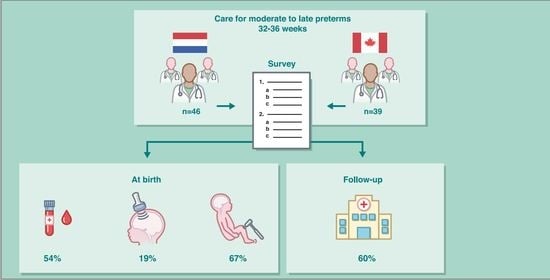
Neurological Surveillance in Moderate-Late Preterm Infants—Results from a Dutch–Canadian Survey
Abstract
1. Introduction
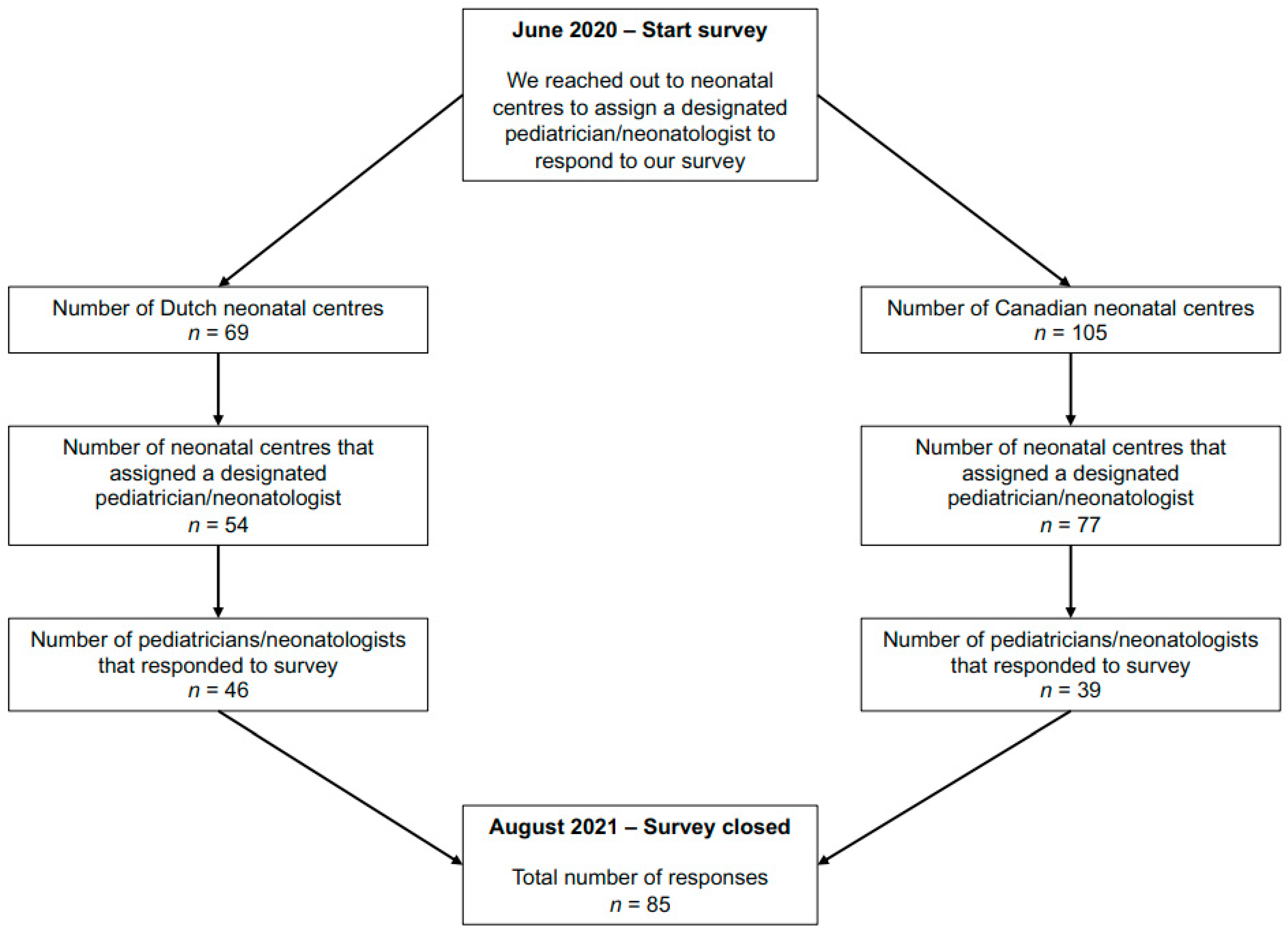
2. Materials and Methods
3. Results
3.1. Admission Criteria
3.2. Laboratory Testing
3.3. Neuroimaging
3.4. Neurological Examination
3.5. Follow-Up Program
3.6. Care for MLPT Infants Admitted to the Postpartum Maternity Ward
3.7. Differences between Dutch and Canadian Practices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.-B.; Kinney, M.; Lawn, J.; the Born Too Soon Preterm Birth Action Group. Born Too Soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, S2. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Natarajan, G.; Shankaran, S. Short- and Long-Term Outcomes of Moderate and Late Preterm Infants. Am. J. Perinatol. 2016, 33, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Mitha, A.; Chen, R.; Altman, M.; Johansson, S.; Stephansson, O.; Bolk, J. Neonatal Morbidities in Infants Born Late Preterm at 35–36 Weeks of Gestation: A Swedish Nationwide Population-based Study. J. Pediatr. 2021, 233, 43–50.e5. [Google Scholar] [CrossRef]
- Scheuchenegger, A.; Lechner, E.; Wiesinger-Eidenberger, G.; Weissensteiner, M.; Wagner, O.; Schimetta, W.; Resch, B. Short-term mor-bidities in moderate and late preterm infants. Klin. Padiatr. 2014, 226, 216–220. [Google Scholar]
- Johnson, S.; Evans, T.A.; Draper, E.S.; Field, D.J.; Manktelow, B.N.; Marlow, N.; Matthews, R.; Petrou, S.; Seaton, S.E.; Smith, L.K.; et al. Neurodevelopmental outcomes following late and moderate prematurity: A popula-tion-based cohort study. Arch. Dis. Child. Fetal. Neonatal Ed. 2015, 100, F301–F308. [Google Scholar] [CrossRef]
- Kerstjens, J.M.; de Winter, A.F.; Sollie, K.M.; Bocca-Tjeertes, I.F.; Potijk, M.R.; Reijneveld, S.A.; Bos, A.F. Maternal and Pregnancy-Related Factors Associated With Developmental Delay in Moderately Preterm–Born Children. Obstet. Gynecol. 2013, 121, 727–733. [Google Scholar] [CrossRef]
- Boyle, J.D. Born just a few weeks early: Does it matter? Fetal Neonatal 2013, 98, 85–88. [Google Scholar] [CrossRef]
- van Baar, A.L.; Vermaas, J.; Knots, E.; de Kleine, M.J.K.; Soons, P. Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics 2009, 124, 251–257. [Google Scholar] [CrossRef]
- Cserjesi, R.; Van Braeckel, K.N.; Butcher, P.R.; Kerstjens, J.M.; Reijneveld, S.A.; Bouma, A.; Geuze, R.H.; Bos, A.F. Functioning of 7-Year-Old Children Born at 32 to 35 Weeks’ Gestational Age. Pediatrics 2012, 130, e838–e846. [Google Scholar] [CrossRef]
- Bogicevic, L.; Verhoeven, M.; van Baar, A.L. Toddler skills predict moderate-to-late preterm born children’s cognition and behaviour at 6 years of age. PLoS ONE 2019, 14, e0223690. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Nadal, S.; Bosch, L. Cognitive and Learning Outcomes in Late Preterm Infants at School Age: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Kerstjens, J.M.; de Winter, A.F.; Bocca-Tjeertes, I.F.; Vergert, E.M.T.; Reijneveld, S.A.; Bos, A.F. Developmental Delay in Moderately Preterm-Born Children at School Entry. J. Pediatr. 2011, 159, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Boswinkel, V.; Nijboer-Oosterveld, J.; Nijholt, I.M.; Edens, M.A.; Mulder-de Tollenaer, S.M.; Boomsma, M.F.; de Vries, L.S.; van Wezel-Meijler, G. A systematic review on brain injury and altered brain development in moderate-late preterm infants. Early Hum. Dev. 2020, 148, 105094. [Google Scholar] [CrossRef] [PubMed]
- Neonatale Neuro-Imaging. Nederlandse Vereniging voor Kindergeneeskunde. Available online: https://neonatology.eu/sites/neonatology.eu/files/neonatale_neuroimaging_versie_1_5_feb_2015.pdf (accessed on 7 May 2022).
- Guillot, M.; Chau, V.; Lemyre, B. Routine imaging of the preterm neonatal brain. Paediatr. Child Health 2020, 25, 249–255, Erratum in Paediatr. Child Health 2021, 26, 259. [Google Scholar] [CrossRef]
- Hand, I.L.; Shellhaas, R.A.; Milla, S.S.; Committee on fetus and newborn, section on neurology, section on radiology. Routine Neuroimaging of the Preterm Brain. Pediatrics 2020, 146. [Google Scholar] [CrossRef]
- Reymundo, M.G.; Suazo, J.A.H.; Aguilar, M.J.C.; Faura, F.J.S.; Galiana, G.G.; Peinador, Y.M.; Moya, A.J.; Guasch, X.D. Follow-up recommendations for the late preterm infant. An. Pediatría 2019, 90, 318.e1–318.e8. [Google Scholar] [CrossRef]
- Te Vroeg en/of Small for Gestational Age (SGA) Geboren Kinderen. Available online: https://richtlijnendatabase.nl/gerelateerde_documenten/f/15122/Samenvatting%20JGZ.pdf (accessed on 28 May 2022).
- Jefferies, A.L. Canadian Paediatric Society, Fetus and Newborn Committee. Going home: Facilitating discharge of the preterm infant. Paediatr. Child Health 2014, 19, 31–42. [Google Scholar] [CrossRef]
- Fleming, P.F.; Arora, P.; Mitting, R.; Aladangady, N. A national survey of admission practices for late preterm infants in England. BMC Pediatr. 2014, 14, 150. [Google Scholar] [CrossRef][Green Version]
- Narvey, M.R.; Marks, S.D. The screening and management of newborns at risk for low blood glucose. Paediatr. Child Health 2019, 24, 536–544. [Google Scholar] [CrossRef]
- Postnatale Zorg in de Algemene Kindergeneeskunde. Available online: https://richtlijnendatabase.nl/richtlijn/postnatale_zorg_in_de_algemene_kindergeneeskunde/startpagina_-_postnatale_zorg_in_de_algemene_kindergeneeskunde.html (accessed on 28 May 2022).
- Kerstjens, J.M.; Bocca-Tjeertes, I.F.; de Winter, A.F.; Reijneveld, S.A.; Bos, A.F. Neonatal Morbidities and Developmental Delay in Moderately Preterm-Born Children. Pediatrics 2012, 130, e265–e272. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Farahbakhsh, N.; Shastri, S.; Sharma, P. Biomarkers for diagnosis of neonatal sepsis: A literature review. J. Matern. Fetal Neonatal Med. 2017, 31, 1646–1659. [Google Scholar] [CrossRef]
- Khan, F. C-reactive Protein as a Screening Biomarker in Neonatal Sepsis. J. Coll. Physicians Surg. Pak. 2019, 29, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Barrington, K.J.; Sankaran, K.; Canadian Paediatric Society, Fetus and Newborn Committee. Guidelines for detection, manage-ment and prevention of hyperbilirubinemia in term and late preterm newborn infants. Paediatr. Child Health 2007, 12 (Suppl. B), 1B–12B. [Google Scholar] [CrossRef]
- Hyperbilirubinemie, Preventie, Diagnostiek en Behandeling bij de Pasgeborene, Geboren na een Zwangerschapsduur van Meer dan 35 Weken. Available online: https://www.nvk.nl/themas/kwaliteit/richtlijnen/richtlijn?componentid=6881307&tagtitles=Maag-Darm-Leverziekten%2B(MDL),Neonatologie (accessed on 28 May 2022).
- Guillot, M.; Sebastianski, M.; Lemyre, B. Comparative performance of head ultrasound and MRI in detecting preterm brain in-jury and predicting outcomes: A systematic review. Acta Paediatr. 2021, 110, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.J.; Doyle, L.W.; Boyd, R.N. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev. Med. Child Neurol. 2008, 50, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.; Ricci, M.; Picilli, M.; Cordaro, B.; Mercuri, E. Early Neurological Assessment and Long-Term Neuromotor Outcomes in Late Preterm Infants: A Critical Review. Medicina 2020, 56, 475. [Google Scholar] [CrossRef]
- Phillips, R.M.; on behalf of The National Perinatal Association; Goldstein, M.; Hougland, K.; Nandyal, R.; Pizzica, A.; Santa-Donato, A.; Staebler, S.; Stark, A.R.; Treiger, T.M.; et al. Multidisciplinary guidelines for the care of late preterm infants. J. Perinatol. 2013, 33, S5–S22. [Google Scholar] [CrossRef]
- Khan, K.A.; Petrou, S.; Dritsaki, M.; Johnson, S.J.; Manktelow, B.; Draper, E.S.; Smith, L.K.; Seaton, S.E.; Marlow, N.; Dorling, J.; et al. Economic costs associated with moderate and late preterm birth: A prospective population-based study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1495–1505. [Google Scholar] [CrossRef]
- Speer, R.; Schaefer, E.; Aholoukpe, M.; Leslie, D.; Gandhi, C. Trends in Costs of Birth Hospitalization and Readmissions for Late Preterm Infants. Children 2021, 8, 127. [Google Scholar] [CrossRef]
- Bridgemohan, C.; Bauer, N.S.; Nielsen, B.A.; DeBattista, A.; Ruch-Ross, H.; Paul, L.B.; Roizen, N. A Workforce Survey on Developmen-tal-Behavioral Pediatrics. Pediatrics 2018, 141, e20172164. [Google Scholar] [CrossRef] [PubMed]
- Short, H.L.; Taylor, N.; Thakore, M.; Piper, K.; Baxter, K.; Heiss, K.F.; Raval, M.V. A survey of pediatric surgeons’ practices with enhanced recovery after children’s surgery. J. Pediatr. Surg. 2018, 53, 418–430. [Google Scholar] [CrossRef] [PubMed]
| Dutch Centres | Canadian Centres | Total | |
|---|---|---|---|
| Response rate | 46/69 (67) | 39/105 (37) | 85/174 (49) |
| n = 46 | n = 39 | n = 85 | |
| Position | |||
| Paediatrician | 22 (48) | 9 (23) | 31 (36) |
| Neonatologist | 23 (50) | 29 (74) | 52 (61) |
| Missing data | 1 (2) | 1 (3) | 2 (2) |
| Number of years working as a paediatrician or neonatologist | |||
| <5 years | 5 (11) | 4 (10) | 9 (11) |
| 5–10 years | 13 (28) | 10 (26) | 23 (27) |
| 10–15 years | 14 (30) | 6 (15) | 20 (24) |
| >15 years | 14 (30) | 18 (46) | 32 (38) |
| Missing data | 0 | 1 (3) | 1 (1) |
| Setting | |||
| Level III neonatal centre | 6 (13) | 20(51) | 25 (29) |
| Level I-II neonatal centre | 40 (87) | 19 (49) | 60 (71) |
| Number of incubators/beds (in total) | |||
| <5 beds | 6 (13) | 2 (5) | 8 (9) |
| 5–12 beds | 12 (26) | 6 (15) | 18 (21) |
| 10–15 beds | 12 (26) | 5 (13) | 17 (20) |
| 15–20 beds | 13 (28) | 7 (18) | 20 (24) |
| >20 beds | 3 (7) | 19 (49) | 22 (26) |
| Indication | Number of Centres Performing cUS on Indication n = 39 |
|---|---|
| (Suspected) seizures | 36 (92) |
| Other neurological symptoms (such as jitteriness, irritability, excessive crying, abnormal muscle tone, lethargy) | 36 (92) |
| Suspected sepsis | 7 (18) |
| Confirmed sepsis | 16 (41) |
| Suspected meningitis | 22 (56) |
| Confirmed meningitis | 32 (82) |
| Anaemia, infant needing PRBC transfusion | 20 (51) |
| Hyperbilirubinemia, infant needing exchange transfusion | 21 (54) |
| Antenatal diagnosis or suspicion of brain anomaly | 37 (95) |
| Dysmorphisms | 34 (87) |
| Other (multiple answers per centre) | 15 (39) Intrauterine growth restriction/dysmaturity: 5 (13) BW < 1500 g: 3 (8) Unexplained apnea: 3 (8) Monochorionic twins: 2 (5) Resuscitation: 2 (5) Fetal therapy: 1 (3) Hydrocephalus: 1 (3) Micro-/macrocephaly: 1 (3) Severe hypoglycaemia: 1 (3) Severe thrombocytopenia: 1 (3) Suspected congenital cytomegalovirus: 1 (3) |
| Indication for Follow-Up n = 51 | Follow-Up Performed by (Multiple Answers Were Possible) n = 51 | ||
|---|---|---|---|
| GA < 35 weeks | 26 (51) | Paediatrician/-neonatologist | 49 (96) |
| GA < 34 weeks | 8 (16) | Paediatric nurse | 7 (14) |
| GA < 33 weeks | 9 (18) | Paediatric resident | 3 (6) |
| GA < 37 weeks: | 3 (6) | ||
| GA < 36 weeks: | 2 (4) | ||
| Other | Low BW 38 (75) Medical conditions: 23 (45) Feeding difficulties: 2 (4) Psycho-social circumstances: 1 (2) | Paediatric nurse practitioner/ physician assistant | 11 (22) |
| Content of follow-up visit n = 51 | Collaboration with other disciplines or services (multiple answers were possible) n = 51 | ||
| Measuring weight and height | 49 (96) | No | 10 (20) |
| Physical examination | 48 (94) | Yes | 41 (80) |
| Neurological examination | 44 (86) | Physiotherapist | 30/41 (73) |
| Answering parents’ questions | 48 (94) | Psychologist | 7/41 (17) |
| Developmental assessment | 46 (90) | Speech therapist | 19/41 (46) |
| Other | Start iron supplementation: 2 (4) Screening for postnatal depression: 1 (2) | Other | Dietician: 5 (10) Well baby doctor or nurse: 3 (6) Social worker: 3 (6) Occupational therapist: 3 (6) Vaccinations: 3 (6) |
| Duration of follow-up program | n = 51 | ||
| 1 month | 1 (2) | ||
| 3–12 months | 17 (33) | ||
| When the child starts walking | 12 (24) | ||
| Unknown | 2 (4) | ||
| Other | 19 (37) Up till 18 months: 1 (2) Up till 24 months: 4 (8) Between 24–36 months: 1 (2) Between 24–48 months: 1 (2) Up till 36 months: 2 (4) Up till 42 months: 2 (4) Up till 48 months: 2 (4) Up till 54 months: 1 (2) Depending on medical condition and history: 4 (8) First year in neonatal centre, after this the infant will enrol in a special program at the ‘well baby’ clinic: 1 (2) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüse-Ruijter, M.F.; Boswinkel, V.; Consoli, A.; Nijholt, I.M.; Boomsma, M.F.; de Vries, L.S.; van Wezel-Meijler, G.; Leijser, L.M. Neurological Surveillance in Moderate-Late Preterm Infants—Results from a Dutch–Canadian Survey. Children 2022, 9, 846. https://doi.org/10.3390/children9060846
Krüse-Ruijter MF, Boswinkel V, Consoli A, Nijholt IM, Boomsma MF, de Vries LS, van Wezel-Meijler G, Leijser LM. Neurological Surveillance in Moderate-Late Preterm Infants—Results from a Dutch–Canadian Survey. Children. 2022; 9(6):846. https://doi.org/10.3390/children9060846
Chicago/Turabian StyleKrüse-Ruijter, Martine F., Vivian Boswinkel, Anna Consoli, Ingrid M. Nijholt, Martijn F. Boomsma, Linda S. de Vries, Gerda van Wezel-Meijler, and Lara M. Leijser. 2022. "Neurological Surveillance in Moderate-Late Preterm Infants—Results from a Dutch–Canadian Survey" Children 9, no. 6: 846. https://doi.org/10.3390/children9060846
APA StyleKrüse-Ruijter, M. F., Boswinkel, V., Consoli, A., Nijholt, I. M., Boomsma, M. F., de Vries, L. S., van Wezel-Meijler, G., & Leijser, L. M. (2022). Neurological Surveillance in Moderate-Late Preterm Infants—Results from a Dutch–Canadian Survey. Children, 9(6), 846. https://doi.org/10.3390/children9060846