Biopsychosocial Contributors to Parent Behaviors during Child Venipuncture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Demographics
2.3.2. Child–Adult Medical Procedure Interaction Scale (CAMPIS) and CAMPIS-R
Transcription
CAMPIS Training
CAMPIS Coding
2.3.3. Pain Catastrophizing Scale for Parents State (PCS-P-State)
2.3.4. Short Form of the State-Trait Anxiety Inventory-State (SF-STAI-State)
2.3.5. Electrocardiogram (ECG)
2.4. Data Preparation
2.5. Analytic Plan
2.5.1. RCT Group Variable
2.5.2. Assumptions and Covariates
2.5.3. Main Analyses
3. Results
3.1. Participants
3.2. Descriptive Statistics
3.3. Moderation Analyses
4. Discussion
4.1. Strengths and Limitations
4.2. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vervoort, T.; Trost, Z. Examining Affective-Motivational Dynamics and Behavioral Implications Within The Interpersonal Context of Pain. J. Pain 2017, 18, 1174–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, K.D. Toward the Social Communication Model of Pain. In Social and Interpersonal Dynamics in Pain: We Don’t Suffer Alone; Vervoort, T., Karos, K., Trost, Z., Prkachin, K.M., Eds.; Springer: Cham, Switzerland, 2018; pp. 23–41. ISBN 9783319783406. [Google Scholar]
- Blount, R.L.; Sturges, J.W.; Powers, S.W. Analysis of child and adult behavioral variations by phase of medical procedure. Behav. Ther. 1990, 21, 33–48. [Google Scholar] [CrossRef]
- Mahoney, L.; Ayers, S.; Seddon, P. The association between parent’s and healthcare professional’s behavior and children’s coping and distress during venepuncture. J. Pediatr. Psychol. 2010, 35, 985–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobol-Kwapińska, M.; Sobol, M.; Woźnica-Niesobska, E. Parental behavior and child distress and pain during pediatric medical procedures: Systematic review and meta-analysis. Health Psychol. 2020, 39, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Blount, R.L.; Cohen, L.L.; Frank, N.C.; Bachanas, P.J.; Smith, A.J.; Manimala, M.R.; Pate, J.T. The child-adult medical procedure interaction scale-revised: An assessment of validity. J. Pediatr. Psychol. 1997, 22, 73–88. [Google Scholar] [CrossRef]
- Moline, R.L.; McMurtry, C.M.; Noel, M.; McGrath, P.J.; Chambers, C.T. Parent-child interactions during pediatric venipuncture: Investigating the role of parent traits, beliefs, and behaviors in relation to child outcomes. Can. J. Pain 2021, 5, 151–165. [Google Scholar] [CrossRef]
- Caes, L.; Goubert, L.; Devos, P.; Verlooy, J.; Benoit, Y.; Vervoort, T. Personal distress and sympathy differentially influence health care professional and parents’ estimation of child procedure-related pain. Pain Med. 2016, 18, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Vervoort, T.; Goubert, L.; Eccleston, C.; Verhoeven, K.; De Clercq, A.; Buysse, A.; Crombez, G. The effects of parental presence upon the facial expression of pain: The moderating role of child pain catastrophizing. Pain 2008, 138, 277–285. [Google Scholar] [CrossRef]
- Goubert, L.; Eccleston, C.; Vervoort, T.; Jordan, A.; Crombez, G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): A preliminary validation. Pain 2006, 123, 254–263. [Google Scholar] [CrossRef]
- Birnie, K.A.; Chambers, C.T.; Chorney, J.; Fernandez, C.V.; McGrath, P.J. Dyadic analysis of child and parent trait and state pain catastrophizing in the process of children’s pain communication. Pain 2016, 157, 938–948. [Google Scholar] [CrossRef]
- Caes, L.; Vervoort, T.; Devos, P.; Verlooy, J.; Benoit, Y.; Goubert, L. Parental distress and catastrophic thoughts about child pain: Implications for parental protective behavior in the context of child leukemia-related medical procedures. Clin. J. Pain 2014, 30, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.C.; Blount, R.L.; Smith, A.J.; Manimala, M.R.; Martin, J.K. Parent and staff behavior, previous child medical experience, and maternal anxiety as they relate to child procedural distress and coping. J. Pediatr. Psychol. 1995, 20, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Balzarotti, S.; Biassoni, F.; Colombo, B.; Ciceri, M.R. Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biol. Psychol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Mertgen, A. Vagal Tank theory: The three Rs of cardiac vagal control functioning—Resting, reactivity, and recovery. Front. Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantin, K.L.; Moline, R.L.; Labonte, L.; McMurtry, C.M. A multi-method approach to understand parent behaviors during child acute pain. J. Psychophysiol. 2021, 36, 28–41. [Google Scholar] [CrossRef]
- Vervoort, T.; Trost, Z.; Sütterlin, S.; Caes, L.; Moors, A. Emotion regulatory function of parent attention to child pain and associated implications for parental pain control behaviour. Pain 2014, 155, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Vervoort, T.; Karos, K.; Johnson, D.; Sütterlin, S.; Van Ryckeghem, D. Parental emotion and pain control behaviour when faced with child’s pain: The emotion regulatory role of parental pain-related attention-set shifting and heart rate variability. Pain 2019, 160, 322–333. [Google Scholar] [CrossRef] [Green Version]
- Constantin, K.L.; Moline, R.L.; Riddell, R.P.; Spence, J.R.; Fiacconi, C.M.; Lupo-Flewelling, K.; McMurtry, C.M. Parent and child self- and co-regulation during pediatric venipuncture: Exploring heart rate variability and the effects of a mindfulness intervention. Dev. Psychobiol. 2022, 64, e22277. [Google Scholar] [CrossRef]
- DiLorenzo, M.G.; Waxman, J.A.; Flora, D.B.; Schmidt, L.A.; Garfield, H.; Flanders, D.; Weinberg, E.; Savlov, D.; Pillai Riddell, R. Distinct trajectories of caregiver-toddler physiological attunement during routine vaccinations: Evidence from parallel-process growth mixture modeling. 2022; under review. [Google Scholar]
- Moline, R.L.; Constantin, K.; Chambers, C.T.; Powell, D.; Lewis, S.P.; Laurignano, L.; McMurtry, C.M. A brief mindfulness intervention for parents and children before pediatric venipuncture: A randomized controlled trial. 2022; under review. [Google Scholar]
- Constantin, K.L.; Lupo-Flewelling, K.; Moline, R.L.; McMurtry, C.M. Child emotion regulation capacity moderates the association between parent behaviors and child distress during pediatric venipuncture. J. Pediatr. Psychol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Moline, R.L.; Chambers, C.; McMurtry, C.M. Study protocol for a randomized controlled trial of a child and parent mindfulness intervention for pediatric venipuncture. Paediatr. Neonatal Pain 2020, 3, 20–28. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 20, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blount, R.L.; Corbin, S.M.; Sturges, J.W.; Wolfe, V.V.; Prater, J.M.; Denise James, L. The relationship between adults’ behavior and child coping and distress during BMA/LP procedures: A sequential analysis. Behav. Ther. 1989, 20, 585–601. [Google Scholar] [CrossRef]
- Bai, J.; Swanson, K.M.; Santacroce, S.J. Observational coding systems of parent-child interactions during painful procedures: A systematic review. Pain Pract. 2018, 18, 130–145. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, C.M.; Chambers, C.T.; McGrath, P.J.; Asp, E. When “don’t worry” communicates fear: Children’s perceptions of parental reassurance and distraction during a painful medical procedure. Pain 2010, 150, 52–58. [Google Scholar] [CrossRef]
- Durand, H.; Birnie, K.A.; Noel, M.; Vervoort, T.; Goubert, L.; Boerner, K.E.; Chambers, C.T.; Caes, L. State versus trait: Validating state assessment of child and parental catastrophic thinking about children’s acute pain. J. Pain 2016, 18, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Marteau, T.M.; Bekker, H. The development of a six-item short-form of the state scale of the Spielberger State—Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 1992, 31, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Goedhart, A.D.; Van Der Sluis, S.; Houtveen, J.H.; Willemsen, G.; De Geus, E.J.C. Comparison of time and frequency domain measures of RSA in ambulatory recordings. Psychophysiology 2007, 44, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; SAGE Publications: Thousand Oaks, CA, USA, 2013; ISBN 1446249174. [Google Scholar]
- Quintana, D.S.; Heathers, J.A.J. Considerations in the assessment of heart rate variability in biobehavioral research. Front. Psychol. 2014, 5, 805. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guildford Press: New York, NY, USA, 2013. [Google Scholar]
- Dahlquist, L.M.; Power, T.G.; Cox, C.N.; Fernbach, D.J. Parenting and child distress during cancer procedures: A multidimensional assessment. Child. Heal. Care 1994, 23, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Blount, R.L. Commentary: Acute pediatric procedural pain, distress, and coping. J. Pediatr. Psychol. 2019, 44, 798–802. [Google Scholar] [CrossRef]
- Thorson, K.R.; West, T.V.; Mendes, W.B. Measuring physiological influence in dyads: A guide to designing, implementing, and analyzing dyadic physiological studies. Psychol. Methods 2018, 23, 595–616. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.L.; Van Roon, A.; Riese, H.; Thio, C.; Oostenbroek, E.; Westrik, I.; De Geus, E.J.C.; Gansevoort, R.; Lefrandt, J.; Nolte, I.M.; et al. Validity of (Ultra-)Short Recordings for Heart Rate Variability Measurements. PLoS ONE 2015, 10, e0138921. [Google Scholar] [CrossRef] [Green Version]
- Constantin, K.; Moline, R.L.; McMurtry, C.M. The Role of Nonverbal Features of Caregiving Behaviors. In Social and Interpersonal Dynamics in Pain: We Don’t Suffer Alone; Vervoort, T., Karos, K., Trost, Z., Prkachin, K.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 295–323. ISBN 978-3-319-78340-6. [Google Scholar]
- McMurtry, C.M.; Noel, M.; Chambers, C.T.; McGrath, P.J. Children’s fear during procedural pain: Preliminary investigation of the Children’s Fear Scale. Heal. Psychol. 2011, 30, 780–788. [Google Scholar] [CrossRef]
- Failo, A.; Giannotti, M.; Venuti, P. Associations between attachment and pain: From infant to adolescent. SAGE Open Med. 2019, 7, 205031211987777. [Google Scholar] [CrossRef]
- Corscadden, L.; Levesque, J.F.; Lewis, V.; Strumpf, E.; Breton, M.; Russell, G. Factors associated with multiple barriers to access to primary care: An international analysis. Int. J. Equity Health 2018, 17, 28. [Google Scholar] [CrossRef]
- Lasser, K.E.; Himmelstein, D.U.; Woolhandler, S. Access to care, health status, and health disparities in the United States and Canada: Results of a Cross-National Population Based Survey. Am. J. Public Health 2006, 96, 1300–1307. [Google Scholar] [CrossRef]
- Moore, S.V.; Lecarie, E.K.; Davis, M.C.; Lemery-Chalfant, K. The effectiveness of parental distraction during children’s acute pain: The moderating effect of socioeconomic status. Eur. J. Pain 2020, 24, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef]
- Groß, D.; Kohlmann, C.W. Increasing Heart Rate Variability through Progressive Muscle Relaxation and Breathing: A 77-Day Pilot Study with Daily Ambulatory Assessment. Int. J. Environ. Res. Public Health 2021, 18, 11357. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, T.L.; Miles, E.; Sheeran, P. Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol. Bull. 2012, 138, 775–808. [Google Scholar] [CrossRef] [PubMed]
| Variable | M (SD) | Range | n | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Catastrophizing | 14.08 (11.73) | 0–44 | 60 | ||||||
| 2. Anxiety | 34.32 (11.76) | 20–60 | 61 | 0.53 ** [0.32, 0.69] | |||||
| 3. Parent baseline HRV a | 7.00 (1.05) | 4.81–8.98 | 51 | −0.15 [−0.41, 0.13] | −0.24 [−0.48, 0.03] | ||||
| 4. Child coping behavior | 3.09 (1.91) | 0–8.39 | 54 | −0.14 [−0.40, 0.14] | −0.23 [−0.46, 0.05] | −0.17 [−0.45, 0.14] | |||
| 5. Child distress behavior b | 1.46 (1.74) | 0–10.00 | 54 | 0.31 * [0.04, 0.53] | 0.08 [−0.19, 0.34] | −0.11 [−0.40, 0.19] | 0.24 [−0.02, 0.47] | ||
| 6. Coping-promoting behavior b | 3.33 (2.01) | 0.57–9.45 | 54 | 0.05 [−0.22, 0.32] | −0.16 [−0.41, 0.12] | −0.38 * [−0.61, −0.09] | 0.62 ** [0.40, 0.78] | −0.06 [−0.35, 0.23] | |
| 7. Distress-promoting behavior b | 0.87 (0.92) | 0–3.83 | 54 | 0.35 * [0.08, 0.57] | 0.22 [−0.05, 0.46] | −0.38 * [−0.61, −0.09] | 0.16 [−0.14, 0.43] | 0.52 ** [0.27, 0.71] | 0.18 [−0.11, 0.50] |
| Predictor Variable | b (SE) | t | p | 95% Confidence Interval for b | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Model 1 Predictor = Parent Catastrophizing, Criterion = Parent Coping-Promoting Behavior | |||||
| Parent catastrophizing | 0.00 (0.02) | 0.16 | 0.88 | −0.04 | 0.04 |
| Parent baseline HRV | −0.37 (0.23) | −1.59 | 0.12 | −0.84 | 0.10 |
| Parent catastrophizing X parent baseline HRV | −0.02 (0.02) | −0.87 | 0.38 | −0.06 | 0.02 |
| Covariates | |||||
| Child coping behaviors | 0.64 (0.12) | 5.54 | <0.001 | 0.41 | 0.88 |
| Parent medication | −0.51 (0.48) | −1.06 | 0.29 | −1.48 | 0.46 |
| Model 2 Predictor = Parent Catastrophizing, Criterion = Parent Distress-Promoting Behavior | |||||
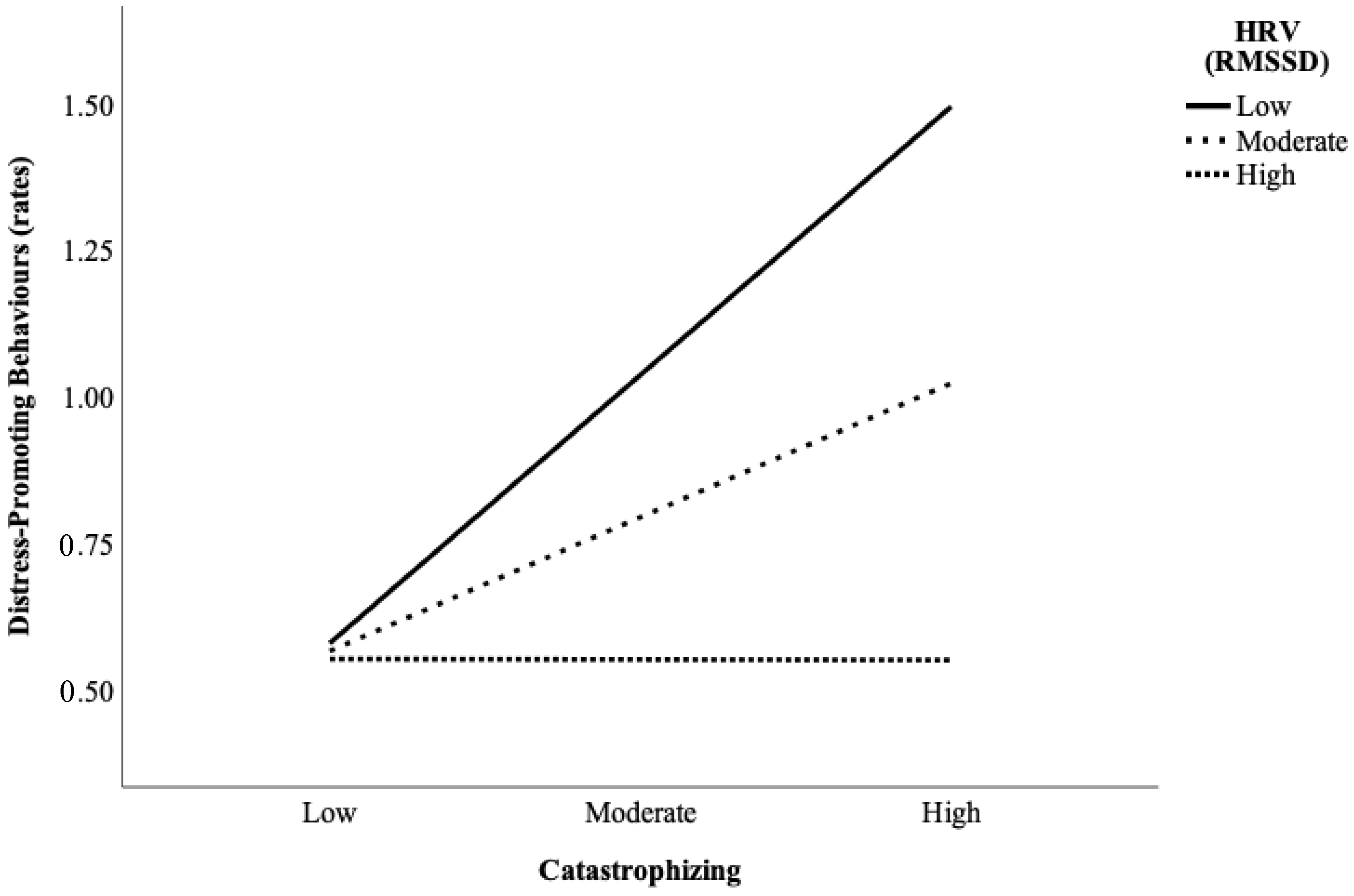
| Parent catastrophizing | 0.02 (0.01) | 1.92 | 0.06 | −0.00 | 0.03 |
| Parent baseline HRV | −0.25 (0.10) | −2.40 | 0.02 | −0.46 | −0.04 |
| Parent catastrophizing X parent baseline HRV | −0.02 (0.01) | −2.03 | 0.05 a | −0.04 | −0.00 |
| Covariates | |||||
| Child distress behaviors | 0.25 (0.06) | 4.23 | <0.001 | 0.13 | 0.37 |
| Parent medication | −0.33 (0.21) | −1.52 | 0.14 | −0.76 | 0.11 |
| Model 3 Predictor = Parent Anxiety, Criterion = Parent Coping-Promoting Behavior | |||||
| Parent anxiety | −0.00 (0.02) | −0.05 | 0.96 | −0.05 | 0.04 |
| Parent baseline HRV | −0.37 (0.24) | −1.53 | 0.13 | −0.86 | 0.12 |
| Parent anxiety X parent baseline HRV | −0.01 (0.02) | −0.65 | 0.52 | −0.05 | 0.03 |
| Covariates | |||||
| Child coping behaviors | 0.65 (0.12) | 5.45 | <0.001 | 0.41 | 0.88 |
| Parent medication | −0.53 (0.48) | −1.11 | 0.27 | −1.50 | 0.44 |
| Model 4 Predictor = Parent Anxiety, Criterion = Parent Distress-Promoting Behavior | |||||
| Parent anxiety | 0.02 (0.01) | 1.52 | 0.14 | −0.01 | 0.04 |
| Parent baseline HRV | −0.22 (0.12) | −1.94 | 0.06 | −0.46 | 0.01 |
| Parent anxiety X parent baseline HRV | −0.01 (0.01) | −0.71 | 0.48 | −0.03 | 0.01 |
| Covariates | |||||
| Child distress behaviors | 0.16 (0.04) | 3.96 | <0.001 | 0.08 | 0.24 |
| Parent medication | −0.40 (0.23) | −1.77 | 0.09 | −0.87 | 0.06 |
| Predictor Variable | b (SE) | t | p | 95% Confidence Interval for b | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Model 5 Criterion = Parent Coping-Promoting Behavior | |||||
| Child distress | −0.11 (0.22) | −0.68 | 0.50 | −0.44 | 0.22 |
| Parent baseline HRV | −0.46 (0.31) | −2.06 | 0.05 | −0.90 | −0.01 |
| Child distress X HRV | 0.12 (0.14) | 1.24 | 0.22 | −0.08 | 0.32 |
| Covariates | |||||
| Child coping behavior | 0.69 (0.11) | 6.32 | <0.001 | 0.47 | 0.91 |
| Parent medication | −0.35 (0.43) | −0.77 | 0.44 | −1.26 | 0.56 |
| Model 6 Criterion = Parent Distress-Promoting Behavior | |||||
| Child distress | 0.28 (0.08) | 3.46 | <0.01 | 0.12 | 0.44 |
| Parent baseline HRV | −0.29 (0.11) | −2.58 | 0.01 | −0.51 | −0.06 |
| Child distress × HRV | −0.01 (0.05) | −0.16 | 0.88 | −0.11 | 0.10 |
| Covariates | |||||
| Parent medication | −0.38 (0.23) | −1.65 | 0.11 | −0.84 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, K.L.; Moline, R.L.; Pillai Riddell, R.; Spence, J.R.; McMurtry, C.M. Biopsychosocial Contributors to Parent Behaviors during Child Venipuncture. Children 2022, 9, 1000. https://doi.org/10.3390/children9071000
Constantin KL, Moline RL, Pillai Riddell R, Spence JR, McMurtry CM. Biopsychosocial Contributors to Parent Behaviors during Child Venipuncture. Children. 2022; 9(7):1000. https://doi.org/10.3390/children9071000
Chicago/Turabian StyleConstantin, Kaytlin L., Rachel L. Moline, Rebecca Pillai Riddell, Jeffrey R. Spence, and C. Meghan McMurtry. 2022. "Biopsychosocial Contributors to Parent Behaviors during Child Venipuncture" Children 9, no. 7: 1000. https://doi.org/10.3390/children9071000
APA StyleConstantin, K. L., Moline, R. L., Pillai Riddell, R., Spence, J. R., & McMurtry, C. M. (2022). Biopsychosocial Contributors to Parent Behaviors during Child Venipuncture. Children, 9(7), 1000. https://doi.org/10.3390/children9071000








