Long-Term Health Associated with Small and Large for Gestational Age Births among Young Thai Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
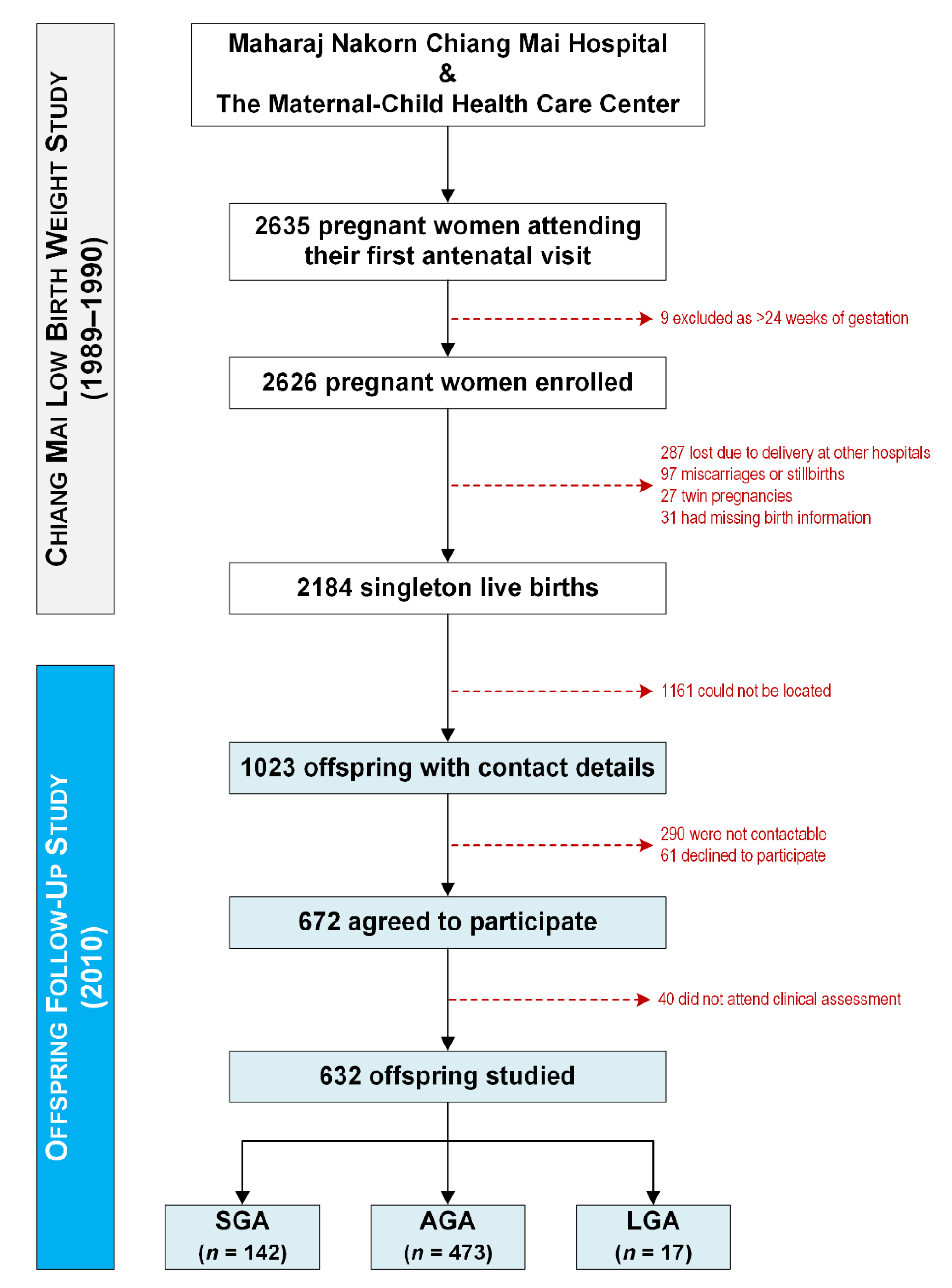
2.2. Study Design
2.3. Statistical Analyses
3. Results
3.1. Study Population
3.2. Anthropometry
3.3. Cardiometabolic Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beaumont, R.N.; Kotecha, S.J.; Wood, A.R.; Knight, B.A.; Sebert, S.; McCarthy, M.I.; Hattersley, A.T.; Järvelin, M.-R.; Timpson, N.J.; Freathy, R.M. Common maternal and fetal genetic variants show expected polygenic effects on risk of small-or large-for-gestational-age (SGA or LGA), except in the smallest 3% of babies. PLoS Genet. 2020, 16, e1009191. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, R.; Mohammed, S.H.; Safabakhsh, M.; Mohseni, F.; Monfared, Z.S.; Seyyedi, J.; Mejareh, Z.N.; Alizadeh, S. Birth weight and risk of cardiovascular disease incidence in adulthood: A dose-response meta-analysis. Curr. Atheroscler. Rep. 2020, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Guo, C.; Li, Q.; Liu, Y.; Sun, X.; Yin, Z.; Li, H.; Chen, X.; Liu, X.; Zhang, D. Birth weight and risk of type 2 diabetes: A dose-response meta-analysis of cohort studies. Diabetes/Metab. Res. Rev. 2019, 35, e3144. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Zinkhan, E. Short- and long-term implications of small for gestational age. Obstet. Gynecol. Clin. N. Am. 2021, 48, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.; van Elburg, R.; van der Beek, E.M. Macrosomia and large for gestational age in Asia: One size does not fit all. J. Obstetr. Gynaecol. Res. 2021, 47, 1929–1945. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef]
- Kozuki, N.; Katz, J.; Lee, A.C.; Vogel, J.P.; Silveira, M.F.; Sania, A.; Stevens, G.A.; Cousens, S.; Caulfield, L.E.; Christian, P.; et al. Short maternal stature increases the risk of small-for-gestational-age and preterm births in low- and middle-income countries: Individual participant data meta-analysis and population attributable fraction. J. Nutr. 2015, 145, 2542–2550. [Google Scholar]
- Tzioumis, E.; Kay, M.C.; Bentley, M.E.; Adair, L.S. Prevalence and trends in the childhood dual burden of malnutrition in low-and middle-income countries, 1990–2012. Public Health Nutr. 2016, 19, 1375–1388. [Google Scholar] [CrossRef]
- Namwongprom, S.; Rerkasem, K.; Wongthanee, A.; Pruenglampoo, S.; Mangklabruks, A. Relationship between total body adiposity assessed by dual-energy X-ray absorptiometry, birth weight and metabolic syndrome in young Thai adults. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 252–257. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Rerkasem, K.; Rattanatanyong, P.; Rerkasem, A.; Wongthanee, A.; Rungruengthanakit, K.; Mangklabruks, A.; Mutirangura, A. Higher Alu methylation levels in catch-up growth in twenty-year-old offsprings. PLoS ONE 2015, 10, e0120032. [Google Scholar]
- Chiang Mai Low Birth Weight Study Group. The risk factors of low birth weight infants in the northern part of Thailand. J. Med. Assoc. Thail. 2012, 95, 358–365. [Google Scholar]
- Rerkasem, A.; Maessen, S.E.; Wongthanee, A.; Pruenglampoo, S.; Mangklabruks, A.; Sripan, P.; Derraik, J.G.B.; Rerkasem, K. Caesarean delivery is associated with increased blood pressure in young adult offspring. Sci. Rep. 2021, 11, 10201. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight.; length.; and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Jordan, S.; Lim, L.; Seubsman, S.A.; Bain, C.; Sleigh, A. Secular changes and predictors of adult height for 86 105 male and female members of the Thai Cohort Study born between 1940 and 1990. J. Epidemiol. Community Health 2012, 66, 75–80. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypert. 2013, 31, 1281–1357. [Google Scholar] [CrossRef]
- Jeyabalan, A.; Heazell, A. Management of isolated hypertension in pregnancy. In Hypertension in Pregnancy; Heazell, A., Norwitz, E., Kenny, L., Baker, P., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 79–96. [Google Scholar]
- Palmsten, K.; Buka, S.L.; Michels, K.B. Maternal pregnancy-related hypertension and risk for hypertension in offspring later in life. Obstetr. Gynecol. 2010, 116, 858–864. [Google Scholar] [CrossRef]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
- Carrascosa, A.; Vicens-Calvet, E.; Yeste, D.; Espadero, R.M.; Ulied, A.; Alpera, R.; Álvarez, E.; Bel, J.; Borrajo, E.; Borrás, V. Children born small for gestational age (SGA) who fail to achieve catch up growth by 2–8 years of age are short from infancy to adulthood: Data from a cross-sectional study of 486 Spanish children. Pediatr. Endocrinol. Rev. 2006, 4, 15–27. [Google Scholar]
- Saenger, P.; Czernichow, P.; Hughes, I.; Reiter, E.O. Small for gestational age: Short stature and beyond. Endocr. Rev. 2007, 28, 219–251. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B.; Lindberg, A.; Board, K.I. Height at start, first-year growth response and cause of shortness at birth are major determinants of adult height outcomes of short children born small for gestational age and Silver-Russell syndrome treated with growth hormone: Analysis of data from KIGS. Horm. Res. Paediatr. 2010, 74, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Campisi, S.C.; Carbone, S.E.; Zlotkin, S. Catch-up growth in full-term small for gestational age infants: A systematic review. Adv. Nutr. 2019, 10, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Leger, J.; Limoni, C.; Collin, D.; Czernichow, P. Prediction factors in the determination of final height in subjects born small for gestational age. Pediatr. Res. 1998, 43, 808–812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaquet, D.; Collin, D.; Levy-Marchal, C.; Czernichow, P. Adult height distribution in subjects born small for gestational age. Horm. Res. Paediatr. 2004, 62, 92–96. [Google Scholar] [CrossRef]
- Waldman, L.A.; Chia, D.J. Towards identification of molecular mechanisms of short stature. Int. J. Pediatr. Endocrinol. 2013, 2013, 19. [Google Scholar] [CrossRef]
- Leger, J.; Levy-Marchal, C.; Bloch, J.; Pinet, A.; Chevenne, D.; Porquet, D.; Collin, D.; Czernichow, P. Reduced final height and indications for insulin resistance in 20 year olds born small for gestational age: Regional cohort study. BMJ 1997, 315, 341–347. [Google Scholar] [CrossRef]
- Arends, N.J.; Boonstra, V.H.; Duivenvoorden, H.J.; Hofman, P.L.; Cutfield, W.S.; Hokken-Koelega, A.C. Reduced insulin sensitivity and the presence of cardiovascular risk factors in short prepubertal children born small for gestational age (SGA). Clin. Endocrinol. 2005, 62, 44–50. [Google Scholar] [CrossRef]
- Putzker, S.; Bechtold-Dalla Pozza, S.; Kugler, K.; Schwarz, H.P.; Bonfig, W. Insulin resistance in young adults born small for gestational age (SGA). J. Pediatr. Endocrinol. Metab. 2014, 27, 253–259. [Google Scholar] [CrossRef]
- Newsome, C.A.; Shiell, A.W.; Fall, C.H.D.; Phillips, D.I.W.; Shier, R.; Law, C.M. Is birth weight related to later glucose and insulin metabolism?—A systematic review. Diabet. Med. 2003, 20, 339–348. [Google Scholar] [CrossRef]
- Derraik, J.G.B.; Maessen, S.E.; Gibbins, J.D.; Cutfield, W.S.; Lundgren, M.; Ahlsson, F. Large-for-gestational-age phenotypes and obesity risk in adulthood: A study of 195,936 women. Sci. Rep. 2020, 10, 2157. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, W.; Sundet, J.M.; Tambs, K. Birth weight and the risk of overweight in young men born at term. Am. J. Hum. Biol. 2015, 27, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Forsen, T.; Tuomilehto, J.; Osmond, C.; Barker, D. Size at birth, childhood growth and obesity in adult life. Int. J. Obes. 2001, 25, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Schellong, K.; Schulz, S.; Harder, T.; Plagemann, A. Birth weight and long-term overweight risk: Systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 2012, 7, e47776. [Google Scholar] [CrossRef]
- Yu, Z.B.; Han, S.P.; Zhu, G.Z.; Zhu, C.; Wang, X.J.; Cao, X.G.; Guo, X.R. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 525–542. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.F.; Mu, M.; Sheng, J. Birth weight and overweight/obesity in adults: A meta-analysis. Eur. J. Pediatr. 2012, 171, 1737–1746. [Google Scholar] [CrossRef]
- Catalano, P.M. Obesity and pregnancy—The propagation of a viscous cycle? J. Clin. Endocr. Metabol. 2003, 88, 3505–3506. [Google Scholar] [CrossRef]
- Kuciene, R.; Dulskiene, V.; Medzioniene, J. Associations between high birth weight, being large for gestational age, and high blood pressure among adolescents: A cross-sectional study. Eur. J. Nutr. 2018, 57, 373–381. [Google Scholar] [CrossRef]
- Mu, M.; Wang, S.F.; Sheng, J.; Zhao, Y.; Li, H.Z.; Hu, C.L.; Tao, F.B. Birth weight and subsequent blood pressure: A meta-analysis. Arch. Cardiovasc. Dis. 2012, 105, 99–113. [Google Scholar] [CrossRef]
- Sjöholm, P.; Pahkala, K.; Davison, B.; Niinikoski, H.; Raitakari, O.; Juonala, M.; Singh, G.R. Birth weight for gestational age and later cardiovascular health: A comparison between longitudinal Finnish and indigenous Australian cohorts. Ann. Med. 2021, 53, 2060–2071. [Google Scholar] [CrossRef]
- Tanner, J.; Goldstein, H.; Whitehouse, R. Standards for children’s height at ages 2–9 years allowing for height of parents. Arch. Dis. Child. 1970, 45, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Hughes, I.P.; Davies, P.S. Proposed new target height equations for use in Australian growth clinics. J. Paediatr. Child. Health 2008, 44, 613–617. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | SGA | AGA | LGA | |
|---|---|---|---|---|
| n | 142 (22.5%) | 473 (74.8%) | 17 (2.7%) | |
| Sex | Male | 71 (50%) | 212 (45%) | 8 (47%) |
| Female | 71 (50%) | 261 (55%) | 9 (53%) | |
| Delivery | Vaginal delivery | 122 (86%) | 439 (93%) | 14 (82%) |
| Caesarean section | 20 (14%) | 34 (7%) | 3 (18%) | |
| Age (years) | 20.5 ± 0.5 | 20.6 ± 0.5 | 20.6 ± 0.5 | |
| Birth weight (kg) | 2.55 ± 0.27 | 3.08 ± 0.36 | 3.75 ± 0.66 | |
| Birth weight Z-score | −1.78 ± 0.40 | −0.24 ± 0.61 | 1.69 ± 0.33 | |
| Birth length (cm) | 47.3 ± 2.1 | 49.2 ± 4.3 | 51.6 ± 4.2 | |
| Birth length Z-score | −1.18 ± 0.98 | 0.21 ± 1.15 | 1.98 ± 1.22 | |
| Gestational age (weeks) | 39.6 ± 1.4 | 39.1 ± 1.7 | 38.4 ± 2.8 | |
| Maternal age at childbirth (years) | 26.5 ± 4.5 | 26.2 ± 4.7 | 28.5 ± 4.2 | |
| Maternal BMI (kg/m2) | 20.62 ± 2.33 | 21.51 ± 2.51 | 22.56 ± 2.95 | |
| Maternal BMI status | Underweight/normal weight | 135 (95%) | 432 (91%) | 12 (71%) |
| Overweight/obesity | 7 (5%) | 41 (9%) | 5 (29%) | |
| Maternal education | Less than high school | 109 (89%) | 366 (91%) | 12 (92%) |
| High school or greater | 14 (11%) | 37 (9%) | 1 (8%) | |
| Paternal education | Less than high school | 97 (80%) | 329 (81%) | 10 (77%) |
| High school or greater | 25 (20%) | 76 (19%) | 3 (23%) | |
| Family income (baht per month) 1 | 2300 [1500, 3500] | 2700 [1675, 4400] | 1700 [1000, 2320] |
| Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| SGA | AGA | LGA | SGA | AGA | LGA | ||
| Anthropometry | Height (cm) | 162.8 (161.4, 164.1) | 164.0 (163.3, 164.8) | 165.6 (161.6, 169.5) | 162.8 (161.9, 163.7) ***† | 164.6 (164.1, 165.1) | 166.1 (163.5, 168.6) |
| Weight (kg) | 55.6 (53.3, 57.9) †† | 57.8 (56.5, 59.1) † | 65.0 (58.3, 71.6) | 56.9 (54.4, 59.4) †† | 59.0 (57.2, 60.9) † | 65.47 (59.3, 71.5) | |
| BMI (kg/m2) | 20.86 (20.15, 21.57) †† | 21.36 (20.97, 21.75) † | 23.72 (21.68, 25.77) | 21.06 (20.37, 21.75) † | 21.36 (20.98, 21.73) † | 23.48 (21.50, 25.46) | |
| Glucose homeostasis | Fasting glucose (mg/dL) | 83 (82, 85) | 83 (82, 83) | 84 (80, 88) | 83 (82, 85) | 83 (82, 83) | 84 (80, 89) |
| Fasting insulin (mIU/L) | 7.8 (6.9, 8.7) | 7.2 (6.7, 7.6) | 9.6 (6.9, 13.1) | 7.8 (7.0, 8.7) | 7.1 (6.7, 7.5) | 9.5 (6.9, 13) | |
| 120-min glucose (mg/dL) | 105 (101, 110) ** | 99 (97, 101) | 96 (86, 107) | 105 (101, 109) ** | 99 (97, 101) | 96 (87, 107) | |
| HOMA-IR | 1.60 (1.42, 1.79) | 1.47 (1.37, 1.56) | 1.99 (1.43, 2.76) | 1.60 (1.42, 1.79) | 1.45 (1.36, 1.55) | 1.97 (1.42, 2.74) | |
| Blood pressure | Systolic (mmHg) | 115 (113, 117) | 114 (113, 116) | 117 (111, 123) | 116 (114, 119) | 116 (113, 118) | 117 (112, 123) |
| Diastolic (mmHg) | 75 (73, 76) | 73 (73, 74) † | 79 (74, 84) | 76 (74, 78) | 75 (73, 77) | 80 (75, 85) | |
| Lipid profile | Total cholesterol (mg/dL) | 169 (163, 174) | 168 (165, 172) | 175 (158, 191) | 168 (162, 174) | 168 (165, 172) | 175 (159, 192) |
| HDL (mg/dL) | 56 (53, 58) | 57 (55, 58) | 57 (50, 64) | 56 (53, 58) | 56 (55, 58) | 57 (50, 64) | |
| LDL (mg/dL) | 96 (91, 101) | 95 (93, 98) | 99 (85, 113) | 96 (91, 101) | 96 (93, 98) | 100 (86, 114) | |
| Triglycerides (mg/dL) | 76 (70, 83) | 76 (72, 79) | 82 (65, 104) | 76 (70, 83) | 76 (73, 80) | 83 (66, 105) | |
| SGA | AGA | LGA | |||
|---|---|---|---|---|---|
| Anthropometry | n | 141 | 470 | 17 | |
| BMI status | Underweight/normal weight | 121 (85.8%) | 392 (83.4%) | 11 (64.7%) | |
| Overweight | 12 (8.5%) | 54 (11.5%) | 4 (23.5%) | ||
| Obesity | 8 (5.7%) | 24 (5.1%) | 2 (11.8%) | ||
| Overweight/obesity | 20 (14.2%) | 78 (16.6%) | 6 (35.3%) | ||
| Height | Normal stature | 130 (92.2%) | 455 (96.8%) | 17 (100%) | |
| Short stature | 11 (7.8%) | 15 (3.2%) | nil | ||
| Blood pressure | n | 139 | 463 | 17 | |
| Systolic | Normotension | 120 (86.3%) | 415 (89.6%) | 12 (70.6%) | |
| Prehypertension | 15 (10.8%) | 30 (6.5%) | 4 (23.5%) | ||
| Hypertension | 4 (2.9%) | 18 (3.9%) | 1 (5.9%) | ||
| Abnormal | 19 (13.7%) | 48 (10.4%) | 5 (29.4%) | ||
| Diastolic | Normotension | 119 (85.6%) | 396 (85.5%) | 12 (70.6%) | |
| Prehypertension | 11 (7.9%) | 43 (9.3%) | 4 (23.5%) | ||
| Hypertension | 9 (6.5%) | 24 (5.2%) | 4 (5.9%) | ||
| Abnormal | 20 (14.4%) | 67 (14.5%) | 5 (29.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhag, A.; Rerkasem, A.; Kulprachakarn, K.; Parklak, W.; Angkurawaranon, C.; Rerkasem, K.; Derraik, J.G.B. Long-Term Health Associated with Small and Large for Gestational Age Births among Young Thai Adults. Children 2022, 9, 779. https://doi.org/10.3390/children9060779
Suhag A, Rerkasem A, Kulprachakarn K, Parklak W, Angkurawaranon C, Rerkasem K, Derraik JGB. Long-Term Health Associated with Small and Large for Gestational Age Births among Young Thai Adults. Children. 2022; 9(6):779. https://doi.org/10.3390/children9060779
Chicago/Turabian StyleSuhag, Alisha, Amaraporn Rerkasem, Kanokwan Kulprachakarn, Wason Parklak, Chaisiri Angkurawaranon, Kittipan Rerkasem, and José G. B. Derraik. 2022. "Long-Term Health Associated with Small and Large for Gestational Age Births among Young Thai Adults" Children 9, no. 6: 779. https://doi.org/10.3390/children9060779
APA StyleSuhag, A., Rerkasem, A., Kulprachakarn, K., Parklak, W., Angkurawaranon, C., Rerkasem, K., & Derraik, J. G. B. (2022). Long-Term Health Associated with Small and Large for Gestational Age Births among Young Thai Adults. Children, 9(6), 779. https://doi.org/10.3390/children9060779









