Lower Respiratory Tract Infections in Pediatric Patients with Severe Neurological Impairments: Clinical Observations and Perspectives in a Palliative Care Unit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Patients and Definition of Bacterial LRTIs
2.3. Data and Material Collection
2.4. Antibiotic Therapy
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Isolated Pathogens from Respiratory Specimens
3.3. Antimicrobial Therapy
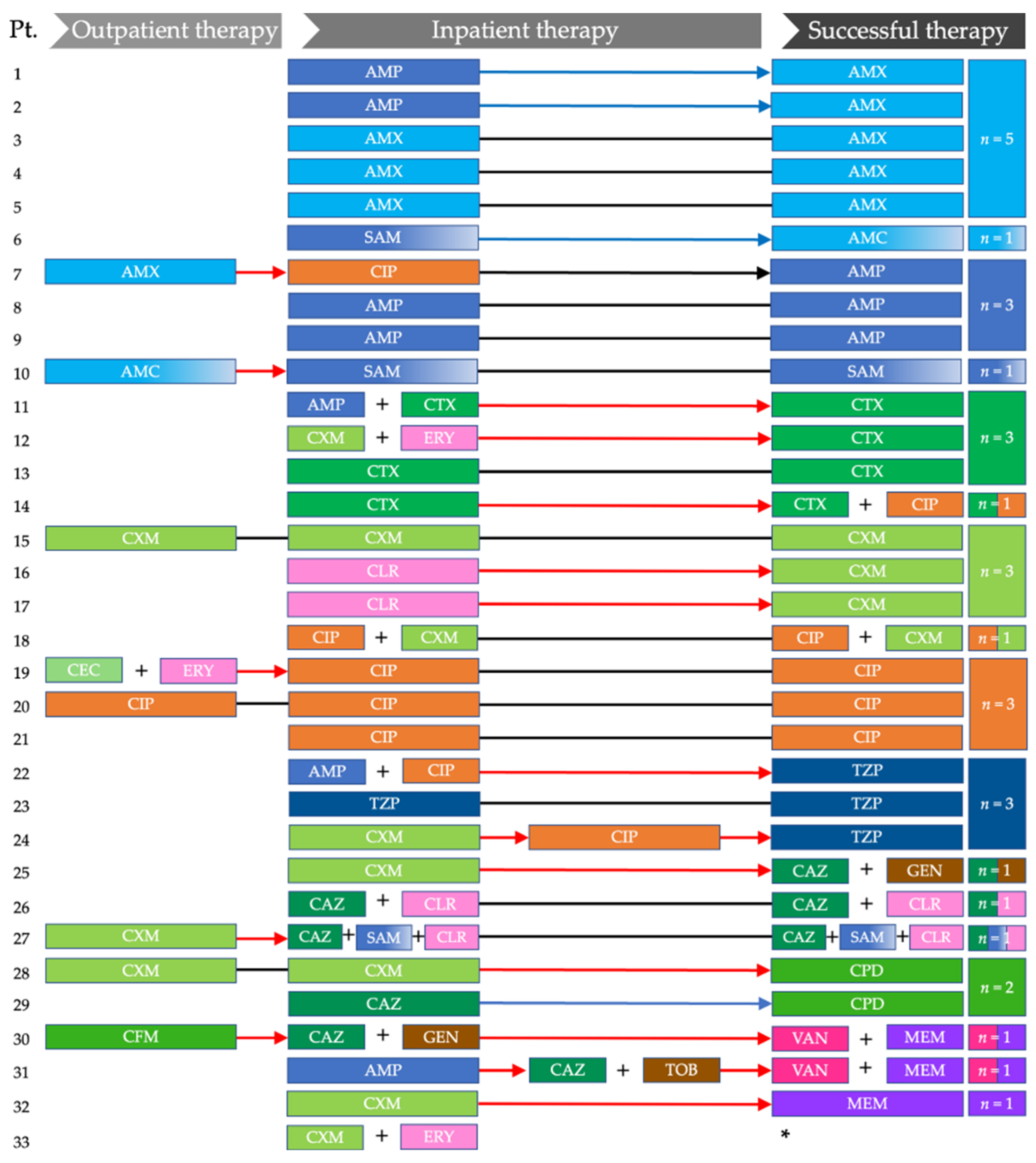
3.3.1. Outpatient Antibiotic Therapy
3.3.2. Initial and Subsequent Inpatient Antibiotic Therapy
3.3.3. Successful Inpatient Antibiotic Therapy
3.3.4. Comparison of In Vitro Sensitivity and Successful Therapies
4. Discussion
4.1. Oral versus Parenteral Antibiotic Therapy
4.2. Relevance of Respiratory Specimens
4.3. Antibiotic Stewardship and Impact of MDRO on Antibiotic Therapy
4.4. Recurrent Pneumonia and Preventive Interventions
4.5. Disease Burden of Pneumonia in SNI Patients and a Pediatric Palliative Care Perspective
4.6. Development of Future Guidelines and Lessons Learned from Our Findings
4.7. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, E.; Kuo, D.Z.; Agrawal, R.; Berry, J.G.; Bhagat, S.K.M.; Simon, T.D.; Srivastava, R. Children with Medical Complexity: An Emerging Population for Clinical and Research Initiatives. Pediatrics 2011, 127, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.A.; Schenker, Y.; Maurer, S.H.; Cook, S.C.; Kavlieratos, D.; Houtrow, A. Pediatric Palliative Care in the Medical Neighborhood for Children with Medical Complexity. Fam. Syst. Health 2019, 37, 107–119. [Google Scholar] [CrossRef]
- Bogetz, J.F.; Lemmon, M.E. Pediatric Palliative Care for Children with Severe Neurological Impairment and Their Families. J. Pain Symptom Manag. 2021, 62, 662–667. [Google Scholar] [CrossRef]
- Garske, D.; Schmidt, P.; Hasan, C.; Wager, J.; Zernikow, B. Inpatient Paediatric Palliative Care—A Retrospective Study. Z Palliativmed 2016, 17, 302–307. [Google Scholar] [CrossRef]
- Hain, R.; Devins, M.; Hastings, R.; Noyes, J. Paediatric Palliative Care: Development and Pilot Study of a ‘Directory’ of Life-Limiting Conditions. BMC Palliat. Care 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Feudtner, C.; Kang, T.I.; Hexem, K.R.; Friedrichsdorf, S.J.; Osenga, K.; Siden, H.; Friebert, S.E.; Hays, R.M.; Dussel, V.; Wolfe, J. Pediatric Palliative Care Patients: A Prospective Multicenter Cohort Study. Pediatrics 2011, 127, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Hoell, J.I.; Weber, H.; Warfsmann, J.; Trocan, L.; Gagnon, G.; Danneberg, M.; Balzer, S.; Keller, T.; Janßen, G.; Kuhlen, M. Facing the Large Variety of Life-Limiting Conditions in Children. Eur. J. Pediatr. 2019, 178, 1893–1902. [Google Scholar] [CrossRef]
- Allen, J.; Brenner, M.; Hauer, J.; Molloy, E.; McDonald, D. Severe Neurological Impairment: A Delphi Consensus-Based Definition. Eur. J. Paediatr. Neuro. 2020, 29, 81–86. [Google Scholar] [CrossRef]
- Millman, A.J.; Finelli, L.; Bramley, A.M.; Peacock, G.; Williams, D.J.; Arnold, S.R.; Grijalva, C.G.; Anderson, E.J.; McCullers, J.A.; Ampofo, K.; et al. Community-Acquired Pneumonia Hospitalization among Children with Neurologic Disorders. J. Pediatr. 2016, 173, 188–195.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.L.; Haren, K.V.; Rigdon, J.; Saynina, O.; Song, H.; Buu, M.C.; Thakur, Y.; Srinivas, N.; Asch, S.M.; Sanders, L.M. Pneumonia Prevention Strategies for Children with Neurologic Impairment. Pediatrics 2019, 144, e20190543. [Google Scholar] [CrossRef]
- Armann, J.; Doenhardt, M.; Hufnagel, M.; Diffloth, N.; Reichert, F.; Haas, W.; Schilling, J.; Haller, S.; Hübner, J.; Simon, A.; et al. Risk Factors for Hospitalization, Disease Severity and Mortality in Children and Adolescents with COVID-19: Results from a Nationwide German Registry. medRxiv 2021, arXiv:2021.06.07.21258488. Available online: https://www.medrxiv.org/content/10.1101/2021.06.07.21258488v1.article-info (accessed on 18 April 2022).
- Kondrich, J.; Rosenthal, M. Influenza in Children. Curr. Opin. Pediatr. 2017, 29, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Prusseit, J.; Müller, A. Respiratory Syncytial Virus Infection in Children with Neuromuscular Impairment. Open Microbiol. J. 2011, 5, 155–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, J.; Hall, M.; Berry, J.G.; Stone, B.; Ambroggio, L.; Srivastava, R.; Shah, S.S. Diagnostic Testing and Hospital Outcomes of Children with Neurologic Impairment and Bacterial Pneumonia. J. Pediatr. 2016, 178, 156–163.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, C.M. Current Concepts of Respiratory Complications of Neuromuscular Disease in Children. Curr. Opin. Pediatr. 2000, 12, 203–207. [Google Scholar] [CrossRef]
- Panitch, H.B. Viral Respiratory Infections in Children with Technology Dependence and Neuromuscular Disorders. Pediatr. Infect. Dis. J. 2004, 23, S222–S227. [Google Scholar] [CrossRef]
- Koumbourlis, A.C. Scoliosis and the Respiratory System. Paediatr. Respir. Rev. 2006, 7, 152–160. [Google Scholar] [CrossRef]
- Boel, L.; Pernet, K.; Toussaint, M.; Ides, K.; Leemans, G.; Haan, J.; Hoorenbeeck, K.V.; Verhulst, S. Respiratory Morbidity in Children with Cerebral Palsy: An Overview. Dev. Med. Child Neurol. 2019, 61, 646–653. [Google Scholar] [CrossRef]
- Healy, F.; Panitch, H.B. Pulmonary Complications of Pediatric Neurological Diseases. Pediatr. Ann. 2010, 39, 216–224. [Google Scholar] [CrossRef]
- Blackmore, A.M.; Bear, N.; Blair, E.; Gibson, N.; Jalla, C.; Langdon, K.; Moshovis, L.; Steer, K.; Wilson, A.C. Factors Associated with Respiratory Illness in Children and Young Adults with Cerebral Palsy. J. Pediatr. 2016, 168, 151–157.e1. [Google Scholar] [CrossRef]
- Cherchi, C.; Testa, M.B.C.; Deriu, D.; Schiavino, A.; Petreschi, F.; Ullmann, N.; Paglietti, M.G.; Cutrera, R. All You Need Is Evidence: What We Know About Pneumonia in Children with Neuromuscular Diseases. Front. Pediatr. 2021, 9, 625751. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.D.; Richardson, S.E.; Cohen, E.; Mahant, S.; Avitzur, Y.; Carsley, S.; Rapoport, A. Gastric Flora in Gastrostomy Fed Children with Neurological Impairment on Antacid Medication. Children 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Seddon, P.C.; Khan, Y. Respiratory Problems in Children with Neurological Impairment. Arch. Dis. Child 2003, 88, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.-Y.; Chiu, N.-C.; Lee, K.-S.; Chi, H.; Huang, F.-Y.; Huang, D.T.-N.; Chang, L.; Kung, Y.-H.; Huang, C.-Y. Respiratory Tract Infections in Children with Tracheostomy. J. Microbiol. Immunol. Infect. 2020, 53, 315–320. [Google Scholar] [CrossRef]
- Berry, J.G.; Poduri, A.; Bonkowsky, J.L.; Zhou, J.; Graham, D.A.; Welch, C.; Putney, H.; Srivastava, R. Trends in Resource Utilization by Children with Neurological Impairment in the United States Inpatient Health Care System: A Repeat Cross-Sectional Study. PLoS Med. 2012, 9, e1001158. [Google Scholar] [CrossRef] [Green Version]
- Arslan, S.S.; Demir, N.; Karaduman, A.A. Both Pharyngeal and Esophageal Phases of Swallowing Are Associated with Recurrent Pneumonia in Pediatric Patients. Clin Respir J 2018, 12, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Enninger, A.; Schmidt, P.; Hasan, C.; Wager, J.; Zernikow, B. Multidrug-Resistant Organisms in Palliative Care: A Systematic Review. J Palliat Med 2021, 24, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.M.; Alberman, E. Certified Cause of Death in Children and Young Adults with Cerebral Palsy. Arch. Dis. Child 1991, 66, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plioplys, A.V.; Kasnicka, I.; Lewis, S.; Moller, D. Survival Rates Among Children with Severe Neurologic Disabilities. South. Med. J. 1998, 91, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Çakar, M.K.; Cinel, G. The Respiratory Problems of Patients with Cerebral Palsy Requiring Hospitalization: Reasons and Solutions. Pediatr. Pulm. 2021, 56, 1626–1634. [Google Scholar] [CrossRef]
- Hauer, J.M. Treating Dyspnea with Morphine Sulfate in Nonverbal Children with Neurological Impairment. Pediatr. Pulm. 2015, 50, E9–E12. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P.; Alvarez, C.; Fabres, J.; Simonetti, M.; Sánchez, I. Home Oxygen Therapy in Children with Chronic Respiratory Failure. Rev. Médica De Chile 1998, 126, 284–292. [Google Scholar]
- Agrawal, R.; Hall, M.; Cohen, E.; Goodman, D.M.; Kuo, D.Z.; Neff, J.M.; O’Neill, M.; Thomson, J.; Berry, J.G. Trends in Health Care Spending for Children in Medicaid with High Resource Use. Pediatrics 2016, 138, e20160682. [Google Scholar] [CrossRef] [Green Version]
- Blackmore, A.M.; Bear, N.; Blair, E.; Langdon, K.; Moshovis, L.; Steer, K.; Wilson, A.C. Predicting Respiratory Hospital Admissions in Young People with Cerebral Palsy. Arch. Dis. Child 2018, 103, 1119. [Google Scholar] [CrossRef] [Green Version]
- Meehan, E.M.; Reid, S.M.; Williams, K.J.; Freed, G.L.; Sewell, J.R.; Reddihough, D.S. Medical Service Use in Children with Cerebral Palsy: The Role of Child and Family Characteristics. J. Paediatr. Child Health 2016, 52, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.J.; Mamey, M.R.; Koh, J.Y.; Schrager, S.M.; Neely, M.N.; Wu, S. Length of Stay and Hospital Revisit After Bacterial Tracheostomy–Associated Respiratory Tract Infection Hospitalizations. Hosp. Pediatr. 2018, 8, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, M.; Barker, M.; Liese, J.; Adams, O.; Ankermann, T.; Baumann, U.; Brinkmann, F.; Bruns, R.; Dahlheim, M.; Ewig, S.; et al. Guidelines for the Management of Community Acquired Pneumonia in Children and Adolescents (Pediatric Community Acquired Pneumonia, PCAP). Pneumologie 2020, 74, 515–544. [Google Scholar] [CrossRef]
- Bradley, J.S.; Byington, C.L.; Shah, S.S.; Alverson, B.; Carter, E.R.; Harrison, C.; Kaplan, S.L.; Mace, S.E.; McCracken, G.H.; Moore, M.R.; et al. The Management of Community-Acquired Pneumonia in Infants and Children Older Than 3 Months of Age: Clinical Practice Guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 53, e25–e76. [Google Scholar] [CrossRef] [Green Version]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and Reliability of a System to Classify Gross Motor Function in Children with Cerebral Palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Schmidt, P.; Hasan, C.; Mauritz, M.D.; Simon, A.; Stening, K.; Hartenstein-Pinter, A.; Zernikow, B.; Wager, J. Multidrug-resistant Organisms in Paediatric Palliative Care Patients—Prevalence, Risk Factors and the Impact of a Liberal Hygiene Concept. J. Paediatr. Child Health 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Flanders, S.A.; Halm, E.A. Guidelines for Community-Acquired Pneumonia—Are They Reflected in Practice? Treat. Respir. Med. 2004, 3, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Krockow, E.M.; Tarrant, C.; Colman, A.M. Prosociality in the Social Dilemma of Antibiotic Prescribing. Curr. Opin. Psychol. 2021, 44, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Pezeshkpour, P.; Armstrong, N.C.; Mahant, S.; Muthusami, P.; Amaral, J.G.; Parra, D.A.; Temple, M.J.; Connolly, B.L. Evaluation of Implanted Venous Port-a-Caths in Children with Medical Complexity and Neurologic Impairment. Pediatr. Radiol. 2019, 49, 1354–1361. [Google Scholar] [CrossRef]
- Rojas-Reyes, M.; Rugeles, C.G. Oral Antibiotics versus Parenteral Antibiotics for Severe Pneumonia in Children. Cochrane Database Syst. Rev. 2006, CD004979. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, M.; Abu-Raideh, J.; Albalawi, H.; Shalabi, M.; Saleh, S. Effective Oral Combination Treatment for Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli. Microb. Drug Resist. 2019, 25, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Schutze, G.E.; Byington, C.; Maldonado, Y.A.; Barnett, E.D.; Campbell, J.D.; Davies, H.D.; Lynfield, R.; Munoz, F.M.; Committee on Infectious Diseases; et al. The Use of Systemic and Topical Fluoroquinolones. Pediatrics 2016, 138, e20162706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adefurin, A.; Sammons, H.; Jacqz-Aigrain, E.; Choonara, I. Ciprofloxacin Safety in Paediatrics: A Systematic Review. Arch. Dis. Child 2011, 96, 874. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.-H.; Hu, C.-F.; Liu, J.-W.; Chung, C.-H.; Chen, Y.-C.; Sun, C.-A.; Chien, W.-C. The Incidence of Collagen-Associated Adverse Events in Pediatric Population with the Use of Fluoroquinolones: A Nationwide Cohort Study in Taiwan. BMC Pediatr. 2020, 20, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Principi, N.; Esposito, S. Appropriate Use of Fluoroquinolones in Children. Int. J. Antimicrob. Agents 2015, 45, 341–346. [Google Scholar] [CrossRef]
- Murray, M.T.; Beauchemin, M.P.; Neu, N.; Larson, E.L. Prior Antibiotic Use and Acquisition of Multidrug-Resistant Organisms in Hospitalized Children: A Systematic Review. Infect. Control Hosp. Epidemiol. 2019, 40, 1107–1115. [Google Scholar] [CrossRef]
- Zar, H.J.; Andronikou, S.; Nicol, M.P. Advances in the Diagnosis of Pneumonia in Children. BMJ 2017, 358, j2739. [Google Scholar] [CrossRef] [PubMed]
- Rueda, Z.V.; Bermúdez, M.; Restrepo, A.; Garcés, C.; Morales, O.; Roya-Pabón, C.; Carmona, L.F.; Arango, C.; Albarracín, J.L.; López, L.; et al. Induced Sputum as an Adequate Clinical Specimen for the Etiological Diagnosis of Community-Acquired Pneumonia (CAP) in Children and Adolescents. Int. J. Infect. Dis. 2022, 116, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.B.; Cravens, L.B.; Creech, C.B. Advances in Pediatric Antimicrobial Agents Development. Curr. Opin. Pediatr. 2019, 31, 135–143. [Google Scholar] [CrossRef]
- Singh, P.; Holmen, J. Multidrug-Resistant Infections in the Developing World. Pediatric Clin. N. Am. 2022, 69, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Chiotos, K.; Hayes, M.; Gerber, J.S.; Tamma, P.D. Treatment of Carbapenem-Resistant Enterobacteriaceae Infections in Children. J. Pediatr. Infect. Dis. Soc. 2019, 9, 56–66. [Google Scholar] [CrossRef]
- Larson, E.L.; Murray, M.T.; Cohen, B.; Simpser, E.; Pavia, M.; Jackson, O.; Jia, H.; Hutcheon, R.G.; Mosiello, L.; Neu, N.; et al. Behavioral Interventions to Reduce Infections in Pediatric Long-Term Care Facilities: The Keep It Clean for Kids Trial. Behav. Med. 2017, 44, 141–150. [Google Scholar] [CrossRef]
- Saiman, L.; Maykowski, P.; Murray, M.; Cohen, B.; Neu, N.; Jia, H.; Hutcheon, G.; Simpser, E.; Mosiello, L.; Alba, L.; et al. Incidence, Risks, and Types of Infections in Pediatric Long-Term Care Facilities. JAMA Pediatr. 2017, 171, 872. [Google Scholar] [CrossRef]
- Murray, M.T.; Jackson, O.; Cohen, B.; Hutcheon, G.; Saiman, L.; Larson, E.; Neu, N. Impact of Infection Prevention and Control Initiatives on Acute Respiratory Infections in a Pediatric Long-Term Care Facility. Infect. Control Hosp. Epidemiol. 2016, 37, 859–862. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.L.; Jain, M.; Saiman, L.; Neu, N. Antimicrobial Stewardship in Pediatric Post-Acute Care Facilities. Am. J. Infect. Control 2018, 46, 468–470. [Google Scholar] [CrossRef]
- Lemmen, S.W.; Lewalter, K. Antibiotic Stewardship and Horizontal Infection Control Are More Effective than Screening, Isolation and Eradication. Infection 2018, 46, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Brook, I.; Shah, K. Sinusitis in Neurologically Impaired Children. Otolaryngol.—Head Neck Surg. 1998, 119, 357–360. [Google Scholar] [CrossRef]
- Papan, C.; Argentiero, A.; Porwoll, M.; Hakim, U.; Farinelli, E.; Testa, I.; Pasticci, M.B.; Mezzetti, D.; Perruccio, K.; Etshtein, L.; et al. A Host Signature Based on TRAIL, IP-10, and CRP for Reducing Antibiotic Overuse in Children by Differentiating Bacterial from Viral Infections: A Prospective, Multicentre Cohort Study. Clin. Microbiol. Infect. 2021, 28, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, S.C.; Monuteaux, M.C.; Bachur, R.G.; Finn, N.; Neuman, M.I. Negative Chest Radiography and Risk of Pneumonia. Pediatrics 2018, 142, e20180236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onakpoya, I.J.; Hayward, G.; Heneghan, C.J. Antibiotics for Preventing Lower Respiratory Tract Infections in High-risk Children Aged 12 Years and Under. Cochrane Database Syst. Rev. 2015, 9, CD011530. [Google Scholar] [CrossRef] [Green Version]
- Borgnakke, W.S. Does Treatment of Periodontal Disease Influence Systemic Disease? Dent. Clin. N. Am. 2015, 59, 885–917. [Google Scholar] [CrossRef]
- Waldron, C.; Nunn, J.; Phadraig, C.M.G.; Comiskey, C.; Guerin, S.; van Harten, M.T.; Donnelly-Swift, E.; Clarke, M.J. Oral Hygiene Interventions for People with Intellectual Disabilities. Cochrane Database Syst. Rev. 2019, 5, CD012628. [Google Scholar] [CrossRef]
- de Hoog, M.L.A.; Venekamp, R.P.; Damoiseaux, R.A.M.J.; Schilder, A.G.M.; Sanders, E.A.M.; Smit, H.A.; Bruijning-Verhagen, P.C.J.L. Impact of Repeated Influenza Immunization on Respiratory Illness in Children with Preexisting Medical Conditions. Ann. Fam. Med. 2019, 17, 7–13. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | n (%)/Median ± SD or Mean ± SD |
|---|---|
| Sex | |
| Female | 8 (40) |
| Male | 12 (60) |
| Age, years (median ± SD) | 9.6 ± 6.2 |
| Length of hospital stay, days (mean ± SD) | 14.5 ± 11.1 |
| ICD10 2019 | |
| Q04.9 Congenital malformation of brain, unspecified | 8 (40) |
| E70-E83 Metabolic disorders | 2 (10) |
| G93.1 Anoxic brain damage, not elsewhere classified | 3 (15) |
| P91 Other disturbances of cerebral status of newborn | 2 (10) |
| G40.0 Localization-related idiopathic epilepsy and epileptic syndromes with seizures of localized onset | 1 (5) |
| G71.2 Congenital myopathies | 1 (5) |
| Q87.1 Congenital malformation syndromes predominantly associated with short stature | 1 (5) |
| Q89 Other congenital malformations, not elsewhere classified | 1 (5) |
| Q91 Edwards syndrome and Patau syndrome | 1 (5) |
| Additional symptoms | |
| Epilepsy | 18 (90) |
| Cerebral Palsy | 12 (60) |
| Death | 2 (21.2) |
| MDRO colonization on admission | 3 (7.8) |
| Escherichia coli (rectal) | 1 (2.6) |
| Morganella morganii (rectal) | 1 (2.6) |
| MRSA (rectal and respiratory specimen) | 1 (2.6) |
| Respiratory Specimen and Isolated Bacteria | n (%) |
|---|---|
| Type of sample | |
| Sputum | 9 (22) |
| Tracheal secretion | 8 (19.5) |
| Oropharyngeal secretion | 3 (7.2) |
| Bronchial secretion | 2 (4.9) |
| Culture result | |
| No bacterial growth | 7 (31.8) |
| Isolated bacteria (n = 23) | |
| Pseudomonas aeruginosa | 3 (13) |
| Escherichia coli | 3 (13) |
| Klebsiella pneumoniae | 3 (13) |
| Haemophilus influenzae | 2 (8.7) |
| Proteus mirabilis | 2 (8.7) |
| Serratia marcescens | 2 (8.7) |
| Achromobacter xylosoxidans | 1 (4.6) |
| Acinetobacter baumannii | 1 (4.6) |
| Enterobacter aerogenes | 1 (4.6) |
| Enterobacter kobei | 1 (4.6) |
| Group C Streptococcus | 1 (4.6) |
| MRSA | 1 (4.6) |
| Staphylococcus aureus | 1 (4.6) |
| Streptococcus spp. | 1 (4.6) |
| Antimicrobial Therapy | n (%) |
|---|---|
| Outpatient antibiotic therapy n = 8 | |
| Cefuroxime | 2 (25) |
| Amoxicillin | 1 (12.5) |
| Amoxicillin-clavulanic acid | 1 (12.5) |
| Cefaclor | 1 (12.5) |
| Cefaclor and erythromycin | 1 (12.5) |
| Cefixime | 1 (12.5) |
| Ciprofloxacin | 1 (12.5) |
| Initial inpatient antibiotic therapy n = 33 | |
| Ampicillin | 5 (15.2) |
| Cefuroxime | 5 (15.2) |
| Ciprofloxacin | 4 (12.1) |
| Amoxicillin | 3 (9.1) |
| Clarithromycin | 2 (6.1) |
| Ampicillin-sulbactam | 2 (6.1) |
| Cefotaxim | 2 (6.1) |
| Ampicillin and cefotaxim | 1 (3) |
| Amoxicillin and ciprofloxacin | 1 (3) |
| Ceftazidim | 1 (3) |
| Ceftazidim and clarithromycin | 1 (3) |
| Ceftazidim and ampicillin-sulbactam and clarithromycin | 1 (3) |
| Ceftazidim and gentamicin | 1 (3) |
| Cefuroxime and clarithromycin | 1 (3) |
| Cefuroxime and erythromycin | 1 (3) |
| Cefuroxime and ciprofloxacin | 1 (3) |
| Piperacillin/tazobactam | 1 (3) |
| Successful antibiotic therapy n = 32 | |
| Amoxicillin | 5 (15.6) |
| Ampicillin | 3 (9.4) |
| Cefotaxim | 3 (9.4) |
| Cefuroxime | 3 (9.4) |
| Ciprofloxacin | 3 (9.4) |
| Piperacillin-tazobactam | 3 (9.4) |
| Cefpodoxime | 2 (6.3) |
| Meropenem and vancomycin | 2 (6.3) |
| Amoxicillin-clavulanic acid | 1 (3.1) |
| Ampicillin-sulbactam | 1 (3.1) |
| Cefotaxim and ciprofloxacin | 1 (3.1) |
| Ceftazidim and ampicillin-sulbactam and clarithromycin | 1 (3.1) |
| Ceftazidim and clarithromycin | 1 (3.1) |
| Ceftazidim and gentamicin | 1 (3.1) |
| Ciprofloxacin and cefuroxime | 1 (3.1) |
| Meropenem | 1 (3.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauritz, M.D.; Hasan, C.; Schmidt, P.; Simon, A.; Knuf, M.; Zernikow, B. Lower Respiratory Tract Infections in Pediatric Patients with Severe Neurological Impairments: Clinical Observations and Perspectives in a Palliative Care Unit. Children 2022, 9, 852. https://doi.org/10.3390/children9060852
Mauritz MD, Hasan C, Schmidt P, Simon A, Knuf M, Zernikow B. Lower Respiratory Tract Infections in Pediatric Patients with Severe Neurological Impairments: Clinical Observations and Perspectives in a Palliative Care Unit. Children. 2022; 9(6):852. https://doi.org/10.3390/children9060852
Chicago/Turabian StyleMauritz, Maximilian David, Carola Hasan, Pia Schmidt, Arne Simon, Markus Knuf, and Boris Zernikow. 2022. "Lower Respiratory Tract Infections in Pediatric Patients with Severe Neurological Impairments: Clinical Observations and Perspectives in a Palliative Care Unit" Children 9, no. 6: 852. https://doi.org/10.3390/children9060852
APA StyleMauritz, M. D., Hasan, C., Schmidt, P., Simon, A., Knuf, M., & Zernikow, B. (2022). Lower Respiratory Tract Infections in Pediatric Patients with Severe Neurological Impairments: Clinical Observations and Perspectives in a Palliative Care Unit. Children, 9(6), 852. https://doi.org/10.3390/children9060852








