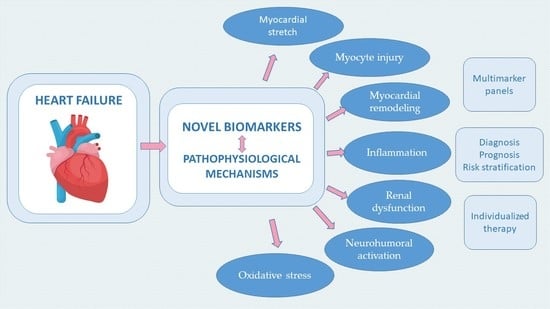
Novel Biomarkers of Heart Failure in Pediatrics
Abstract
1. Introduction
2. Biomarkers of Myocardial Stretch
Midregional Proatrial Natriuretic Peptide (MR-proANP)
3. Biomarkers of Myocyte Injury
3.1. High-Sensitivity Cardiac Troponin (hs-cTn)
3.2. Heart-Type Fatty Acid-Binding Proteins (H-FABPs)
3.3. Glutathione Transferase P1 (GSTP1)
4. Biomarkers of Myocardial Remodeling
4.1. Galectin-3
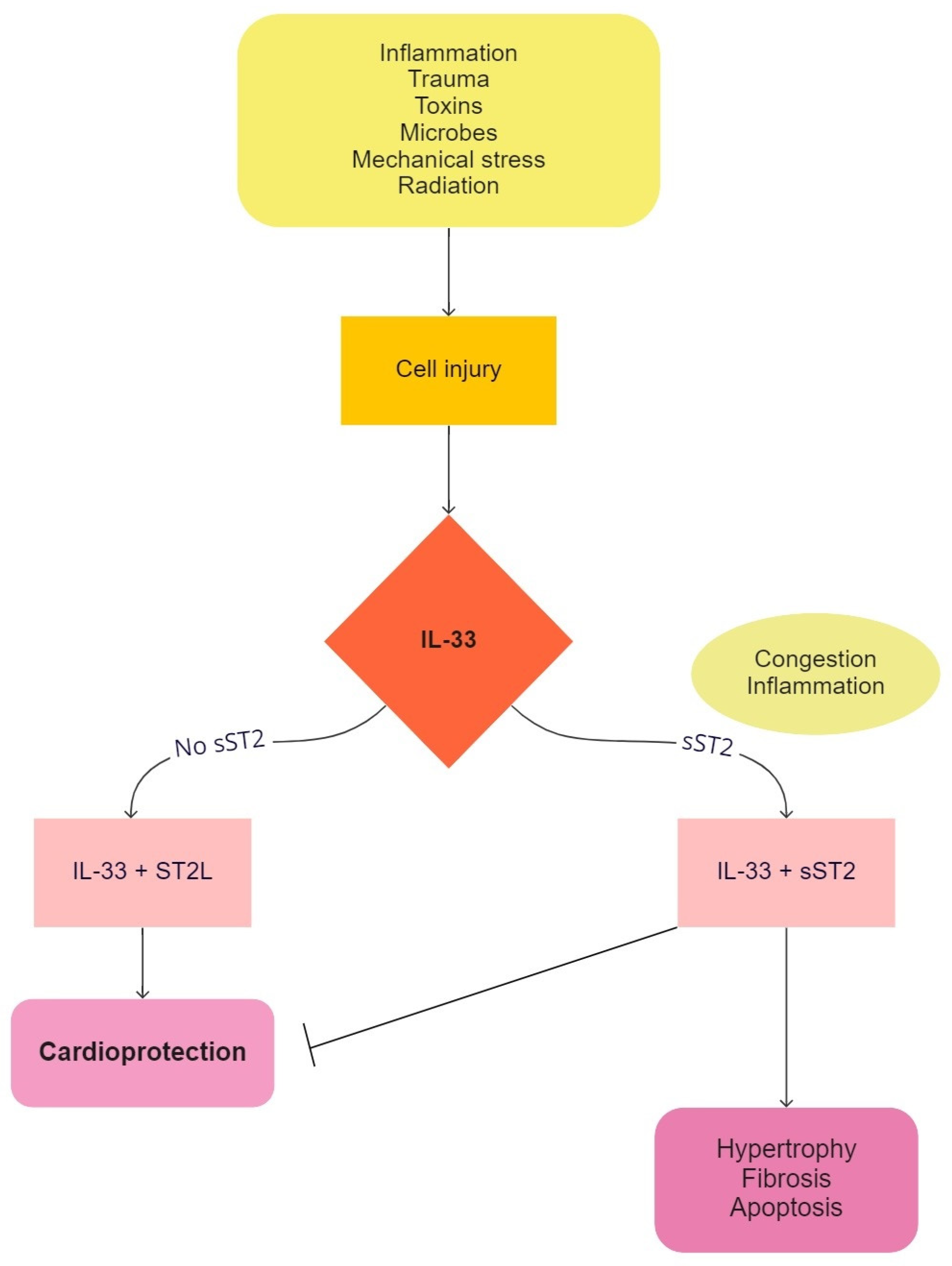
4.2. Soluble Isoform of Suppression of Tumorigenicity 2 (sST2)
4.3. MicroRNAs
5. Biomarkers of Inflammation
5.1. Growth Differentiation Factor-15 (GDF-15)
5.2. Endothelial Microparticles (EMPs) and Endothelial Progenitor Cells (EPCs)
6. Biomarkers of Renal Dysfunction
6.1. Neutrophil Gelatinase-Associated Lipocalin (NGAL)
6.2. Kidney Injury Molecule-1 (KIM-1)
7. Biomarkers of Neurohumoral Activation
7.1. Adrenomedullin (MR-proADM)
7.2. Copeptin
7.3. Matrix Metalloproteinases (MMPs)
8. Biomarkers of Oxidative Stress
8.1. Ceruloplasmin
8.2. Myeloperoxidase (MPO)
9. Biomarkers Determined in Urine
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Simic, D.; Radovanovic, S.; Simic, T. Novel biomarkers of heart failure. Adv. Clin. Chem. 2017, 79, 93–152. [Google Scholar] [PubMed]
- Aimo, A.; Georgiopoulos, G.; Senni, M.; Emdin, M. Searching for diagnostic biomarkers of heart failure with preserved ejection fraction: Methodological issues. Eur. J. Heart Fail. 2020, 22, 1598–1599. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; Braunwald, E. Future of biomarkers in acute coronary syndromes: Moving toward a multimarker strategy. Circulation 2003, 108, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Arab, S.; Gramolini, A.O.; Ping, P.; Kislinger, T.; Stanley, B.; van Eyk, J.; Ouzounian, M.; MacLennan, D.H.; Emili, A.; Liu, P.P. Cardiovascular proteomics: Tools to develop novel biomarkers and potential applications. J. Am. Coll. Cardiol. 2006, 48, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Heart failure. JACC Heart Fail. 2013, 1, 1–20. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Lam, E.; Higgins, V.; Zhang, L.; Chan, M.K.; Bohn, M.K.; Trajcevski, K.; Liu, P.; Adeli, K.; Nathan, P.C. Normative values of high-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide in children and adolescents: A study from the CALIPER cohort. J. Appl. Lab. Med. 2021, 6, 344–353. [Google Scholar] [CrossRef]
- Bonnet, L.; Marquant, E.; Fromonot, J.; Hamouda, I.; Berbis, J.; Godefroy, A.; Vierge, M.; Tsimaratos, M.; Reynaud, R. Copeptin assays in children for the differential diagnosis of polyuria-polydipsia syndrome and reference levels in hospitalized children. Clin. Endocrinol. 2022, 96, 47–53. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Johnson, J.N.; Gray, A.; Wendt, P.; Jefferies, J.L.; Jaffe, A.S.; Donato, L.J.; Saenger, A.K. Soluble ST2 and galectin-3 in pediatric patients without heart failure. Clin. Biochem. 2015, 48, 1337–1340. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Costello-Boerrigter, L.C.; Boerrigter, G.; Redfield, M.M.; Rodeheffer, R.J.; Urban, L.H.; Mahoney, D.W.; Jacobsen, S.J.; Heublein, D.M.; Burnett, J.C., Jr. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: Determinants and detection of left ventricular dysfunction. J. Am. Coll. Cardiol. 2006, 47, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shih, R. Update of pediatric heart failure. Pediatr. Clin. N. Am. 2020, 67, 889–901. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Jankowska, E.A.; Morgenthaler, N.G.; Vassanelli, C.; Zanolla, L.; Rozentryt, P.; Filippatos, G.S.; Doehner, W.; Koehler, F.; Papassotiriou, J.; et al. Comparison of midregional pro-atrial natriuretic peptide with N-terminal pro-B-type natriuretic peptide in predicting survival in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; von Haehling, S.; Anker, S.D. Natriuretic peptides and other biomarkers in chronic heart failure: From BNP, NT-proBNP, and MR-proANP to routine biochemical markers. Int. J. Cardiol. 2009, 132, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Latini, R.; Carbonieri, E.; Moretti, L.; Rossi, M.G.; Ciricugno, S.; Milani, V.; Marchioli, R.; Struck, J.; Bergmann, A.; et al. The predictive value of stable precursor fragments of vasoactive peptides in patients with chronic heart failure: Data from the GISSI-heart failure (GISSI-HF) trial. Eur. J. Heart Fail. 2010, 12, 338–347. [Google Scholar] [CrossRef]
- Dhingra, R.; Vasan, R.S. Biomarkers in cardiovascular disease: Statistical assessment and section on key novel heart failure biomarkers. Trends Cardiovasc. Med. 2017, 27, 123–133. [Google Scholar] [CrossRef]
- Khan, S.Q.; Dhillon, O.; Kelly, D.; Squire, I.B.; Struck, J.; Quinn, P.; Morgenthaler, N.G.; Bergmann, A.; Davies, J.E.; Ng, L.L. Plasma N-terminal B-Type natriuretic peptide as an indicator of long-term survival after acute myocardial infarction: Comparison with plasma midregional pro-atrial natriuretic peptide: The LAMP (Leicester Acute Myocardial Infarction Peptide) study. J. Am. Coll. Cardiol. 2008, 51, 1857–1864. [Google Scholar] [CrossRef][Green Version]
- Konstantinidis, K.; Whelan, R.S.; Kitsis, R.N. Mechanisms of cell death in heart disease. Arter. Thromb. Vasc. Biol. 2012, 32, 1552–1562. [Google Scholar] [CrossRef]
- Parmacek, M.S.; Solaro, R.J. Biology of the troponin complex in cardiac myocytes. Prog. Cardiovasc. Dis. 2004, 47, 159–176. [Google Scholar] [CrossRef]
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J.; Bio-Markers, IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Cardiac troponin assays: Guide to understanding analytical characteristics and their impact on clinical care. Clin. Chem. 2017, 63, 73–81. [Google Scholar] [CrossRef]
- Missov, E.; Calzolari, C.; Pau, B. Circulating cardiac troponin I in severe congestive heart failure. Circulation 1997, 96, 2953–2958. [Google Scholar] [CrossRef] [PubMed]
- Ilva, T.; Lassus, J.; Siirila-Waris, K.; Melin, J.; Peuhkurinen, K.; Pulkki, K.; Nieminen, M.S.; Mustonen, H.; Porela, P.; Harjola, V.P. Clinical significance of cardiac troponins I and T in acute heart failure. Eur. J. Heart Fail. 2008, 10, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Jarolim, P. High sensitivity cardiac troponin assays in the clinical laboratories. Clin. Chem. Lab. Med. 2015, 53, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Giannitsis, E.; Kurz, K.; Hallermayer, K.; Jarausch, J.; Jaffe, A.S.; Katus, H.A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin. Chem. 2010, 56, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Chenevier-Gobeaux, C.; Bailleul, S.; Mzabi, A.; Blanc, M.C.; Lefevre, G. Upper reference limits of high-sensitivity cardiac troponin T in a general population: Comparison with those of sensitive cardiac troponin I. Clin. Lab. 2013, 59, 333–336. [Google Scholar] [CrossRef]
- Masson, S.; Anand, I.; Favero, C.; Barlera, S.; Vago, T.; Bertocchi, F.; Maggioni, A.P.; Tavazzi, L.; Tognoni, G.; Cohn, J.N.; et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: Data from 2 large randomized clinical trials. Circulation 2012, 125, 280–288. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Li, V.W.; Lai, C.T.; Shin, V.Y.; Keung, W.; Cheuk, D.K.; Kwong, A.; Li, R.A.; Chan, G.C. Circulating high-sensitivity troponin T and microRNAs as markers of myocardial damage during childhood leukaemia treatment. Pediatr. Res. 2021, 89, 1245–1252. [Google Scholar] [CrossRef]
- Pelsers, M.M.; Hermens, W.T.; Glatz, J.F. Fatty acid-binding proteins as plasma markers of tissue injury. Clin. Chim. Acta 2005, 352, 15–35. [Google Scholar] [CrossRef]
- Kleine, A.H.; Glatz, J.F.; Van Nieuwenhoven, F.A.; Van der Vusse, G.J. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol. Cell. Biochem. 1992, 116, 155–162. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Takahashi, T.; Okuyama, H.; Takabatake, N.; Nozaki, N.; Hirono, O.; Tsunoda, Y.; Shishido, T.; et al. Combination of heart-type fatty acid binding protein and brain natriuretic peptide can reliably risk stratify patients hospitalized for chronic heart failure. Circ. J. 2005, 69, 922–927. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.P.; Wang, W.D.; Ma, S.C.; Wang, L.Y.; Qiao, L.Y.; Zhang, L.P. Changes of heart-type fatty acid-binding protein in children with chronic heart failure and its significance. Zhongguo Dang Dai Er Ke Za Zhi 2013, 15, 99–101. [Google Scholar] [PubMed]
- Simic, T.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Mimic-Oka, J. Glutathione S-transferases in kidney and urinary bladder tumors. Nat. Rev. Urol. 2009, 6, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, Y.; Xue, B.; Luo, L.; Shen, J.; Zhang, S.; Jiang, Y.; Yin, Z. Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene 2006, 25, 5787–5800. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, Y.; Feng, Q.; Zhang, H.; Xue, B.; Shen, J.; Ye, Y.; Han, X.; Ma, H.; Xu, J.; et al. Recombinant protein glutathione S-transferases P1 attenuates inflammation in mice. Mol. Immunol. 2009, 46, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Salama, M.; Rosenhek, R.; Gmeiner, M.; Perkmann, T.; Steindl, J.; Aharinejad, S. Serum glutathione S-transferase P1 1 in prediction of cardiac function. J. Card. Fail. 2012, 18, 253–261. [Google Scholar] [CrossRef]
- Tripaydonis, A.; Conyers, R.; Elliott, D.A. Pediatric anthracycline-induced cardiotoxicity: Mechanisms, pharmacogenomics, and pluripotent stem-cell modeling. Clin. Pharmacol. Ther. 2019, 105, 614–624. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P.; Iraqi, W. Extracellular matrix fibrotic markers in heart failure. Heart Fail. Rev. 2010, 15, 319–329. [Google Scholar] [CrossRef]
- Elola, M.T.; Wolfenstein-Todel, C.; Troncoso, M.F.; Vasta, G.R.; Rabinovich, G.A. Galectins: Matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol. Life Sci. 2007, 64, 1679–1700. [Google Scholar] [CrossRef]
- van Kimmenade, R.R.; Januzzi, J.L., Jr.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.; Holzendorf, V.; Wachter, R.; Nolte, K.; Schmidt, A.G.; Kraigher-Krainer, E.; Duvinage, A.; Unkelbach, I.; Dungen, H.D.; Tschope, C.; et al. Galectin-3 in patients with heart failure with preserved ejection fraction: Results from the Aldo-DHF trial. Eur. J. Heart Fail. 2015, 17, 214–223. [Google Scholar] [CrossRef]
- Kotby, A.A.; Youssef, O.I.; Elmaraghy, M.O.; El Sharkawy, O.S. Galectin-3 in children with chronic heart failure with normal and reduced ejection fraction: Relationship to disease severity. Pediatr. Cardiol. 2017, 38, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chan, W.L.; Leung, B.P.; Huang, F.; Wheeler, R.; Piedrafita, D.; Robinson, J.H.; Liew, F.Y. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 1998, 187, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.; Bayes-Genis, A.; Beltran-Troncoso, P.; Caravaca-Perez, P.; Conde-Martel, A.; Crespo-Leiro, M.G.; Delgado, J.F.; Diez, J.; Formiga, F.; Manito, N. Sacubitril-valsartan, clinical benefits and related mechanisms of action in heart failure with reduced ejection fraction. A review. Front. Cardiovasc. Med. 2021, 8, 754499. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschope, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 391–412. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Pascual-Figal, D.; Daniels, L.B. ST2 testing for chronic heart failure therapy monitoring: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115 (Suppl. S7), 70B–75B. [Google Scholar] [CrossRef]
- Dieplinger, B.; Egger, M.; Gegenhuber, A.; Haltmayer, M.; Mueller, T. Analytical and clinical evaluation of a rapid quantitative lateral flow immunoassay for measurement of soluble ST2 in human plasma. Clin. Chim. Acta 2015, 451 Pt B, 310–315. [Google Scholar] [CrossRef][Green Version]
- Berezin, A.E. Circulating biomarkers in heart failure. Adv. Exp. Med. Biol. 2018, 1067, 89–108. [Google Scholar]
- Ovchinnikova, E.S.; Schmitter, D.; Vegter, E.L.; Ter Maaten, J.M.; Valente, M.A.; Liu, L.C.; van der Harst, P.; Pinto, Y.M.; de Boer, R.A.; Meyer, S.; et al. Signature of circulating microRNAs in patients with acute heart failure. Eur. J. Heart Fail. 2016, 18, 414–423. [Google Scholar] [CrossRef]
- Watson, C.J.; Gupta, S.K.; O’Connell, E.; Thum, S.; Glezeva, N.; Fendrich, J.; Gallagher, J.; Ledwidge, M.; Grote-Levi, L.; McDonald, K.; et al. MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure. Eur. J. Heart Fail. 2015, 17, 405–415. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef]
- Miyamoto, S.D.; Karimpour-Fard, A.; Peterson, V.; Auerbach, S.R.; Stenmark, K.R.; Stauffer, B.L.; Sucharov, C.C. Circulating microRNA as a biomarker for recovery in pediatric dilated cardiomyopathy. J. Heart Lung Transpl. 2015, 34, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B.; et al. Inflammatory markers and onset of cardiovascular events: Results from the Health ABC study. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Gaggin, H.K.; Januzzi, J.L., Jr. Biomarkers and diagnostics in heart failure. Biochim. Biophys. Acta 2013, 1832, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Stahrenberg, R.; Edelmann, F.; Mende, M.; Kockskamper, A.; Dungen, H.D.; Luers, C.; Binder, L.; Herrmann-Lingen, C.; Gelbrich, G.; Hasenfuss, G.; et al. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur. J. Heart Fail. 2010, 12, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.L.; Tan, K.C.B.; Au, P.C.M.; Li, G.H.Y.; Cheung, B.M.Y. Evaluation of GDF15 as a therapeutic target of cardiometabolic diseases in human: A Mendelian randomization study. EBioMedicine 2019, 41, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Zhang, X.; Zhang, J.; Zhou, L.; Zhou, T.T.; Zhang, J.W. Diagnostic value of growth differentiation factor-15 and beta2-microglobulin in children with congenital heart disease combined with chronic heart failure and its relationship with cardiac function. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8096–8103. [Google Scholar]
- Berezin, A.E.; Kremzer, A.A.; Berezina, T.A.; Martovitskaya, Y.V. Pattern of circulating microparticles in chronic heart failure patients with metabolic syndrome: Relevance to neurohumoral and inflammatory activation. BBA Clin. 2015, 4, 69–75. [Google Scholar] [CrossRef][Green Version]
- Tan, K.; Sethi, S.K. Biomarkers in cardiorenal syndromes. Transl. Res. 2014, 164, 122–134. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Mori, K.; Li, J.Y.; Kalandadze, A.; Cohen, D.J.; Devarajan, P.; Barasch, J. Dual action of neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2007, 18, 407–413. [Google Scholar] [CrossRef]
- Aghel, A.; Shrestha, K.; Mullens, W.; Borowski, A.; Tang, W.H. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J. Card. Fail. 2010, 16, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Masson, S.; Hillege, H.L.; Maggioni, A.P.; Voors, A.A.; Opasich, C.; van Veldhuisen, D.J.; Montagna, L.; Cosmi, F.; Tognoni, G.; et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur. Heart J. 2011, 32, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S.; Mueller, C.; Fitzgerald, R.; Brikhan, R.; Hiestand, B.C.; Iqbal, N.; Clopton, P.; van Veldhuisen, D.J. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: The NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur. J. Heart Fail. 2011, 13, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Tawfeek, M.S.; Raafat, D.M.; Saad, K.; Idriss, N.K.; Sayed, S.; Fouad, D.A.; El-Houfey, A.A. Plasma levels of neutrophil gelatinase-associated lipocalin in children with heart failure. Ther. Adv. Cardiovasc. Dis. 2016, 10, 30–36. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Kidney injury molecule-1. Curr. Opin. Crit. Care 2010, 16, 556–561. [Google Scholar] [CrossRef]
- Ichimura, T.; Asseldonk, E.J.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef]
- Park, M.; Vittinghoff, E.; Liu, K.D.; Shlipak, M.G.; Hsu, C.Y. Urine biomarkers neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) have different patterns in heart failure exacerbation. Biomark. Insights 2013, 8, 15–18. [Google Scholar] [CrossRef]
- Jungbauer, C.G.; Birner, C.; Jung, B.; Buchner, S.; Lubnow, M.; von Bary, C.; Endemann, D.; Banas, B.; Mack, M.; Boger, C.A.; et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur. J. Heart Fail. 2011, 13, 1104–1110. [Google Scholar] [CrossRef]
- Baek, H.S.; Lee, Y.; Jang, H.M.; Cho, J.; Hyun, M.C.; Kim, Y.H.; Hwang, S.K.; Cho, M.H. Variation in clinical usefulness of biomarkers of acute kidney injury in young children undergoing cardiac surgery. Clin. Exp. Pediatr. 2020, 63, 151–156. [Google Scholar] [CrossRef]
- Chatterjee, N.A.; Singh, J.P. Novel interventional therapies to modulate the autonomic tone in heart failure. JACC Heart Fail. 2015, 3, 786–802. [Google Scholar] [CrossRef]
- Kitamura, K.; Sakata, J.; Kangawa, K.; Kojima, M.; Matsuo, H.; Eto, T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem. Biophys. Res. Commun. 1993, 194, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Cheung, B.M.; Leung, R.; Wang, Q.; Lai, W.H.; Lau, C.P. Increase in plasma adrenomedullin in patients with heart failure characterised by diastolic dysfunction. Heart 2001, 86, 155–160. [Google Scholar] [PubMed]
- Caruhel, P.; Mazier, C.; Kunde, J.; Morgenthaler, N.G.; Darbouret, B. Homogeneous time-resolved fluoroimmunoassay for the measurement of midregional proadrenomedullin in plasma on the fully automated system BRAHMS KRYPTOR. Clin. Biochem. 2009, 42, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Hala, A.; Amal, A.M. Study of adrenomedullin in children with heart failure. Alex. J. Pediatr. 2005, 19, 17. [Google Scholar]
- Neuhold, S.; Huelsmann, M.; Strunk, G.; Stoiser, B.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Moertl, D.; Berger, R.; Pacher, R. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: Prediction of death at different stages of the disease. J. Am. Coll. Cardiol. 2008, 52, 266–272. [Google Scholar] [CrossRef]
- Karki, K.B.; Towbin, J.A.; Philip, R.R.; Harrell, C.; Tadphale, S.; Shah, S.; Saini, A. Copeptin: A novel biomarker in pediatric heart failure due to cardiomyopathies. Circulation 2019, 140, A11217. [Google Scholar]
- Elhewala, A.A.; Sanad, M.; Soliman, A.M.; Sami, M.M.; Ahmed, A.A. Matrix metalloproteinase-9 in pediatric rheumatic heart disease with and without heart failure. Biomed. Rep. 2021, 14, 4. [Google Scholar] [CrossRef]
- Radovanovic, S.; Krotin, M.; Simic, D.V.; Mimic-Oka, J.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Ninkovic, N.; Ivanovic, B.; Simic, T. Markers of oxidative damage in chronic heart failure: Role in disease progression. Redox Rep. 2008, 13, 109–116. [Google Scholar] [CrossRef][Green Version]
- Grassi, D.; Ferri, L.; Desideri, G.; Di Giosia, P.; Cheli, P.; Del Pinto, R.; Properzi, G.; Ferri, C. Chronic hyperuricemia, uric acid deposit and cardiovascular risk. Curr. Pharm. Des. 2013, 19, 2432–2438. [Google Scholar] [CrossRef]
- Bielli, P.; Calabrese, L. Structure to function relationships in ceruloplasmin: A ‘moonlighting’ protein. Cell Mol. Life Sci. 2002, 59, 1413–1427. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, H.; Zhou, Y.; Cheng, G.; Xu, G. Ceruloplasmin and the extent of heart failure in ischemic and nonischemic cardiomyopathy patients. Mediat. Inflamm. 2013, 2013, 348145. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Tong, W.; Troughton, R.W.; Martin, M.G.; Shrestha, K.; Borowski, A.; Jasper, S.; Hazen, S.L.; Klein, A.L. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J. Am. Coll. Cardiol. 2007, 49, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.L.; Pathik, B.; Loke, I.W.; Squire, I.B.; Davies, J.E. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am. Heart J. 2006, 152, 94–101. [Google Scholar] [CrossRef]
- El-Alameey, I.R.; Mahmoud, R.A.; Kairy, S.A.; Medany, E.A. Significance of myeloperoxidase in the onset of cardiovascular disease among obese children and adolescents. Biomed. Pharmacol. J. 2019, 12, 1647–1659. [Google Scholar] [CrossRef]
- de Boer, R.A.; Lok, D.J.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef]
- Ng, L.L.; Geeranavar, S.; Jennings, S.C.; Loke, I.; O’Brien, R.J. Diagnosis of heart failure using urinary natriuretic peptides. Clin. Sci. 2004, 106, 129–133. [Google Scholar] [CrossRef]
- Ouwerkerk, W.; Zwinderman, A.H.; Ng, L.L.; Demissei, B.; Hillege, H.L.; Zannad, F.; van Veldhuisen, D.J.; Samani, N.J.; Ponikowski, P.; Metra, M.; et al. Biomarker-guided versus guideline-based treatment of patients with heart failure: Results from BIOSTAT-CHF. J. Am. Coll. Cardiol. 2018, 71, 386–398. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; French, F. Pharmacogenomics of heart failure. Methods Mol. Biol. 2014, 1175, 245–257. [Google Scholar]
- Yogasundaram, H.; Alhumaid, W.; Dzwiniel, T.; Christian, S.; Oudit, G.Y. Cardiomyopathies and genetic testing in heart failure: Role in defining phenotype-targeted approaches and management. Can. J. Cardiol. 2021, 37, 547–559. [Google Scholar] [CrossRef] [PubMed]

| Myocardial Stretch | Myocyte Injury | Myocardial Remodeling | Inflammation | Renal Dysfunction | Neurohumoral Activation | Oxidative Stress |
|---|---|---|---|---|---|---|
| BNP 1 | CTn 5 (TnI 6, TnT 7) | galectin-3 | GDF-15 12 | NGAL 19 | MR-proADM 24 | ceruloplasmin |
| NT-proBNP 2 | hs-cTn 8 | sST2 11 | EMPs 13 | KIM-1 20 | copeptin | MPO 26 |
| ANP 3 | H-FABPs 9 | microRNAs | EPCs 14 | cystatin C | MMPs 25 | SUA 27 |
| MR-proANP 4 | GSTP1 10 | CRP 15 | IL-18 21 | vitamin D3 | ||
| hs-CRP 16 | L-FABP 22 | 8-hydroxy-2-0-deoxyguanosine | ||||
| TNF-α 17 | NAG 23 | |||||
| IL-6 18 | β-2 microglobulin | |||||
| glutathione-S-transferase |
| Biomarker | Adult Population | Pediatric Population |
|---|---|---|
| BNP 1 | <35 ng/L | |
| NT-proBNP 2 | <125 ng/L | <3569 ng/L (0–1 Y 11) <178 ng/L (1–19 Y) |
| MR-proANP 3 | <40 pmol/L | |
| HsTnT 4 | <14 ng/L | <78 ng/L (0–6 M 12) <34 ng/L (6 M–1 Y) <6 ng/L (1–19 Y) |
| HsTnI 5 | <6 ng/L | <93.8 ng/L (<1 M) <52.1 ng/L (1–12 M) <48.1 ng/L (1–12 Y) <3.9 ng/L (13–18 Y) |
| H-FABPs 6 | <19 ng/mL | |
| Galectin-3 | <22.1 ng/mL | <33 ng/mL |
| sST2 7 | <49.3 ng/mL (male) <33.5 ng/mL (female) | <50 ng/mL |
| GDF-15 8 | <584 pg/mL | |
| NGAL 9 | <50 ng/mL | |
| MR-proADM 10 | <0.55 nmol/L | |
| Copeptin | <11.25 pmol/L | <13.1 pmol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senekovič Kojc, T.; Marčun Varda, N. Novel Biomarkers of Heart Failure in Pediatrics. Children 2022, 9, 740. https://doi.org/10.3390/children9050740
Senekovič Kojc T, Marčun Varda N. Novel Biomarkers of Heart Failure in Pediatrics. Children. 2022; 9(5):740. https://doi.org/10.3390/children9050740
Chicago/Turabian StyleSenekovič Kojc, Teja, and Nataša Marčun Varda. 2022. "Novel Biomarkers of Heart Failure in Pediatrics" Children 9, no. 5: 740. https://doi.org/10.3390/children9050740
APA StyleSenekovič Kojc, T., & Marčun Varda, N. (2022). Novel Biomarkers of Heart Failure in Pediatrics. Children, 9(5), 740. https://doi.org/10.3390/children9050740