What Is New on Paediatric Echocardiography for the Diagnosis, Management and Follow-Up of the Multisystem Inflammatory Syndrome Associated with COVID-19?
Abstract
:1. Introduction
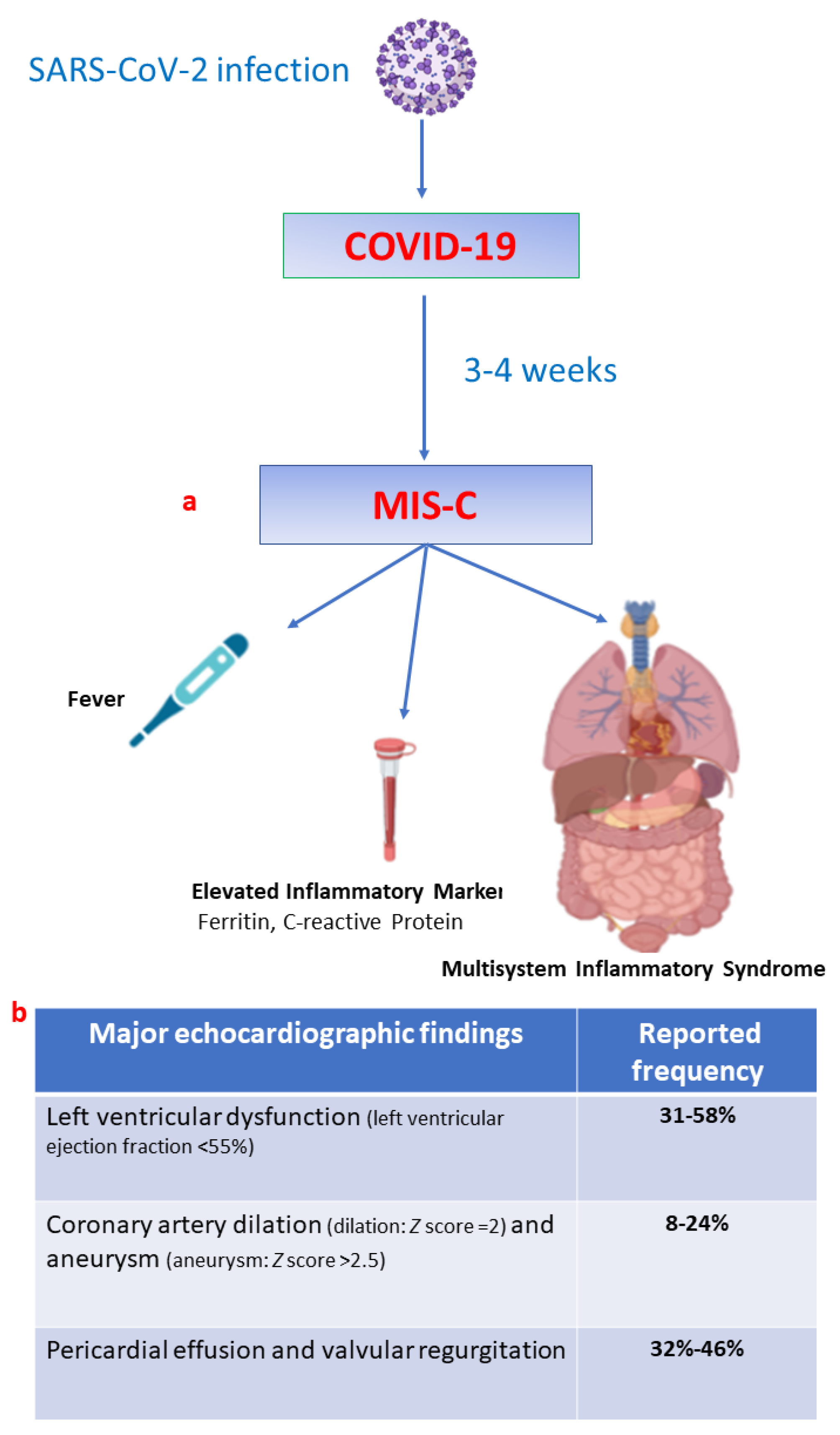
2. MIS-C
3. Conventional Echocardiography for the Diagnosis, Management and Follow-Up of MIS-C
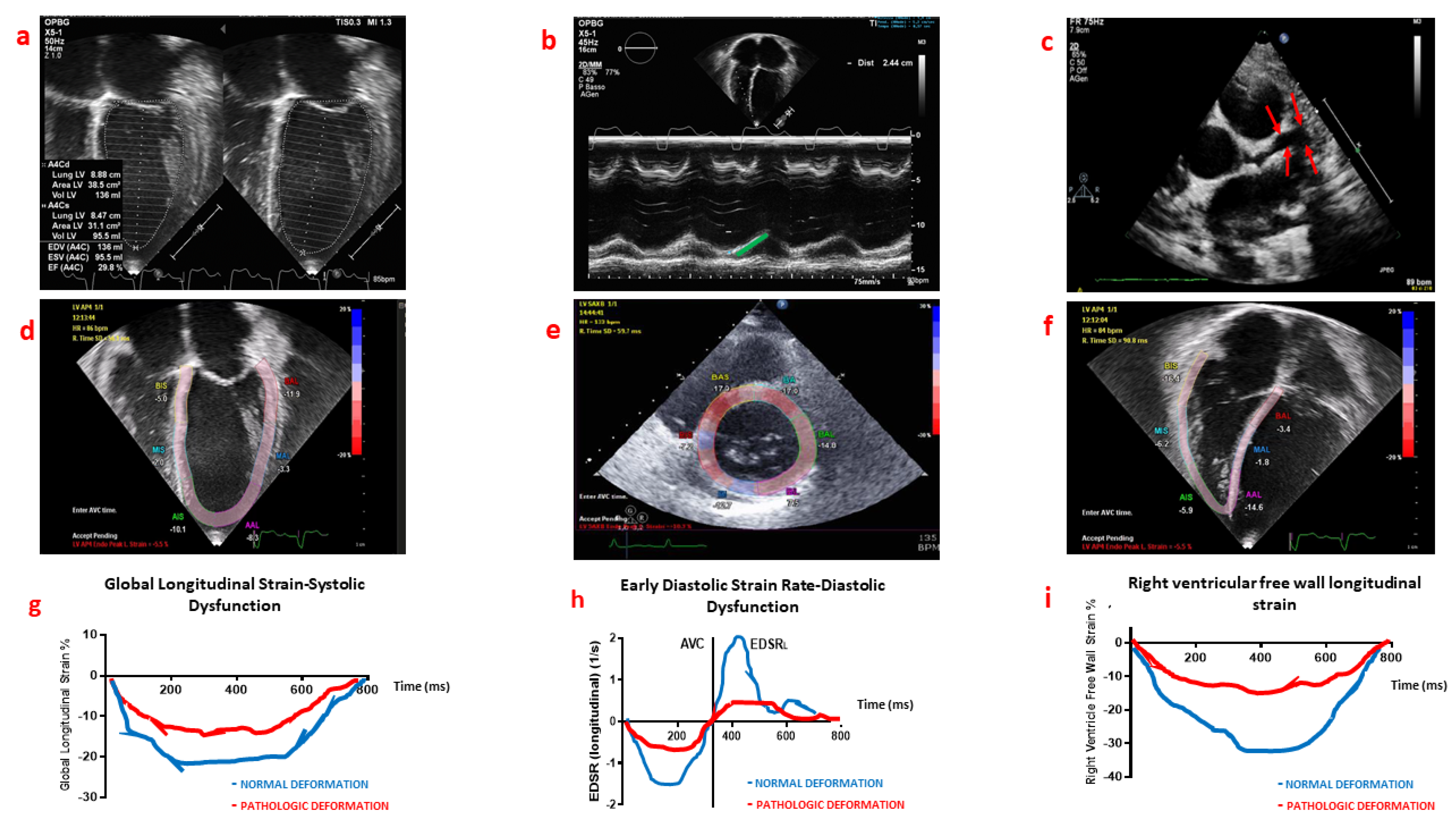
4. Myocardial Deformation Analysis
5. MIS-C Follow-Up
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Di Nardo, M.; van Leeuwen, G.; Loreti, A.; Barbieri, M.A.; Guner, Y.; Locatelli, F.; Ranieri, V.M. A literature review of 2019 novel coronavirus (SARS-CoV2) infection in neonates and children. Pediatr. Res. 2020, 89, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19—Associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Di Nardo, M.; Hoskote, A.; Thiruchelvam, T.; Lillie, J.; Horan, M.; Belda Hofheinz, S.; Dupic, L.; Gimeno, R.; de Piero, M.E.; Lo Coco, V.; et al. Extracorporeal Membrane Oxygenation in Children with Coronavirus Disease 2019: Preliminary Report from the Collaborative European Chapter of the Extracorporeal Life Support Organization Prospective Survey. Asaio J. 2021, 67, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Valentini, P.; Sodero, G.; Buonsenso, D. The Relationship between COVID-19 and Innate Immunity in Children: A Review. Children 2021, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Tissières, P.; Teboul, J.L. SARS-CoV-2 post-infective myocarditis: The tip of COVID-19 immune complications? Ann. Intensiv. Care 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, T.; Tremoulet, A.H.; Burns, J.C.; Saidi, A.; Dionne, A.; Lang, S.M.; Newburger, J.W.; de Ferranti, S.; Friedman, K.G. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation 2021, 143, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, D.; Kauffman, H.L.; Wang, Y.; Calderon-Anyosa, R.; Nadaraj, S.; Elias, M.D.; White, T.J.; Torowicz, D.L.; Yubbu, P.; Giglia, T.M.; et al. Echocardiographic Findings in Pediatric Multisystem Inflammatory Syndrome Associated With COVID-19 in the United States. J. Am. Coll. Cardiol. 2020, 76, 1947–1961. [Google Scholar] [CrossRef] [PubMed]
- Chinali, M.; Franceschini, A.; Ciancarella, P.; Lisignoli, V.; Curione, D.; Ciliberti, P.; Esposito, C.; Del Pasqua, A.; Rinelli, G.; Secinaro, A. Echocardiographic two-dimensional speckle tracking identifies acute regional myocardial edema and sub-acute fibrosis in pediatric focal myocarditis with normal ejection fraction: Comparison with cardiac magnetic resonance. Sci. Rep. 2020, 10, 11321. [Google Scholar] [CrossRef] [PubMed]
- Sirico, D.; Basso, A.; Reffo, E.; Cavaliere, A.; Castaldi, B.; Sabatino, J.; Meneghel, A.; Martini, G.; Da Dalt, L.; Zulian, F.; et al. Early Echocardiographic and Cardiac MRI Findings in Multisystem Inflammatory Syndrome in Children. J. Clin. Med. 2021, 10, 3360. [Google Scholar] [CrossRef] [PubMed]
- Schranz, D.; Voelkel, N.F. “Nihilism” of chronic heart failure therapy in children and why effective therapy is withheld. Eur. J. Pediatr. 2016, 175, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Blondiaux, E.; Parisot, P.; Redheuil, A.; Tzaroukian, L.; Levy, Y.; Sileo, C.; Schnuriger, A.; Lorrot, M.; Guedj, R.; Ducou le Pointe, H. Cardiac MRI in Children with Multisystem Inflammatory Syndrome Associated with COVID-19. Radiology 2020, 297, E283–E288. [Google Scholar] [CrossRef] [PubMed]
- Sanil, Y.; Misra, A.; Safa, R.; Blake, J.M.; Eddine, A.C.; Balakrishnan, P.; Garcia, R.U.; Taylor, R.; Dentel, J.N.; Ang, J.; et al. Echocardiographic Indicators Associated with Adverse Clinical Course and Cardiac Sequelae in Multisystem Inflammatory Syndrome in Children with COVID-19. J. Am. Soc. Echocardiogr. 2021, 34, 862–876. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nardo, M.; Franceschini, A.; Tissieres, P.; Chinali, M. What Is New on Paediatric Echocardiography for the Diagnosis, Management and Follow-Up of the Multisystem Inflammatory Syndrome Associated with COVID-19? Children 2022, 9, 146. https://doi.org/10.3390/children9020146
Di Nardo M, Franceschini A, Tissieres P, Chinali M. What Is New on Paediatric Echocardiography for the Diagnosis, Management and Follow-Up of the Multisystem Inflammatory Syndrome Associated with COVID-19? Children. 2022; 9(2):146. https://doi.org/10.3390/children9020146
Chicago/Turabian StyleDi Nardo, Matteo, Alessio Franceschini, Pierre Tissieres, and Marcello Chinali. 2022. "What Is New on Paediatric Echocardiography for the Diagnosis, Management and Follow-Up of the Multisystem Inflammatory Syndrome Associated with COVID-19?" Children 9, no. 2: 146. https://doi.org/10.3390/children9020146
APA StyleDi Nardo, M., Franceschini, A., Tissieres, P., & Chinali, M. (2022). What Is New on Paediatric Echocardiography for the Diagnosis, Management and Follow-Up of the Multisystem Inflammatory Syndrome Associated with COVID-19? Children, 9(2), 146. https://doi.org/10.3390/children9020146





