | N/A |
| Based on indication | 6 (6.0%) “Twice a day” | 4 (6.5%) “Only once” |
| 4 (4.0%) “Special cases” | 4 (6.5%) “Meconium ileus” | |
| 2 (2.0%) “Three times per day” | 1 (1.6%) “Two times” | |
| 2 (2.0%) “Every 48 h” | 1 (1.6%) “Three times every 48 h” | |
| 1 (1.6%) “Meconium plugging” | ||
| Duration of use | n = 100 # | N/A |
| Until passing meconium at least once | 35 (35.0%) | |
| Until passing transitional stool | 45 (45.0%) | |
| Until passing milk stool | 3 (3.0%) | |
| Until full enteral feeds | 2 (2.0%) | |
| Others | 15 (15.0%) “One or two spontaneous | |
| bowel movements per day” | ||
| 9 (9.0%) “Based on individual decisions” | ||
| Agents used | n = 100 # | N/A |
| Normal saline | 76 (76.0%) | |
| Contrast agent | 67 (67.0%) | |
| Glycerin | 41 (41.0%) | |
| Acetylcysteine | 23 (23.0%) | |
| Glucose 5% | 22 (22.0%) | |
| Lipid solution | 9 (9.0%) | |
| Breast milk | 5 (5.0%) | |
| Others | 2 (2.0%) “Glucose and glycerin” | |
| 2 (2.0%) “Tween 0.5%” | ||
| 1 (1.0%) “Glucose and acetylcysteine” | ||
| 1 (1.0%) “Glucose 10%” | ||
| 1 (1.0%) “Glycerin and distilled water” | ||
| 1 (1.0%) “Normal saline and glycerin” | ||
| 1 (1.0%) “Normal saline and acetylcysteine” | ||
| 1 (1.0%) “Ringer’s solution and PEG” | ||
| Type of contrast agent | n = 67 | n = 63 |
| Low osmolar | 40 (59.7%) | 39 (61.9%) |
| High osmolar | 24 (35.8%) | 22 (34.9%) |
| Both low and high osmolar | 3 (4.5%) | 2 (3.2%) |
| Total (n = 102) | Polyethylene Glycol n (%) | Maltodextrin n (%) |
|---|---|---|
| Use | ||
| Yes | 47 (46.1%) | 9 (8.8%) |
| No | 55 (53.9%) | 93 (91.2%) |
| Intended use | n = 47 | N/A |
| Prophylactic | 16 (34.0%) | |
| Therapeutic | 31 (66.0%) | |
| Target population | n = 47 | n = 9 |
| All preterm infants | 11 (23.4%) | 1 (11.1%) |
| <32 weeks of gestation | 11 (23.4%) | 2 (22.2%) |
| <28 weeks of gestation | 15 (31.9%) | 2 (22.2%) |
| <1500 g birth weight | 14 (29.8%) | 0 (0.0%) |
| <1000 g birth weight | 11 (23.4%) | 2 (22.2%) |
| Other criteria | 4 (8.5%) “Small for gestational age” | 1 (11.1%) “Small for gestational age” |
| 3 (6.4%) “If other interventions failed” | 1 (11.1%) “Impaired intestinal motility” | |
| 2 (4.3%) “Only in rare cases” | ||
| 1 (2.1%) “Meconium plugging” | ||
| Initiation | n = 47 | n = 9 |
| First postnatal day | 8 (17.0%) | 6 (66.7%) |
| Given postnatal day | 14 (29.8%) Postnatal day 3.0 (1.0–8.0) | 1 (11.1%) Postnatal day 3.0 (3.0–3.0) |
| No or little meconium passed until | 25 (53.2%) Postnatal day 3.0 (2.0–14.0) | 2 (22.2%) Postnatal day 4.0 (3.0–5.0) |
| Duration of use | n = 47 | n = 9 |
| Until passing meconium at least once | 12 (25.5%) | 2 (22.2%) |
| Until passing transitional stool | 18 (38.3%) | 5 (55.6%) |
| Until passing milk stool | 4 (8.5%) | 0 (0.0%) |
| Until full enteral feeds | 9 (19.1%) | 0 (0.0%) |
| Others | 3 (6.4%) “Multiple bowel movements” | 1 (11.1%) “First two feeds with maltodex- |
| 1 (2.1%) “14 days” | trin, then one feed consisting of a 1:1 mix- | |
| 1 (2.1%) “28 days” | ture of maltodextrin and milk | |
| 1 (2.1%) “42 days” | or formula” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gross, M.; Hummler, H.; Haase, B.; Quante, M.; Wiechers, C.; Poets, C.F. Interventions for Promoting Meconium Passage in Very Preterm Infants—A Survey of Current Practice at Tertiary Neonatal Centers in Germany. Children 2022, 9, 1122. https://doi.org/10.3390/children9081122
Gross M, Hummler H, Haase B, Quante M, Wiechers C, Poets CF. Interventions for Promoting Meconium Passage in Very Preterm Infants—A Survey of Current Practice at Tertiary Neonatal Centers in Germany. Children. 2022; 9(8):1122. https://doi.org/10.3390/children9081122
Chicago/Turabian StyleGross, Maximilian, Helmut Hummler, Bianca Haase, Mirja Quante, Cornelia Wiechers, and Christian F. Poets. 2022. "Interventions for Promoting Meconium Passage in Very Preterm Infants—A Survey of Current Practice at Tertiary Neonatal Centers in Germany" Children 9, no. 8: 1122. https://doi.org/10.3390/children9081122
APA StyleGross, M., Hummler, H., Haase, B., Quante, M., Wiechers, C., & Poets, C. F. (2022). Interventions for Promoting Meconium Passage in Very Preterm Infants—A Survey of Current Practice at Tertiary Neonatal Centers in Germany. Children, 9(8), 1122. https://doi.org/10.3390/children9081122






