The Impact of Preterm Birth on Sleep through Infancy, Childhood and Adolescence and Its Implications
Abstract
1. Introduction
2. Sleep during the Neonatal Period
2.1. Sleep Ontogeny during the Neonatal Period
2.2. Methodological Approaches to Assessing Sleep in Preterm Neonates
2.3. Impact of NICU Environment on Sleep in Preterm Infants
2.4. Summary: Sleep during the Neonatal Period
3. Sleep during Childhood and Adolescence
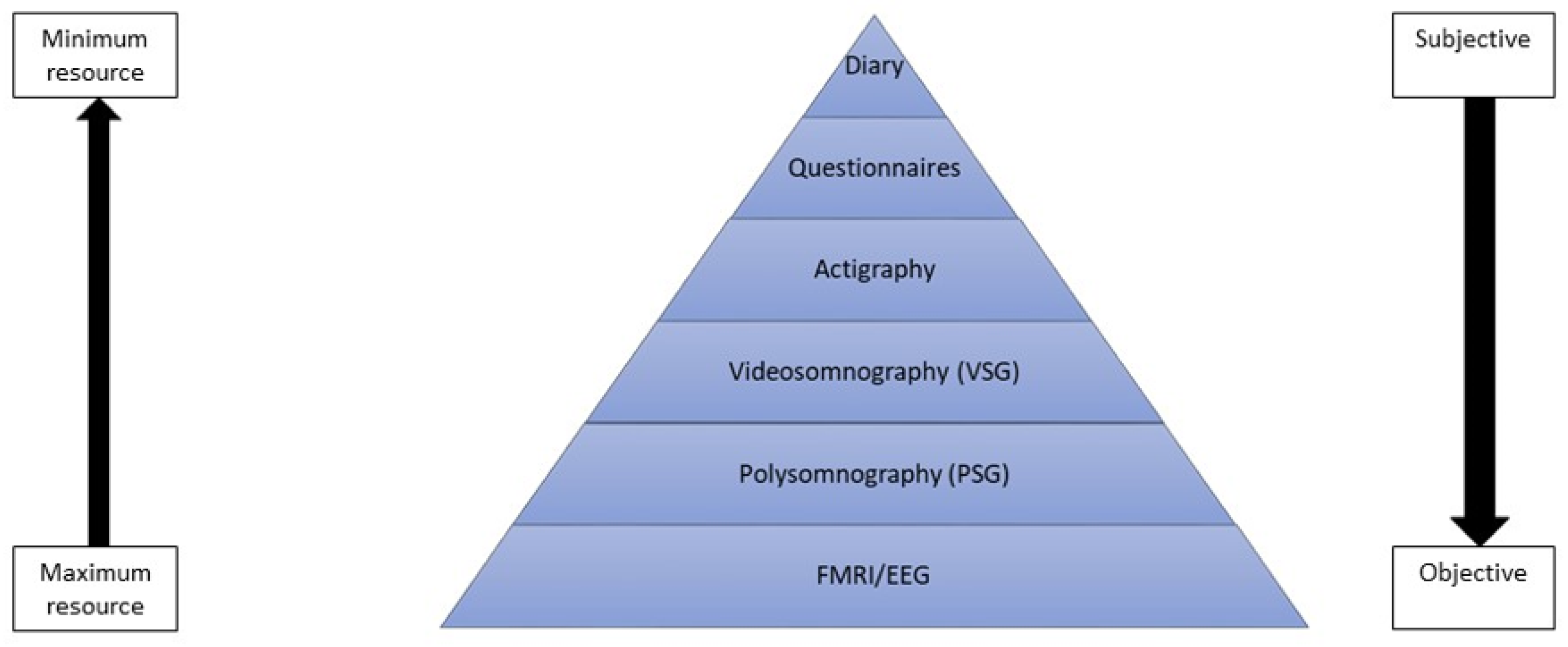
3.1. Assessing Sleep and Its Disorders in Childhood and Adolescence
3.1.1. Objective Measures of Sleep
3.1.2. Subjective Measures of Sleep
3.2. Sleep in Childhood and Adolescence following Preterm Birth
3.2.1. Sleep during Infancy and the Pre-School Period
3.2.2. Sleep during Middle Childhood and Adolescence
3.2.3. Sleep-Disordered Breathing in Childhood following Preterm Birth
3.3. Summary: Sleep and Sleep Disordered Breathing in Children Born Preterm
4. Sleep and Cognitive and Socio-Emotional Problems in Children Born Very Preterm
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Johnson, S.; Marlow, N. Preterm birth and childhood psychiatric disorders. Pediatr. Res. 2011, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Alterman, N.; Johnson, S.; Carson, C.; Petrou, S.; Rivero-Arias, O.; Kurinczuk, J.J.; Macfarlane, A.; Boyle, E.; Quigley, M.A. Gestational age at birth and child special educational needs: A UK representative birth cohort study. Arch. Dis. Child 2021, 106, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Twilhaar, E.S.; de Kieviet, J.F.; Aarnoudse-Moens, C.S.; van Elburg, R.M.; Oosterlaan, J. Academic performance of children born preterm: A meta-analysis and meta-regression. Arch. Dis. Child.-Fetal Neonatal Ed. 2018, 103, F322–F330. [Google Scholar] [CrossRef] [PubMed]
- Kostović, I.; Sedmak, G.; Judaš, M. Neural histology and neurogenesis of the human fetal and infant brain. Neuroimage 2019, 188, 743–773. [Google Scholar] [CrossRef]
- Bonan, K.C.S.d.C.; Pimentel Filho, J.d.C.; Tristão, R.M.; Jesus, J.A.L.d.; Campos Junior, D. Sleep deprivation, pain and prematurity: A review study. Arq. Neuro-Psiquiatr. 2015, 73, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Zores, C.; Langlet, C.; Escande, B.; Astruc, D.; Dufour, A. Moderate acoustic changes can disrupt the sleep of very preterm infants in their incubators. Acta Paediatr. 2013, 102, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, D.Y.; Weiss, M.D. Sleep disturbances in newborns. Children 2017, 4, 90. [Google Scholar] [CrossRef]
- de Vries, J.I.; Visser, G.H.; Prechtl, H.F. Fetal behaviour in early pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1986, 21, 271–276. [Google Scholar] [CrossRef]
- Bourel-Ponchel, E.; Hasaerts, D.; Challamel, M.-J.; Lamblin, M.-D. Behavioral-state development and sleep-state differentiation during early ontogenesis. Neurophysiol. Clin. 2021, 51, 89–98. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.H.; Mulder, E.J.H. Fetal sleep organization: A biological precursor of self-regulation in childhood and adolescence? Biol. Psychol. 2012, 89, 584–590. [Google Scholar] [CrossRef]
- Peirano, P.; Algarín, C.; Uauy, R. Sleep-wake states and their regulatory mechanisms throughout early human development. J. Pediatrics 2003, 143, 70–79. [Google Scholar] [CrossRef]
- Bennet, L.; Walker, D.W.; Horne, R.S. Waking up too early–the consequences of preterm birth on sleep development. J. Physiol. 2018, 596, 5687–5708. [Google Scholar] [CrossRef] [PubMed]
- de Groot, E.R.; Knoop, M.S.; van den Hoogen, A.; Wang, X.; Long, X.; Pillen, S.; Benders, M.; Dudink, J. The value of cardiorespiratory parameters for sleep state classification in preterm infants: A systematic review. Sleep Med. Rev 2021, 58, 101462. [Google Scholar] [CrossRef] [PubMed]
- Vecchierini, M.-F.; d’Allest, A.-M.; Verpillat, P. EEG patterns in 10 extreme premature neonates with normal neurological outcome: Qualitative and quantitative data. Brain Dev. 2003, 25, 330–337. [Google Scholar] [CrossRef]
- Bourel-Ponchel, E.; Gueden, S.; Hasaerts, D.; Héberlé, C.; Malfilâtre, G.; Mony, L.; Vignolo-Diard, P.; Lamblin, M.-D. Normal EEG during the neonatal period: Maturational aspects from premature to full-term newborns. Neurophysiol. Clin. 2021, 51, 61–88. [Google Scholar] [CrossRef]
- Scher, M.S.; Johnson, M.W.; Holditch-Davis, D. Cyclicity of neonatal sleep behaviors at 25 to 30 weeks’ postconceptional age. Pediatric Res. 2005, 57, 879–882. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. The AASM manual for the scoring of sleep and associated events. In Rules, Terminology and Technical Specifications; Anders, T., Emde, R., Parmelee, A.H., Eds.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007; Volume 37. [Google Scholar]
- Cailleau, L.; Weber, R.; Cabon, S.; Flamant, C.; Roué, J.-M.; Favrais, G.; Gascoin, G.; Thollot, A.; Esvan, M.; Porée, F. Quiet sleep organization of very preterm infants is correlated with postnatal maturation. Front. Pediatrics 2020, 8, 559658. [Google Scholar] [CrossRef]
- Klebermass, K.; Olischar, M.; Waldhoer, T.; Fuiko, R.; Pollak, A.; Weninger, M. Amplitude-integrated EEG pattern predicts further outcome in preterm infants. Pediatric Res. 2011, 70, 102–108. [Google Scholar] [CrossRef]
- Arditi-Babchuk, H.; Feldman, R.; Eidelman, A.I. Rapid eye movement (REM) in premature neonates and developmental outcome at 6 months. Infant Behav. Dev. 2009, 32, 27–32. [Google Scholar] [CrossRef]
- Gertner, S.; Greenbaum, C.W.; Sadeh, A.; Dolfin, Z.; Sirota, L.; Ben-Nun, Y. Sleep–wake patterns in preterm infants and 6 month’s home environment: Implications for early cognitive development. Early Hum. Dev. 2002, 68, 93–102. [Google Scholar] [CrossRef]
- Derbin, M.; McKenna, L.; Chin, D.; Coffman, B.; Bloch-Salisbury, E. Actigraphy: Metrics reveal it is not a valid tool for determining sleep in neonates. J. Sleep Res. 2022, 31, e13444. [Google Scholar] [CrossRef] [PubMed]
- Joosten, K.; de Goederen, R.; Pijpers, A.; Allegaert, K. Sleep related breathing disorders and indications for polysomnography in preterm infants. Early Hum. Dev. 2017, 113, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Dereymaeker, A.; Pillay, K.; Vervisch, J.; De Vos, M.; Van Huffel, S.; Jansen, K.; Naulaers, G. Review of sleep-EEG in preterm and term neonates. Early Hum. Dev. 2017, 113, 87–103. [Google Scholar] [CrossRef] [PubMed]
- de Groot, E.R.; Bik, A.; Sam, C.; Wang, X.; Shellhaas, R.A.; Austin, T.; Tataranno, M.L.; Benders, M.J.N.L.; van den Hoogen, A.; Dudink, J. Creating an optimal observational sleep stage classification system for very and extremely preterm infants. Sleep Med. Rev. 2022, 90, 167–175. [Google Scholar] [CrossRef]
- Werth, J.; Atallah, L.; Andriessen, P.; Long, X.; Zwartkruis-Pelgrim, E.; Aarts, R.M. Unobtrusive sleep state measurements in preterm infants–A review. Sleep Med. Rev. 2017, 32, 109–122. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, S.H.; Na, J.Y.; Lim, Y.-H.; Cho, S.H.; Cho, S.H.; Park, H.-K. Non-contact sleep/wake monitoring using impulse-radio ultrawideband radar in neonates. Front. Pediatrics 2021, 9, 782623. [Google Scholar] [CrossRef]
- Paul, M.; Karthik, S.; Joseph, J.; Sivaprakasam, M.; Kumutha, J.; Leonhardt, S.; Antink, C.H. Non-contact sensing of neonatal pulse rate using camera-based imaging: A clinical feasibility study. Physiol. Meas 2020, 41, 024001. [Google Scholar] [CrossRef]
- Bremmer, P.; Byers, J.F.; Kiehl, E. Noise and the premature infant: Physiological effects and practice implications. J. Obstet. Gynecol. Neonatal Nurs. 2003, 32, 447–454. [Google Scholar] [CrossRef]
- Axelin, A.; Kirjavainen, J.; Salanterä, S.; Lehtonen, L. Effects of pain management on sleep in preterm infants. Eur. J. Pain 2010, 14, 752–758. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Hodgman, J.E.; Rybine, D.; Fabrikant, G.; Corwin, M.; Crowell, D.; Weese-Mayer, D.E.; Group, C.S. Sleep architecture in term and preterm infants beyond the neonatal period: The influence of gestational age, steroids, and ventilatory support. Sleep 2005, 28, 1428–1436. [Google Scholar] [CrossRef]
- Galland, B.C.; Short, M.A.; Terrill, P.; Rigney, G.; Haszard, J.J.; Coussens, S.; Foster-Owens, M.; Biggs, S.N. Establishing normal values for pediatric nighttime sleep measured by actigraphy: A systematic review and meta-analysis. Sleep 2018, 41, zsy017. [Google Scholar] [CrossRef] [PubMed]
- Acebo, C.; Sadeh, A.; Seifer, R.; Tzischinsky, O.; Wolfson, A.R.; Hafer, A.; Carskadon, M.A. Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep 1999, 22, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, L.J.; Montgomery-Downs, H.E.; Insana, S.P.; Walsh, C.M. Use of actigraphy for assessment in pediatric sleep research. Sleep Med. Rev. 2012, 16, 463–475. [Google Scholar] [CrossRef]
- Becker, S.P.; Sidol, C.A.; Van Dyk, T.R.; Epstein, J.N.; Beebe, D.W. Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: A systematic review. Sleep Med. Rev. 2017, 34, 94–121. [Google Scholar] [CrossRef] [PubMed]
- Halal, C.S.; Bassani, D.G.; Santos, I.S.; Tovo-Rodrigues, L.; Del-Ponte, B.; Silveira, M.F.; Bertoldi, A.D.; Barros, F.C.; Nunes, M.L. Maternal perinatal depression and infant sleep problems at 1 year of age: Subjective and actigraphy data from a population-based birth cohort study. J. Sleep Res. 2021, 30, e13047. [Google Scholar] [CrossRef]
- Kaditis, A.G.; Alvarez, M.L.A.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H. Obstructive sleep disordered breathing in 2-to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2016, 47, 69–94. [Google Scholar] [CrossRef]
- Kaditis, A.G.; Alvarez, M.L.A.; Boudewyns, A.; Abel, F.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I. ERS statement on obstructive sleep disordered breathing in 1-to 23-month-old children. Eur. Respir. J 2017, 50, 1700985. [Google Scholar] [CrossRef]
- Hill, C.M.; Evans, H.J. The investigation of sleep disordered breathing: Seeing through a glass, darkly? Arch. Dis. Child. 2016, 101, 1082–1083. [Google Scholar] [CrossRef][Green Version]
- Hahn, M.; Joechner, A.K.; Roell, J.; Schabus, M.; Heib, D.P.; Gruber, G.; Peigneux, P.; Hoedlmoser, K. Developmental changes of sleep spindles and their impact on sleep-dependent memory consolidation and general cognitive abilities: A longitudinal approach. Dev. Sci. 2019, 22, e12706. [Google Scholar] [CrossRef]
- Russo, K.; Greenhill, J.; Burgess, S. Home (Level 2) polysomnography is feasible in children with suspected sleep disorders. Sleep Med. Rev. 2021, 88, 157–161. [Google Scholar] [CrossRef]
- Sen, T.; Spruyt, K. Pediatric sleep tools: An updated literature review. Front. Psychiatry 2020, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Spruyt, K.; Gozal, D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: A review of currently available instruments. Sleep Med. Rev. 2011, 15, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep N. Y. 2000, 23, 1043–1052. [Google Scholar] [CrossRef]
- Bruni, O.; Ottaviano, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sleep Disturbance Scale for Children (SDSC) Construct ion and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J. Sleep Res. 1996, 5, 251–261. [Google Scholar] [CrossRef]
- Biggs, S.; Kennedy, J.D.; Martin, A.J.; Van den Heuvel, C.; Lushington, K. Psychometric properties of an omnibus sleep problems questionnaire for school-aged children. Sleep Med. Rev. 2012, 13, 390–395. [Google Scholar] [CrossRef]
- Sadeh, A. A brief screening questionnaire for infant sleep problems: Validation and findings for an Internet sample. Pediatrics 2004, 113, e570–e577. [Google Scholar] [CrossRef]
- Mindell, J.A.; Gould, R.A.; Tikotzy, L.; Leichman, E.S.; Walters, R.M. Norm-referenced scoring system for the Brief Infant Sleep Questionnaire–Revised (BISQ-R). Sleep Med. Rev. 2019, 63, 106–114. [Google Scholar] [CrossRef]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. Rev. 2000, 1, 21–32. [Google Scholar] [CrossRef]
- Smith, D.L.; Gozal, D.; Hunter, S.J.; Kheirandish-Gozal, L. Frequency of snoring, rather than apnea–hypopnea index, predicts both cognitive and behavioral problems in young children. Sleep Med. Rev. 2017, 34, 170–178. [Google Scholar] [CrossRef]
- Akkus, P.Z.; Bahtiyar-Saygan, B. Do preterm babies sleep differently than their peers? Sleep characteristics and their associations with maternal depression and parenting stress. Sleep Med. Rev. 2022, 90, 109–116. [Google Scholar] [CrossRef]
- Asaka, Y.; Takada, S. Activity-based assessment of the sleep behaviors of VLBW preterm infants and full-term infants at around 12 months of age. Brain Dev. 2010, 32, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Asaka, Y.; Takada, S. Relation between sleep status of preterm infants aged 1–2 years and mothers’ parenting stress. Pediatrics Int. 2013, 55, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.S.; Humphreys, J.S.; Gringras, P.; Taheri, S.; Scott, N.; Emond, A.; Henderson, J.; Fleming, P.J. Childhood sleep duration and associated demographic characteristics in an English cohort. Sleep 2012, 35, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Cam, S.; Ovali, F. Impact of sleep behaviors on social and emotional problems in three-year-old children born prematurely. Sleep Med. Rev. 2020, 74, 173–178. [Google Scholar] [CrossRef]
- Caravale, B.; Sette, S.; Cannoni, E.; Marano, A.; Riolo, E.; Devescovi, A.; De Curtis, M.; Bruni, O. Sleep characteristics and temperament in preterm children at two years of age. J. Clin. Sleep Med. 2017, 13, 1081–1088. [Google Scholar] [CrossRef]
- Gössel-Symank, R.; Grimmer, I.; Korte, J.; Siegmund, R. Actigraphic monitoring of the activity-rest behavior of preterm and full-term infants at 20 months of age. Chronobiol. Int. 2004, 21, 661–671. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Paiva, T.; Hsu, J.-F.; Kuo, M.-C.; Guilleminault, C. Sleep and breathing in premature infants at 6 months post-natal age. BMC Pediatrics 2014, 14, 1–6. [Google Scholar] [CrossRef]
- Lupini, F.; Leichman, E.S.; Lee, C.; Mindell, J.A. Sleep patterns, problems, and ecology in young children born preterm and full-term and their mothers. Sleep Med. Rev. 2021, 81, 443–450. [Google Scholar] [CrossRef]
- Shimada, M.; Segawa, M.; Higurashi, M.; Akamatsu, H. Development of the sleep and wakefulness rhythm in preterm infants discharged from a neonatal care unit. Pediatric Res. 1993, 33, 159–163. [Google Scholar] [CrossRef]
- Wolke, D.; Söhne, B.; Riegel, K.; Ohrt, B.; Österlund, K. An epidemiologic longitudinal study of sleeping problems and feeding experience of preterm and term children in southern Finland: Comparison with a southern German population sample. J. Pediatrics 1998, 133, 224–231. [Google Scholar] [CrossRef]
- Romeo, D.M.; Leo, G.; Lapenta, L.; Leone, D.; Turrini, I.; Brogna, C.; Gallini, F.; Cota, F.; Vento, G.; Mercuri, E. Sleep disorders in low-risk preschool very preterm children. Sleep Med. Rev. 2019, 63, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Durankus, F.; Ciftdemir, N.A.; Ozbek, U.V.; Duran, R.; Acunas, B. Comparison of sleep problems between term and preterm born preschool children. Sleep Med. Rev. 2020, 75, 484–490. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, A.; Nixon, E. “Weathering the storm:” Mothers’ and fathers’ experiences of parenting a preterm infant. Infant Ment. Health J. 2019, 40, 573–587. [Google Scholar] [CrossRef]
- Visser, S.S.; van Diemen, W.J.; Kervezee, L.; van den Hoogen, A.; Verschuren, O.; Pillen, S.; Benders, M.J.; Dudink, J. The relationship between preterm birth and sleep in children at school age: A systematic review. Sleep Med. Rev. 2021, 57, 101447. [Google Scholar] [CrossRef]
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.A.; Kotagal, S.; Lloyd, R.M.; Malow, B.A.; Maski, K.; Nichols, C.; Quan, S.F. Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2016, 12, 785–786. [Google Scholar] [CrossRef]
- Trickett, J.; Bernardi, M.; Fahy, A.; Lancaster, R.; Larsen, J.; Ni, Y.; Suonpera, E.; Wolke, D.; Marlow, N.; Johnson, S. Disturbed sleep in children born extremely preterm is associated with behavioural and emotional symptoms. Sleep Med. Rev. 2021, 85, 157–165. [Google Scholar] [CrossRef]
- Brockmann, P.E.; Poggi, H.; Martinez, A.; D’Apremont, I.; Moore, R.; Smith, D.; Gozal, D. Perinatal antecedents of sleep disturbances in schoolchildren. Sleep 2020, 43, zsaa021. [Google Scholar] [CrossRef]
- Chan, M.; Wong, T.C.; Weichard, A.; Nixon, G.M.; Walter, L.M.; Horne, R.S. Sleep macro-architecture and micro-architecture in children born preterm with sleep disordered breathing. Pediatric Res. 2020, 87, 703–710. [Google Scholar] [CrossRef]
- Trickett, J.; Bernardi, M.; Fahy, A.; Lancaster, R.; Larsen, J.; Ni, Y.; Suonpera, E.; Wolke, D.; Marlow, N.; Johnson, S. Neuropsychological abilities underpinning academic attainment in children born extremely preterm. Child Neuropsychol. 2022, 1–22. [Google Scholar] [CrossRef]
- Stangenes, K.M.; Fevang, S.K.; Grundt, J.; Donkor, H.M.; Markestad, T.; Hysing, M.; Elgen, I.B.; Bjorvatn, B. Children born extremely preterm had different sleeping habits at 11 years of age and more childhood sleep problems than term-born children. Acta Paediatr. 2017, 106, 1966–1972. [Google Scholar] [CrossRef]
- Biggs, S.N.; Meltzer, L.J.; Tapia, I.E.; Traylor, J.; Nixon, G.M.; Horne, R.S.; Doyle, L.W.; Asztalos, E.; Mindell, J.A.; Marcus, C.L. Sleep/wake patterns and parental perceptions of sleep in children born preterm. J. Clin. Sleep Med. 2016, 12, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Hibbs, A.M.; Storfer-Isser, A.; Rosen, C.; Ievers-Landis, C.E.; Taveras, E.M.; Redline, S. Advanced sleep phase in adolescents born preterm. Behav. Sleep Med. 2014, 12, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Randler, C.; Faßl, C.; Kalb, N. From Lark to Owl: Developmental changes in morningness-eveningness from new-borns to early adulthood. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Beckmann, J.; Gandhi, R.; Hurst, J.R.; Morris, J.K.; Marlow, N. Growth to early adulthood following extremely preterm birth: The EPICure study. Arch. Dis. Child.-Fetal Neonatal Ed. 2020, 105, 496–503. [Google Scholar] [CrossRef]
- Crump, C.; Friberg, D.; Li, X.; Sundquist, J.; Sundquist, K. Preterm birth and risk of sleep-disordered breathing from childhood into mid-adulthood. Int. J. Epidemiol. 2019, 48, 2039–2049. [Google Scholar] [CrossRef]
- Rosen, C.L.; Larkin, E.K.; Kirchner, H.L.; Emancipator, J.L.; Bivins, S.F.; Surovec, S.A.; Martin, R.J.; Redline, S. Prevalence and risk factors for sleep-disordered breathing in 8-to 11-year-old children: Association with race and prematurity. J. Pediatrics 2003, 142, 383–389. [Google Scholar] [CrossRef]
- Tapia, I.E.; Shults, J.; Doyle, L.W.; Nixon, G.M.; Cielo, C.M.; Traylor, J.; Marcus, C.L.; Group, C.f.A.o.P.S.S. Perinatal risk factors associated with the obstructive sleep apnea syndrome in school-aged children born preterm. Sleep 2016, 39, 737–742. [Google Scholar] [CrossRef]
- Sadras, I.; Reiter, J.; Fuchs, N.; Erlichman, I.; Gozal, D.; Gileles-Hillel, A. Prematurity as a risk factor of sleep-disordered breathing in children younger than two years: A retrospective case-control study. J. Clin. Sleep Med 2019, 15, 1731–1736. [Google Scholar] [CrossRef]
- Hibbs, A.M.; Johnson, N.L.; Rosen, C.L.; Kirchner, H.L.; Martin, R.; Storfer-Isser, A.; Redline, S. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm. J. Pediatrics 2008, 153, 176–182. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Developmental Follow-Up of Children and Young People Born Preterm: NICE Guideline NG72; National Institute for Health and Care Excellence: London, UK, 2017. [Google Scholar]
- Horne, R.S.; Sun, S.; Yiallourou, S.R.; Fyfe, K.L.; Odoi, A.; Wong, F.Y. Comparison of the longitudinal effects of persistent periodic breathing and apnoea on cerebral oxygenation in term-and preterm-born infants. J. Physiol. 2018, 596, 6021–6031. [Google Scholar] [CrossRef]
- Emancipator, J.L.; Storfer-Isser, A.; Taylor, H.G.; Rosen, C.L.; Kirchner, H.; Johnson, N.L.; Zambito, A.M.; Redline, S. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch. Pediatrics Adolesc. Med. 2006, 160, 203–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamal, M.; Tamana, S.K.; Smithson, L.; Ding, L.; Lau, A.; Chikuma, J.; Mariasine, J.; Lefebvre, D.L.; Subbarao, P.; Becker, A.B. Phenotypes of sleep-disordered breathing symptoms to two years of age based on age of onset and duration of symptoms. Sleep Med. 2018, 48, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Garagozzo, A.; Hunter, S.J. Cognition in pediatric SDB—Yes, no, maybe? Pediatric Pulmonol. 2021. [CrossRef] [PubMed]
- Mietchen, J.J.; Bennett, D.P.; Huff, T.; Hedges, D.W.; Gale, S.D. Executive function in pediatric sleep-disordered breathing: A meta-analysis. J. Int. Neuropsychol. Soc. 2016, 22, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ando, A.; Ohta, H.; Yoshimura, Y.; Nakagawa, M.; Asaka, Y.; Nakazawa, T.; Mitani, Y.; Oishi, Y.; Mizushima, M.; Adachi, H. Sleep maturation influences cognitive development of preterm toddlers. Sci. Rep. 2021, 11, 1–9. [Google Scholar]
- Manacero, S.; Nunes, M.L. Longitudinal study of sleep behavior and motor development in low-birth-weight preterm children from infancy to preschool years. J. Pediatr. 2021, 97, 44–51. [Google Scholar] [CrossRef]
- Anders, T.; Iosif, A.-M.; Schwichtenberg, A.; Tang, K.; Goodlin-Jones, B. Sleep and daytime functioning: A short-term longitudinal study of three preschool-age comparison groups. Am. J. Intellect. Dev. Disabil. 2012, 117, 275–290. [Google Scholar] [CrossRef]
- Poirier, A.; Corkum, P. Night-to-night variability of sleep in children with ADHD and typically developing controls. J. Atten. Disord. 2018, 22, 942–946. [Google Scholar] [CrossRef]
- Langberg, J.M.; Breaux, R.P.; Cusick, C.N.; Green, C.D.; Smith, Z.R.; Molitor, S.J.; Becker, S.P. Intraindividual variability of sleep/wake patterns in adolescents with and without attention-deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 2019, 60, 1219–1229. [Google Scholar] [CrossRef]
- Floros, O.; Axelsson, J.; Almeida, R.; Tigerström, L.; Lekander, M.; Sundelin, T.; Petrovic, P. Vulnerability in executive functions to sleep deprivation is predicted by subclinical attention-deficit/hyperactivity disorder symptoms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 290–298. [Google Scholar] [CrossRef]
- Hagmann-von Arx, P.; Perkinson-Gloor, N.; Brand, S.; Albert, D.; Holsboer-Trachsler, E.; Grob, A.; Weber, P.; Lemola, S. In school-age children who were born very preterm sleep efficiency is associated with cognitive function. Neuropsychobiology 2014, 70, 244–252. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Bayliss, D.M.; Anderson, M.; Campbell, C.; French, N.; McMichael, J.; Reid, C.; Bucks, R.S. The relationship between sleep problems and working memory in children born very preterm. Child. Neuropsychol. 2018, 24, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Möhring, W.; Urfer-Maurer, N.; Brand, S.; Holsboer-Trachsler, E.; Weber, P.; Grob, A.; Lemola, S. The association between sleep and dual-task performance in preterm and full-term children: An exploratory study. Sleep Med. 2019, 55, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, F.M.; Latal, B.; O’Gorman, R.L.; Hagmann, C.F.; Huber, R. Sleep EEG maps the functional neuroanatomy of executive processes in adolescents born very preterm. Cortex 2017, 86, 11–21. [Google Scholar] [CrossRef]
- Stangenes, K.M.; Hysing, M.; Elgen, I.B.; Halvorsen, T.; Markestad, T.; Bjorvatn, B. Sleep problems, behavioural problems and respiratory health in children born extremely preterm: A parental questionnaire study. BMJ Paediatrics Open 2019, 3, e000534. [Google Scholar] [CrossRef]
- Hiscock, H.; Sciberras, E.; Mensah, F.; Gerner, B.; Efron, D.; Khano, S.; Oberklaid, F. Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: Randomised controlled trial. BMJ 2015, 350, h68. [Google Scholar] [CrossRef]
- Clementi, M.A.; Alfano, C.A. An integrated sleep and anxiety intervention for anxious children: A pilot randomized controlled trial. Clin. Child. Psychol. Psychiatry 2020, 25, 945–957. [Google Scholar] [CrossRef]
| Age Range | Sensitivity Range | Specificity Range |
|---|---|---|
| Infants | 83.4–99.3 | 17.0–97.8 |
| Toddler | 87.7 | 76.9 |
| Pre-schoolers | 97.0 | 24.0 |
| Adolescents | 95.0 | 74.5 |
| Multiple ages | 82.2–90.1 | 50.9–72.8 |
| Findings Relative to Term-Born Control Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Method of Assessment of Sleep | Gestational Age | Age at Assessment | N Born Preterm | Night Waking | Sleep Duration Night | Sleep Duration Day | Sleep Timing | Sleep Onset Latency | Parent Perception of Sleep Problem | Other | Study Limitations |
| Sleep following preterm birth in infancy/toddlerhood following discharge from hospital | ||||||||||||
| (Akkus and Bahtiyar-Saygan, 2022) [51] | Parent report: Brief Infant Sleep Questionnaire | <37 weeks of gestation without significant neurodevelopmental problems or severe medical conditions | 6–17 months corrected age | 40 | X both in number of night wakings and duration of nocturnal wakefulness | ↓ | x | Significantly later bedtime in the PT group | x | No significant group difference in the percentage of children who were classified as a poor sleeper (waking >3 times per night, spending more than one hour awake at night, or spending less than 9 h asleep). No significant group difference in bedtime difficulty score | Small sample size, relied on parent-report. Sample not representative of children born <37 weeks with significant neurodevelopmental disorders or medical conditions | |
| (Asaka and Takada, 2010) [52] | Actigraphy | <1500 BW and <32 weeks of gestation without severe illness or congenital abnormality or use of medication with sedative effects | 12 months corrected age | 14 | X both number of wakings and duration of nocturnal wakefulness Higher night-time activity index in PT group | ↓ | x | Earlier bedtimes and wake time in PT group | Greater proportion of parents concerned about child’s sleep in PT group | No significant difference in longest sleep episode or sleep efficiency | Small sample size. Sample not representative of children born <32 weeks with neontal morbidities | |
| (Asaka and Takada, 2013) [53] | Actigraphy | <36 weeks of gestation and <2500 BW. No neurological or developmental problems | 10–22 months corrected age | 21 | X both number of wakings (over 5 min) and duration of nocturnal wakefulness | ↓ in PT group for infants ≤14 months of age X between the groups at over 15 months of age | ↑ infants over 15 months in PT group | x | Greater proportion of parents concerned about child’s sleep in PT group | Sleep efficiency and longest sleep episode (whilst both did not significantly differ between the two groups) significantly accounted for 71% of maternal parenting stress | Small sample size. Sample not representative of children born <36 weeks with developmental problems | |
| (Blair et al., 2012) [54] | Parent-reported sleep duration computed from bedtime and wake time | <37 weeks of gestation | 6 months to 140 months | Approximately 350 at 140 months | x | Only estimated sleep duration from bedtime and wake-up time. Does not account for sleep onset latency of duration of night waking. | ||||||
| (Bulut et al., 2020) [55] | Parent report: Brief Infant Sleep Questionnaire | ≤32 weeks of gestation without developmental delay, intraventricular haemorrhage grades 3 and 4, chronic disease, congenital anomalies, current teething or infections | 12–31 months | 40 | ↑ greater duration waking in PT group ↓ More frequent number of wakings in full-term group | x | x | x | Small sample size, relied on parent-report. Sample not representative of children born ≤32 weeks with neonatal morbidities. Included control group of term-born children | |||
| (Caravale et al., 2017) [56] | Parent report: Brief Infant Sleep Questionnaire and the Sleep Disturbance Scale for Children | <37 weeks of gestation Without cognitive, language, or motor delay or neurosensory impairment or genetic syndrome, or major congenital abnormalities | 13–29 months | 51 | X both frequency of night waking and duration of nocturnal wakefulness | x | x | x | Greater sleep difficulties (nocturnal movement, restless during the night, breathing problems) compared with term group | Small sample size, relied on parent-report. Sample not representative of children born <37 weeks with developmental problems | ||
| (Gössel-Symank, Grimmer, Korte and Siegmund, 2004) [57] | Actigraphy | 24–34 weeks of gestation, birthweight <1500 g. Twins, infants with serious infectious diseases e.g., HIV, genetic disorders, and cerebral palsy were excluded | 20 months | 17 | Infants born PT had significantly less restful sleep (immobile time) and more restless sleep (moving time) than infants born at term | x | ↓ Daily rest phase significantly shorter in infants born preterm | Small sample size, did not use an algorithm to calculate the duration of night waking | ||||
| (Huang et al., 2014) [58] | Brief Infant Sleep Questionnaire | <37 weeks of gestation Without severe medical or congenital problems | 6 months | 68 | ↑ (number of night awakenings) | ↑ | ↑ | x | More time spent crying during the night respiratory symptoms; greater time spent breathing through their mouth and loud-noisy breathing and greater severity of sleep problems in PT versus term group. Of preterm infants, 81% had an apnoea–hypopnea index (AHI) >1 event/hour | Did not compare objective sleep data between the groups. Small sample size. Sample not representative of children born <37 weeks with medical problems | ||
| (Lupini et al., 2021) [59] | Parent report: Brief Infant Sleep Questionnaire | <37 weeks of gestation | 0–36 months | 417 | x | x | x | x | x | Greater for parents of preterm children aged 12–36 months | Relied on parent-report | |
| (Shimada et al., 1993) [60] | Parental observation of daily activities over 14 days every month until 12 months corrected age | <37 weeks of gestation without neonatal morbidities | From corrected age 35–39 weeks to 12 months | 57 | x | x | Delayed development of sleep-wake periodicity when referenced to post-natal weeks, but not post-conceptional weeks. No significant between-group difference in longest sustained sleep and longest sustained wake period at the same corrected ages | Relied on parental observation. Sample not representative of children born <37 weeks with neonatal morbidities | ||||
| (Wolke et al., 1998) [61] | Parent-reported night waking problems | <37 weeks of gestation in two cohorts- South Finland (SF) and South Germany (SG) | 5, 20, and 56 months follow up | 305 (SF) 1703 (SG) | ↓ at 5 months (SF) ↓ at 20 months (SG) No significant differences at 20 and 56 months in SF cohort and at 5 and 56 months in SG cohort | Relied on parental report of night waking | ||||||
| Sleep in the preschool period | ||||||||||||
| (Romeo et al., 2019) a [62] | Parent-reported: Sleep Disturbance Scale for Children | ≤31 weeks of gestation with no history of major medical complications and above 10th percentile for GA-BW | 3–6 years | 146 | Y (Combined difficulty initiating and maintaining sleep score) | Y (Combined difficulty initiating and maintaining sleep score) | Significantly higher scores for sleep-disordered breathing and sleep hyperhidrosis. No significant difference in parasomnias, disorders of excessive somnolence, and nonrestorative sleep scores | Sample not representative of children born ≤31 weeks without neonatal complications. Questionnaire does not differentiate between night waking and sleep duration. Did not include a term-born control group | ||||
| (Durankus et al., 2020) [63] | Parent-report Children’s Sleep Habits Questionnaire (CSHQ) | <37 weeks of gestation, without diagnosed sleep disorder and without major congenital abnormality | 4–6 years | 137 | X on CHSQ scale ↑ More parents in the PT group stated that their child would wake up during sleep | x | ↑ More parents in the PT group stated that their child would wake up early | x | Significantly higher CSHQ total scores in the PT group. No significant difference in bedtime resistance, sleep anxiety, parasomnia, or sleep-disordered breathing subscales. Greater frequency of loud snoring. A greater proportion of children born PT had obstructive sleep apnoea symptoms (mouth breathing, hyponasal speech, loud snoring, difficulty in breathing). A greater proportion of children born very and moderately preterm had CHSQ scores above cut-off for sleep disorder compared with children born late preterm | Relied on parental reports of sleep problems | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trickett, J.; Hill, C.; Austin, T.; Johnson, S. The Impact of Preterm Birth on Sleep through Infancy, Childhood and Adolescence and Its Implications. Children 2022, 9, 626. https://doi.org/10.3390/children9050626
Trickett J, Hill C, Austin T, Johnson S. The Impact of Preterm Birth on Sleep through Infancy, Childhood and Adolescence and Its Implications. Children. 2022; 9(5):626. https://doi.org/10.3390/children9050626
Chicago/Turabian StyleTrickett, Jayne, Catherine Hill, Topun Austin, and Samantha Johnson. 2022. "The Impact of Preterm Birth on Sleep through Infancy, Childhood and Adolescence and Its Implications" Children 9, no. 5: 626. https://doi.org/10.3390/children9050626
APA StyleTrickett, J., Hill, C., Austin, T., & Johnson, S. (2022). The Impact of Preterm Birth on Sleep through Infancy, Childhood and Adolescence and Its Implications. Children, 9(5), 626. https://doi.org/10.3390/children9050626






