Total Esophagogastric and Cologastric Dissociation in Neurologically Normal Children: Systematic Review
Abstract
:1. Introduction
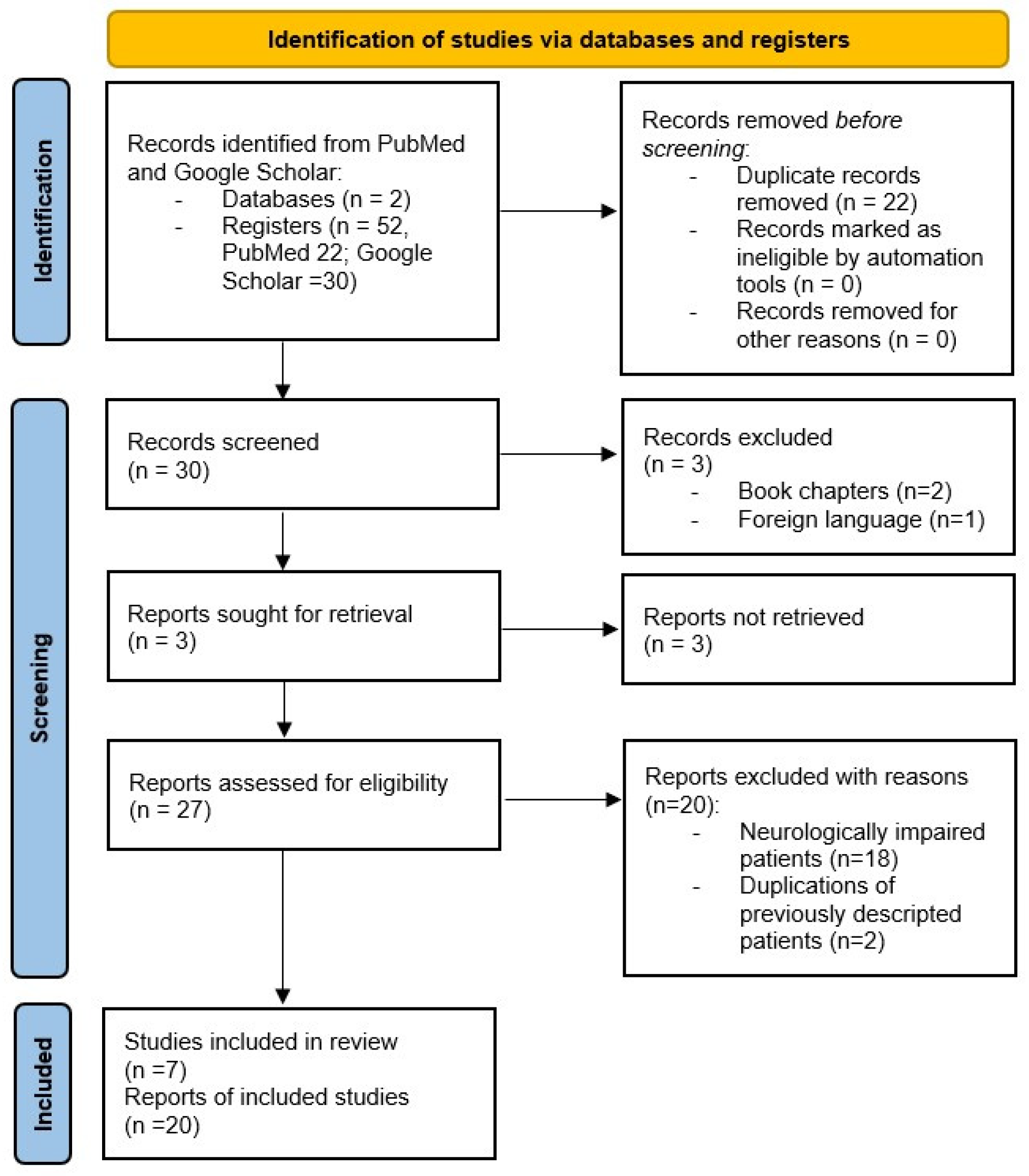
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quitadamo, P.; Papadopoulou, A.; Wenzl, T.; Urbonas, V.; Kneepkens, C.M.F.; Roman, E.; Orel, R.; Pavkov, D.J.; Dias, J.A.; Vandenplas, Y.; et al. European pediatricians’ approach to children with ger symptoms: Survey of the implementation of 2009 NASPGHAN-ESPGHAN guidelines. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.M.; Muniyappa, P. Pediatric gastroesophageal reflux disease in primary care: Evaluation and care update. Curr. Probl. Pediatr. Adolesc. Health Care 2020, 50, 100784. [Google Scholar] [CrossRef] [PubMed]
- Madre, C.; Serhal, L.; Michaud, L.; Bonnevalle, M.; de Lagausie, P.; Gottrand, F.; Bonnard, A.; Hugot, J.-P. Prolonged enteral feeding is often required to avoid long-term nutritional and metabolic complications after esophagogastric dissociation. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Lansdale, N.; McNiff, M.; Morecroft, J.; Kauffmann, L.; Morabito, A. Long-term and “patient-reported” outcomes of total esophagogastric dissociation versus laparoscopic fundoplication for gastroesophageal reflux disease in the severely neurodisabled child. J. Pediatr. Surg. 2015, 50, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Kvello, M.; Knatten, C.K.; Fyhn, T.; Bjørnland, K. Short and long-term outcomes after pediatric redo fundoplication. J. Pediatr. Surg. 2022, 57, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Pacilli, M.; Eaton, S.; Maritsi, D.; Lopez, P.J.; Spitz, L.; Kiely, E.M.; Drake, D.P.; Curry, J.I.; Pierro, A. Factors predicting failure of redo Nissen fundoplication in children. Pediatr. Surg. Int. 2007, 23, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A. Total esophagogastric dissociation: An alternative approach. J. Pediatr. Surg. 1997, 32, 1291–1294. [Google Scholar] [CrossRef]
- Coletta, R.; Aldeiri, B.; Jackson, R.; Morabito, A. Total esophagogastric dissociation (TEGD): Lessons from two decades of experience. J. Pediatr. Surg. 2019, 54, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Orizio, P.; Boroni, G.; Cheli, M.; Colusso, M.M.; Parolini, F.; Bianchi, A.; Alberti, D. Total Oesophagogastric Dissociation in Neurologically Impaired Children: 18 Years’ Experience and Long-term Follow-up. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Garritano, S.; Irino, T.; Scandavini, C.M.; Tsekrekos, A.; Lundell, L.; Rouvelas, I. Long-term functional outcomes after replacement of the esophagus in pediatric patients: A systematic literature review. J. Pediatr. Surg. 2017, 52, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fernandez, S.; Hernandez, F.; Hernandez-Martin, S.; Dominguez, E.; Ortiz, R.; de la Torre, C.; Martinez, L.; Tovar, J.A. Failed nissen fundoplication in children: Causes and management. Eur. J. Pediatr. Surg. 2014, 24, 79–82. [Google Scholar] [PubMed]
- Lall, A.; Morabito, A.; Bianchi, A. “Total Gastric Dissociation (TGD)” in difficult clinical situations. Eur. J. Pediatr. Surg. 2006, 16, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- De Lagausie, P.; Bonnard, A.; Schultz, A.; Van Den Abbeel, T.; Bellaiche, M.; Hartmann, J.F.; Cezard, J.P.; Aigrain, Y. Reflux in esophageal atresia, tracheoesophageal cleft, and esophagocoloplasty: Bianchi’s procedure as an alternative approach. J. Pediatr. Surg. 2005, 40, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Boubnova, J.; Hery, G.; Ughetto, F.; Charpentier, A.; Guys, J.M.; de Lagausie, P. Laparoscopic total esophagogastric dissociation. J. Pediatr. Surg. 2009, 44, e1. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, S.M.; Dakhoub, A.; Jarboe, M.D.; Geiger, J.D. Gastric dissociation for the treatment of congenital microgastria with paraesophageal hiatal hernia. J. Pediatr. Surg. 2011, 46, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Gottrand, M.; Michaud, L.; Guimber, D.; Coopman, S.; Sfeir, R.; Bonnevalle, M.; Leteurtre, E.; Gottrand, F. Barrett esophagus and esophagojejunal anastomotic stenosis as complications of esophagogastric disconnection in children with esophageal atresia. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Bvulani, B.; Numanoglu, A.; Cox, S.; Millar, A. Total Esophageal Gastric Dissociation for the Failed Antireflux Procedure in a Child with Microgastria. Eur. J. Pediatr. Surg. Rep. 2016, 4, 006–009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitz, L. Gastric transposition for oesophageal replacement in children. S. Afr. J. Surg. 2001, 39, 9–13. [Google Scholar]
- Coletta, R.; Mussi, E.; Bianchi, A.; Morabito, A. Modified Oesophago-Gastric Dissociation (M-OGD)—A technical modification. Updates Surg. 2020, 73, 775–778. [Google Scholar] [CrossRef] [PubMed]
| Author | n° | Sex | Mean Age Age Range | Primary Diagnosis | Previous Anti-Reflux Surgery | Gastrostomy (n°) | Gastrostomies Removed (n°) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | YES (n°) | NO (n°) | Redo NF (n°) | ||||||
| De Lagausie [14] | 13 | 6 | 7 | 35 m (14 d–218 m) | 9 EA (6 ECP) | NF (2) | 7 | Y (2) | Yes (13) | Yes (2) |
| 2 TEC | 0 | 2 | N | |||||||
| 2 CBI (2 ECP) | 0 | 2 | N | |||||||
| Lall [12] | 11 | 7 | 4 | 52.7 m (24 m–72 m) | 3 EA (ECP) | 0 | 3 | N | Yes (11) | No (7) but oral feeding “ad libitum” |
| 2 EA short gap | NF (2) | 0 | N | |||||||
| 1 Gastrectomy | 0 | 1 | N | |||||||
| Microgastria | 0 | 1 | N | |||||||
| Congenital stenosis | 0 | 1 | N | |||||||
| Short esophagus and CDH | 0 | 1 | N | |||||||
| 2 TEC | NF (2) | 0 | N | |||||||
| Boubnova [15] | 1 | 0 | 1 | 54 m | EA | NF(1) | 0 | Y | Yes (1) | No (1) |
| Madre [3] | 17 | 11 | 6 | 14.5 m (15 d–206.5 m) | 7 ECP | NF (4) | 3 | N | Yes (17) | Yes (4) |
| 5 EA | NF (4) | 1 | N | |||||||
| 2 TEC | NF (1) | 1 | Y | |||||||
| Microgastria | 0 | 1 | N | |||||||
| CBI | 0 | 1 | N | |||||||
| CHARGE | NF (1) | N | Y | |||||||
| Kunisaki [16] | 1 | 0 | 1 | 11 m | Microgastria | 0 | 1 | N | Yes (1) | Yes (1) |
| Gottrand [17] | 4 | 4 | 0 | 12.9 m (1.5 m–14 m) | 2 EA type III | NF (1) | 1 | N | Yes (4) | No (4) |
| 1 EA and Microgastria | NF (1) | 0 | N | |||||||
| 1 EA Type I | NF (1) | 0 | N | |||||||
| 2 * | N/A | N/A | N/A | 1 EA | N/A | N/A | N/A | N/A | ||
| 1 Laringeal diastema | N/A | N/A | N/A | N/A | ||||||
| Hattori [18] | 1 | 1 | 0 | 7 m | Microgastria | PANF (1) | 0 | N | No (1) | |
| Author | N° Patients | Early Complications (Main Diagnosis; Time after TEGD) | Early Complications’ Treatment | Late Complications (Main Diagnosis; Time after TEGD) | Late Complications’ Treatment |
|---|---|---|---|---|---|
| De Lagausie [14] | 13 | 0 | 0 | 1 Esophagocolic anastomotic stenosis (N/A; N/A) | Pneumatic dilatations |
| 1 Esophagojejunal anastomotic stenosis (N/A; m 3) | Pneumatic dilatations | ||||
| Lall [12] | 11 | 0 | 0 | 1 Peritonitis after small bowel herniation in the thorax (CHD with short esophagus; y 2) | Patient’s death |
| Boubnova [15] | 1 | 0 | 0 | 1 Small bowel paraesophageal herniation (EA; m 6) | Laparoscopic correction |
| Madre [3] | 17 | 1 Peritonitis (Microgastry, day 4) | Laparotomy | Bowel obstruction (EA, m 2) | Laparotomy |
| 2 Esophagojejunal anastomotic stenosis (2 EA, m 6) | 1 Pneumatic dilatation | ||||
| 1 Redo anastomosis | |||||
| 1 Evisceration (EA, day 10) | Laparotomy | 2 Esophagocolic anastomotic stenosis (1 EA y 1,4.5; 1 CBI y 1, 2.5) | Pneumatic dilatations with Mitomycin C application | ||
| 1 Eventration and herniation of jejunal loop (EA; y 3; y 3.5) | Laparotomy | ||||
| Kunisaki [16] | 1 | 0 | 0 | 1 Adhesive small bowel obstruction (Severe microgastria; N/A) | Enterolisis |
| Gottrand [17] | 6 | 0 | 0 | 1 Esophagojejunal stenosis (EA type I; y 1) | Endoscopic dilatation with Mitomycin C application |
| 1 Barret esophagus (EA type III; y 10) | 0 | ||||
| 2 Combined esophagojejunal stenosis and gastric metaplasia (2 EA type III; m 12; y 9) | Endoscopic dilatation, PPI and follow-up | ||||
| Hattori [18] | 1 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negri, E.; Coletta, R.; Bici, K.; Bianchi, A.; Morabito, A. Total Esophagogastric and Cologastric Dissociation in Neurologically Normal Children: Systematic Review. Children 2022, 9, 999. https://doi.org/10.3390/children9070999
Negri E, Coletta R, Bici K, Bianchi A, Morabito A. Total Esophagogastric and Cologastric Dissociation in Neurologically Normal Children: Systematic Review. Children. 2022; 9(7):999. https://doi.org/10.3390/children9070999
Chicago/Turabian StyleNegri, Elisa, Riccardo Coletta, Kejd Bici, Adrian Bianchi, and Antonino Morabito. 2022. "Total Esophagogastric and Cologastric Dissociation in Neurologically Normal Children: Systematic Review" Children 9, no. 7: 999. https://doi.org/10.3390/children9070999
APA StyleNegri, E., Coletta, R., Bici, K., Bianchi, A., & Morabito, A. (2022). Total Esophagogastric and Cologastric Dissociation in Neurologically Normal Children: Systematic Review. Children, 9(7), 999. https://doi.org/10.3390/children9070999







