A Dynamic Approach for Early Risk Prediction of Gram-Negative Bloodstream Infection and Systemic Inflammatory Response Syndrome in Febrile Pediatric Hemato-Oncology Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methodology
2.3. Definitions:
- Fever was defined as a single axillary temperature ≥ 38.3°C for more than one hour or two episodes of fever ≥ 38°C within a 12 h period.
- Severe neutropenia was defined as an absolute neutrophil count ≤ 500/mm3.
- Gr-BSI was defined as one or more blood cultures positive for a Gram-negative bacterial pathogen either in central or peripheral blood samples.
- SIRS was defined according to the criteria defined by the International Pediatric Sepsis Consensus Conference (IPSCC) in 2005 [22]. Leukopenia was not considered a criteria for classifying an episode as SIRS if any treatment that could produce it, such as chemotherapy, had been previously received.
- Henceforth in this article, Gr-BSI and SIRS episodes will be referred to as high-risk episodes (HRE).
- Samples and their resultant variables obtained in the first evaluation and those of the later 12–24 h evaluation will be labeled with the number 1 and 2, respectively (for example CRP-1 or PCT-2). Resultant variables obtained from the calculation of the percentage of variation between moment 1 and 2 following the formula (Value-2−Value-1)/Value 1 × 100, will be labeled as 2vs1 (example CRP-2vs1).
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Biomarkers
3.3. Estimation of Optimal Cut-Off Point
3.4. Logistic Regression Models
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Richardson, L.C.; Henley, S.J.; Wilson, R.J.; Dowling, N.F.; Weir, H.K.; Tai, E.W.; Buchanan Lunsford, N. Pediatric cancer mortality and survival in the United States, 2001–2016. Cancer 2020, 126, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.T.; Armstrong, D.; Bodey, G.P.; Bow, E.J.; Brown, A.E.; Calandra, T.; Feld, R.; Pizzo, P.A.; Rolston, K.V.; Shenep, J.L.; et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin. Infect. Dis. 2002, 34, 730–751. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; Paesmans, M.; Rubenstein, E.B.; Boyer, M.; Elting, L.; Feld, R.; Gallagher, J.; Herrstedt, J.; Rapoport, B.; Rolston, K.; et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J. Clin. Oncol. 2000, 18, 3038–3051. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, P.; Aebi, C.; Hirt, A.; Niggli, F.K.; Nadal, D.; Simon, A.; Ozsahin, H.; Kontny, U.; Kühne, T.; Beck Popovic, M.; et al. Predicting bacteremia in children with cancer and fever in chemotherapy-induced neutropenia: Results of the prospective multicenter SPOG 2003 FN study. Pediatr. Infect. Dis. J. 2011, 30, e114–e119. [Google Scholar] [CrossRef]
- AlAzmi, A.; Jastaniah, W.; AlDabbagh, M.; Elimam, N. A clinical approach to non-neutropenic fever in children with cancer. J. Oncol. Pharm. Pract. 2021, 27, 560–569. [Google Scholar] [CrossRef]
- Ali, B.A.; Hirmas, N.; Tamim, H.; Merabi, Z.; Hanna-Wakim, R.; Muwakkit, S.; Abboud, M.; Solh, H.E.; Saab, R. Approach to Non-Neutropenic Fever in Pediatric Oncology Patients-A Single Institution Study. Pediatr. Blood Cancer 2015, 62, 2167–2171. [Google Scholar] [CrossRef]
- Rackoff, W.R.; Gonin, R.; Robinson, C.; Kreissman, S.G.; Breitfeld, P.B. Predicting the risk of bacteremia in childen with fever and neutropenia. J. Clin. Oncol. 1996, 14, 919–924. [Google Scholar] [CrossRef]
- Klaassen, R.J.; Goodman, T.R.; Pham, B.; Doyle, J.J. “Low-risk” prediction rule for pediatric oncology patients presenting with fever and neutropenia. J. Clin. Oncol. 2000, 18, 1012–1019. [Google Scholar] [CrossRef]
- Santolaya, M.E.; Alvarez, A.M.; Becker, A.; Cofré, J.; Enríquez, N.; O’Ryan, M.; Payá, E.; Pilorget, J.; Salgado, C.; Tordecilla, J.; et al. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. J. Clin. Oncol. 2001, 19, 3415–3421. [Google Scholar] [CrossRef]
- Mian, A.; Becton, D.; Saylors, R.; James, L.; Tang, X.; Bhutta, A.; Prodhan, P. Biomarkers for risk stratification of febrile neutropenia among children with malignancy: A pilot study. Pediatr. Blood Cancer 2012, 59, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ammann, R.A.; Bodmer, N.; Hirt, A.; Niggli, F.K.; Nadal, D.; Simon, A.; Ozsahin, H.; Kontny, U.; Kühne, T.; Popovic, M.B.; et al. Predicting adverse events in children with fever and chemotherapy-induced neutropenia: The prospective multicenter SPOG 2003 FN study. J. Clin. Oncol. 2010, 28, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- te Poele, E.M.; Tissing, W.J.; Kamps, W.A.; de Bont, E.S. Risk assessment in fever and neutropenia in children with cancer: What did we learn? Crit. Rev. Oncol. Hematol. 2009, 72, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Oude Nijhuis, C.; Kamps, W.A.; Daenen, S.M.; Gietema, J.A.; van der Graaf, W.T.; Groen, H.J.; Vellenga, E.; Ten Vergert, E.M.; Vermeulen, K.M.; de Vries-Hospers, H.G.; et al. Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients. J. Clin. Oncol. 2005, 23, 7437–7444. [Google Scholar] [CrossRef]
- Boragina, M.; Patel, H.; Reiter, S.; Dougherty, G. Management of febrile neutropenia in pediatric oncology patients: A Canadian survey. Pediatr. Blood Cancer 2007, 48, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.; Selwood, K.; Lane, S.M.; Skinner, R.; Gibson, F.; Chisholm, J.C.; United Kingdom Children’s Cancer Study Group. Variation in policies for the management of febrile neutropenia in United Kingdom Children’s Cancer Study Group centres. Arch. Dis. Child. 2007, 92, 495–498. [Google Scholar] [CrossRef][Green Version]
- Santolaya, M.E.; Alvarez, A.M.; Aviles, C.L.; Becker, A.; King, A.; Mosso, C.; O’Ryan, M.; Paya, E.; Salgado, C.; Silva, P.; et al. Predictors of severe sepsis not clinically apparent during the first twenty-four hours of hospitalization in children with cancer, neutropenia, and fever: A prospective, multicenter trial. Pediatr. Infect. Dis. J. 2008, 27, 538–543. [Google Scholar] [CrossRef]
- Persson, L.; Söderquist, B.; Engervall, P.; Vikerfors, T.; Hansson, L.O.; Tidefelt, U. Assessment of systemic inflammation markers to differentiate a stable from a deteriorating clinical course in patients with febrile neutropenia. Eur. J. Haematol. 2005, 74, 297–303. [Google Scholar] [CrossRef]
- Badurdeen, S.; Hodge, G.; Osborn, M.; Scott, J.; John-Green, C.; Tapp, H.; Zola, H.; Revesz, T. Elevated Serum Cytokine Levels Using Cytometric Bead Arrays Predict Culture-Positive Infections in Childhood Oncology Patients With Febrile Neutropenia. J. Pediatric Hematol. Oncol. 2012, 34, e36–e38. [Google Scholar] [CrossRef]
- Diepold, M.; Noellke, P.; Duffner, U.; Kontny, U.; Berner, R. Performance of Interleukin-6 and Interleukin-8 serum levels in pediatric oncology patients with neutropenia and fever for the assessment of low-risk. BMC Infect. Dis. 2008, 8, 28. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.; Wingard, J.R.; et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A.; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 11 November 2011).
- Harrell, F.E., Jr. rms: Regression Modeling Strategies. R Package Version 6.1-0. 2020. Available online: https://CRAN.Rproject.org/package=rms (accessed on 11 November 2011).
- OptimalCutpoints; Lopez-Raton, M.; Rodriguez-Alvarez, M.X.; Cadarso-Suarez, C.; Gude-Sampedro, F. OptimalCutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. J. Stat. Softw. 2014, 61, 1–36. Available online: http://www.jstatsoft.org/v61/i08/ (accessed on 11 November 2011). [CrossRef]




| Patients (n = 44) | |
| Female, n (%) | 29 (66%) |
| Ethnicity | Caucasian: 44 (100%) |
| Mean (+/−standard deviation) in years at the first febrile episode | 7.7 (+/−5.26) |
| Solid tumor/HL n (%) ALL/AML/NHL n (%) | 21 (47.7%) 23 (52.3%) |
| Episodes (n = 103) | |
| Solid tumor/HL n (%) ALL/AML/NHL n (%) | 56 (54.4%) 47 (45.6%) |
| Disease status: Complete or Partial Remission n (%) | 64 (62.1%) |
| Severe Neutropenia n (%) | 52 (50.5%) |
| GCS-F: Yes, (%) | 35 (34%) |
| Final diagnoses: HRE n (%) Non-HRE n (%) | 19 (18.5%) 84 (81.5) |
| HRE | Non-HRE | p-Value | |
|---|---|---|---|
| CRP-1 (mg/dL) | 2.3 (1.2–5.15) | 3.15 (1.52–7.22) | 0.451 |
| CRP-2 (mg/dL) | 11.1 (5.65–18.75) | 6.55 (2.98–12.12) | 0.017 |
| CRP-2vs1 (%) | 311.1 (77.66–618) | 55.52 (8.10–154.7) | 0.001 |
| PCT-1 (ng/mL) | 0.61 (0.34–3.71) | 0.19 (0.13–0.35) | <0.001 |
| PCT-2 (ng/mL) | 5.89 (0.96–17.5) | 0.28 (0.17–0.76) | <0.001 |
| PCT-2vs1 (%) | 290.2 (52.5–1077.3) | 16.67 (−6.66–96.67) | <0.001 |
| IL6-1 (pg/mL) | 826 (249–4125.75) | 76 (35.75–139.25) | <0.001 |
| IL6-2 (pg/mL) | 180 (29–341) | 44.5 (23.5–86.75) | 0.039 |
| IL6-2vs1 (%) | −90.02 (−96.8–3.66) | −47.97 (−71.15–2.18) | 0.109 |
| CRP-1 | CRP-2 | CRP-2vs1 | |
|---|---|---|---|
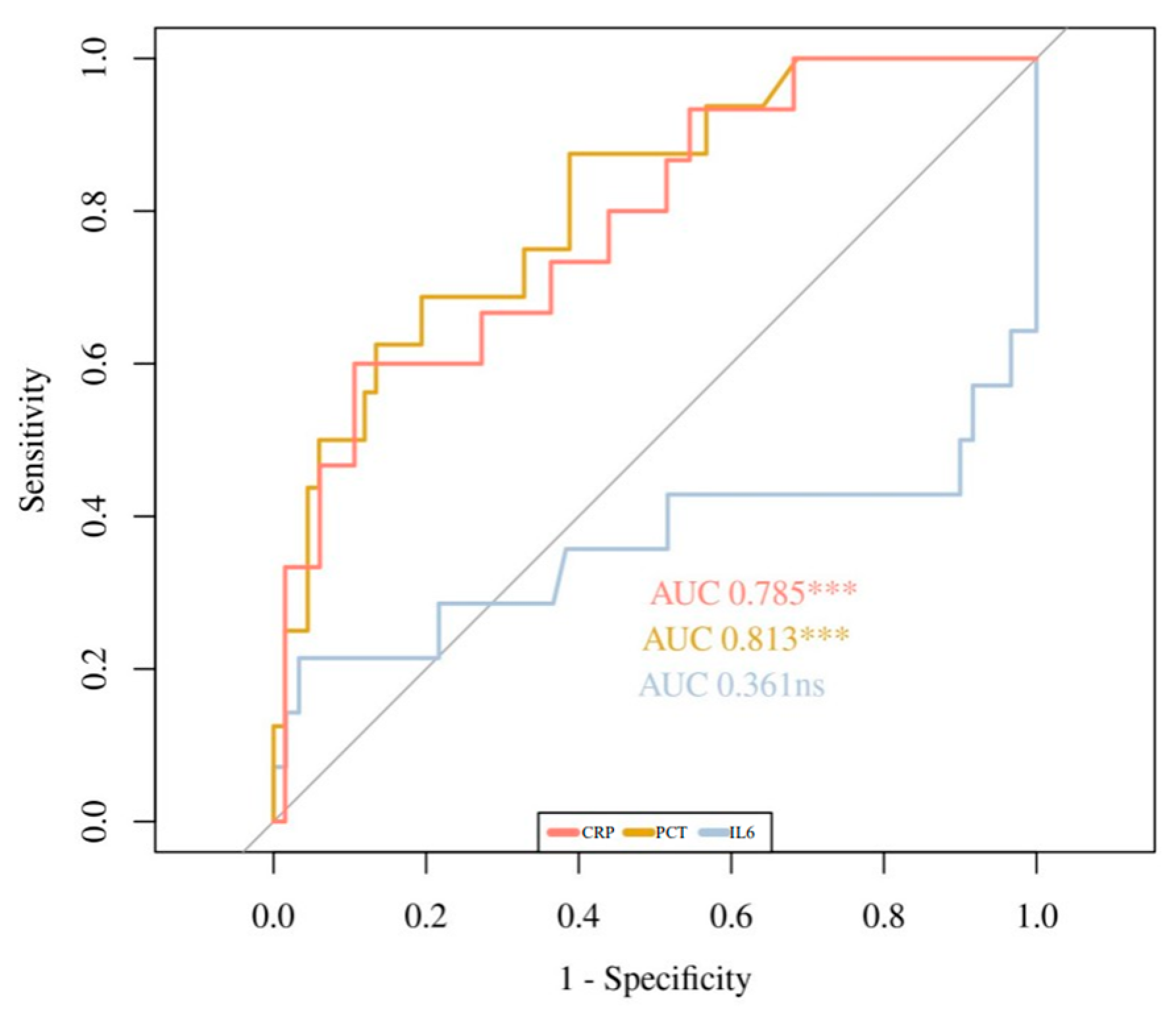
| AUC (CI 95%) | 0.438 (0.267–0.609) | 0.667 (0.532–0.802) | 0.785 (0.655–0.915) |
| Se (%) | 13.33 | 94.74 | 60 |
| Sp (%) | 95.12 | 32.35 | 89.39 |
| PPV (%) | 33.33 | 28.13 | 56.25 |
| NPV (%) | 85.71 | 95.65 | 90.77 |
| Cut-off point | 14.4 mg/dL | 3.5 mg/dL | 291.37% |
| p-value | 0.412 | 0.027 | <0.001 |
| PCT-1 | PCT-2 | PCT-2vs1 | |
| AUC (CI 95%) | 0.805 (0.700–0.910) | 0.836 (0.725–0.947) | 0.812 (0.696–0.928) |
| Se (%) | 81.25 | 78.95 | 68.75 |
| Sp (%) | 68.29 | 79.71 | 80.6 |
| PPV (%) | 33.33 | 51.72 | 45.83 |
| NPV (%) | 94.92 | 93.22 | 91.52 |
| Cut-off point | 0.32 ng/mL | 0.94 ng/mL | 113.64% |
| p-value | <0.001 | <0.001 | <0.001 |
| IL6-1 | IL6-2 | IL6-2vs1 | |
| AUC (CI 95%) | 0.890 (0.791–0.989) | 0.665 (0.474–0.855) | 0.361 (0.138–0.585) |
| Se (%) | 92.86 | 64.71 | 21.43 |
| Sp (%) | 82.5 | 60.65 | 96,67 |
| PPV (%) | 48.15 | 47.82 | 60 |
| NPV (%) | 98.51 | 89.29 | 84.06 |
| Cut-off point | 164 pg/mL | 104 pg/mL | 107.32% |
| p-value | <0.001 | 0.04 | 0.11 |
| Variable (IL-6 Included) | Cut-Off Point | Univariate OR | Multivariate OR |
|---|---|---|---|
| CRP-1 | <14.4 mg/dL | – | – |
| >14.4 mg/dL | 3.00 (0.39–17.09, p = 0.231) | 0.18 (0.01–1.81, p= 0.18 | |
| PCT-1 | <0.32 ng/mL | – | – |
| >0.32 ng/mL | 9.33 (2.73–43.31, p = 0.001) | 4.55 (0.90–27.84, p = 0.076) | |
| IL6-1 | <164 pg/mL | – | – |
| >164 pg/mL | 61.29 (10.90–1159, p < 0.001) | 48.68 (7.92–951.42, p < 0.001) | |
| Variable (IL-6 not Included) | Cut-off point | Univariate OR | Multivariate OR |
| CRP-1 | <14.4 mg/dL | – | – |
| >14.4 mg/dL | 3.00 (0.39–17.09, p = 0.231) | 1.10 (0.14–6.67, p= 0.92 | |
| PCT-1 | <0.32 ng/mL | – | – |
| >0.32 ng/mL | 9.33 (2.73–43.31, p = 0.001) | 8.48 (2.35–40.57, p = 0.002) |
| Variable (IL-6 Included) | Cut-off Point | Univariate OR | Multivariate OR |
|---|---|---|---|
| CRP-2 | <3.5 mg/dL | – | – |
| >3.5 mg/dL | 8.61 (1.61–159.79, p = 0.042) | 1.99 (0.10–76.26, p= 0.664 | |
| PCT-2 | <0.94 ng/mL | – | – |
| >0.94 ng/mL | 14.73 (4.57–58.36, p < 0.001) | 13.01 (1.82–149.13, p = 0.018) | |
| IL6–2 | <104 pg/mL | – | – |
| >104 pg/mL | 7.64 (2.43–26.35, p = 0.001) | 4.55 (0.56–50.59, p = 0.170) | |
| CRP-2vs1 | <291% | – | – |
| >291% | 12.64 (3.59–49.45, p < 0.001) | 31.09 (4.87–355.33, p = 0.001) | |
| PCT-2vs1 | <113% | – | – |
| >113% | 6.92 (2.19–23.84, p = 0.001) | 0.53 (0.04–4.29, p = 0.578) | |
| IL6-2vs1 | <107% | ||
| >107% | 4.83 (0.54–43.68, p = 0.133) | 0.33 (0.00–18.64, p = 0.606) | |
| Variable (IL-6 not Included) | Cut-off point | Univariate OR | Multivariate OR |
| CRP-2 | <3.5 mg/dL | – | – |
| >3.5 mg/dL | 8.61 (1.61–159.79, p = 0.042) | 10.69 (0.79–353.54, p = 0.113) | |
| PCT-2 | <0.94 ng/mL | – | – |
| >0.94 ng/mL | 14.73 (4.57–58.36, p < 0.001) | 9.67 (1.81–78.01, p = 0.014) | |
| CRP-2vs1 | <291% | – | – |
| >291% | 12.64 (3.59–49.45, p < 0.001) | 16.81 (3.34–130.48, p = 0.002) | |
| PCT-2vs1 | <113% | – | – |
| >113% | 6.92 (2.19–23.84, p = 0.001) | 1.74 (0.34–9.00, p = 0.499) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas Rubio, J.A.; Palomo Moraleda, P.; De Lucio Delgado, A.; Solís Sánchez, G.; Prieto García, B.; Rey Galán, C. A Dynamic Approach for Early Risk Prediction of Gram-Negative Bloodstream Infection and Systemic Inflammatory Response Syndrome in Febrile Pediatric Hemato-Oncology Patients. Children 2022, 9, 833. https://doi.org/10.3390/children9060833
Villegas Rubio JA, Palomo Moraleda P, De Lucio Delgado A, Solís Sánchez G, Prieto García B, Rey Galán C. A Dynamic Approach for Early Risk Prediction of Gram-Negative Bloodstream Infection and Systemic Inflammatory Response Syndrome in Febrile Pediatric Hemato-Oncology Patients. Children. 2022; 9(6):833. https://doi.org/10.3390/children9060833
Chicago/Turabian StyleVillegas Rubio, José Antonio, Pilar Palomo Moraleda, Ana De Lucio Delgado, Gonzalo Solís Sánchez, Belén Prieto García, and Corsino Rey Galán. 2022. "A Dynamic Approach for Early Risk Prediction of Gram-Negative Bloodstream Infection and Systemic Inflammatory Response Syndrome in Febrile Pediatric Hemato-Oncology Patients" Children 9, no. 6: 833. https://doi.org/10.3390/children9060833
APA StyleVillegas Rubio, J. A., Palomo Moraleda, P., De Lucio Delgado, A., Solís Sánchez, G., Prieto García, B., & Rey Galán, C. (2022). A Dynamic Approach for Early Risk Prediction of Gram-Negative Bloodstream Infection and Systemic Inflammatory Response Syndrome in Febrile Pediatric Hemato-Oncology Patients. Children, 9(6), 833. https://doi.org/10.3390/children9060833






