COVID-19 Vaccination in Italian Children: The Limits of Parental Rights
Abstract
:1. Introduction
2. Aim and Scope
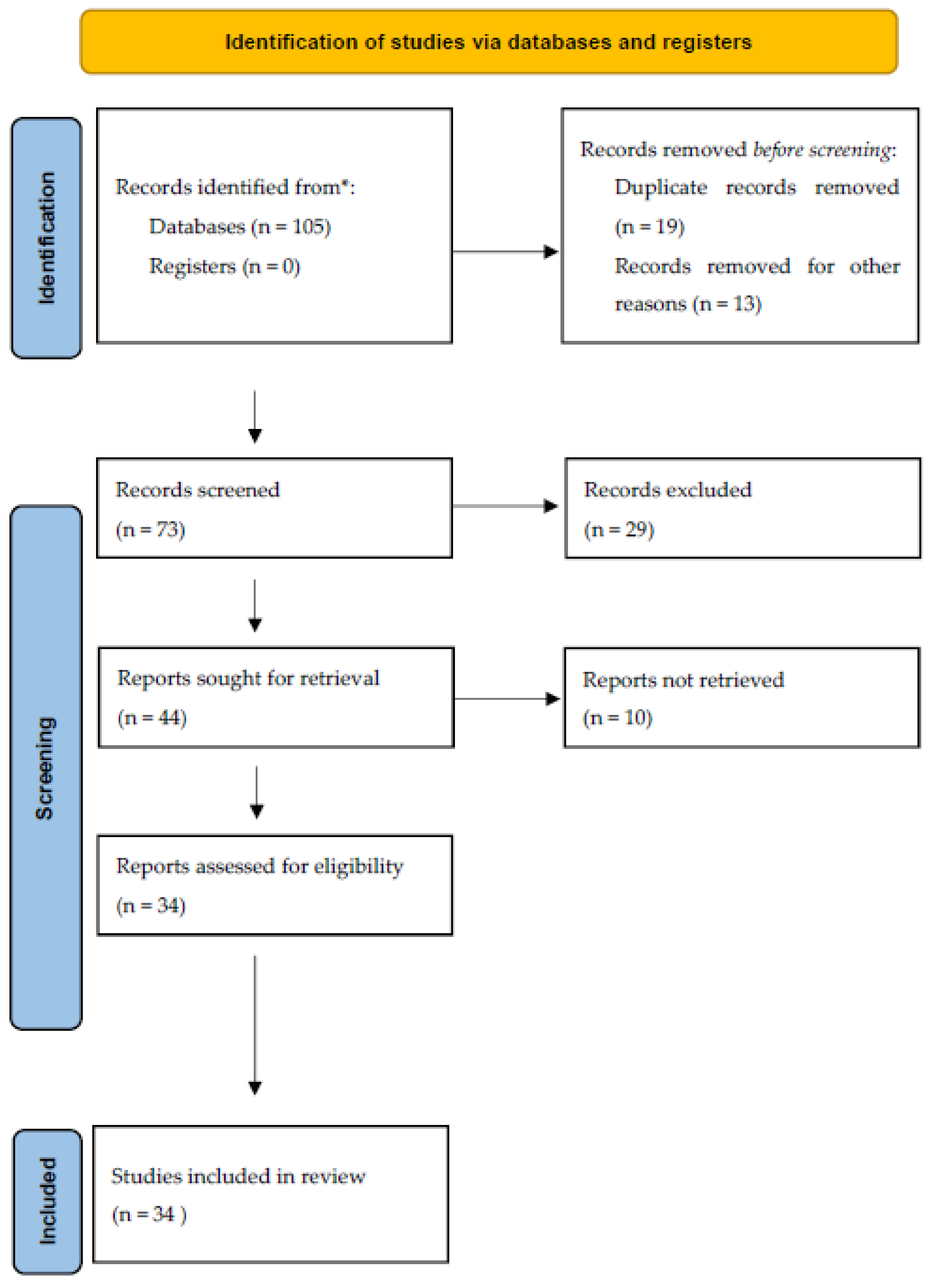
3. Materials and Methods
- -
- Articles published in English;
- -
- Type of paper: Original article, research article, systematic review, review.
4. Results
- -
- Article: 11;
- -
- Review: 5;
- -
- Editorials: 4;
- -
- Letter to the Editor: 4;
- -
- Original article: 3;
- -
- Rapid Communication: 3;
- -
- Research paper: 2;
- -
- Viewpoint: 1;
- -
- Study protocol: 1.
5. Discussion
- -
- In the first place, there is the case in which the parents or a legal guardian belonging to “anti-vax” movements or simply opposed to vaccination due to the prevalence of feelings of fear and anguish, do not want to vaccinate the minor, who instead wants to undergo vaccination;
- -
- There is also the case in which the minor expresses the will not to be vaccinated, and the parents or legal guardian, on the other hand, want it to be carried out.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, T.-P.; Lien, T.-C.; Yang, C.-Y.; Su, Y.L.; Wang, J.-H.; Tsai, S.-L.; Yin, J.-C. Prevalence of psychiatric morbidity and psychological adaptation of the nurses in a structured SARS caring unit during outbreak: A prospective and periodic assessment study in Taiwan. J. Psychiatr. Res. 2007, 41, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kwek, S.-K.; Chew, W.-M.; Ong, K.-C.; Ng, A.W.-K.; Lee, L.; Kaw, G.; Leow, M.K.-S. Quality of life and psychological status in survivors of severe acute respiratory syndrome at 3 months postdischarge. J. Psychosom. Res. 2006, 60, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal–Oral Transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Zuckerman, N.; Nemet, I.; Atari, N.; Kliker, L.; Regev-Yochay, G.; Sapir, E.; Mor, O.; Alroy-Preis, S.; Mendelson, E.; et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Eurosurveillance 2021, 26, 2100557. [Google Scholar] [CrossRef] [PubMed]
- Luxi, N.; Giovanazzi, A.; Capuano, A.; Crisafulli, S.; Cutroneo, P.M.; Fantini, M.P.; Ferrajolo, C.; Moretti, U.; Poluzzi, E.; Raschi, E.; et al. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021, 44, 1247–1269. [Google Scholar] [CrossRef]
- Kiem, C.T.; Andronico, A.; Bosetti, P.; Paireau, J.; Alter, L.; Boëlle, P.-Y.; Fontanet, A.; Lévy-Bruhl, D.; Cauchemez, S. Benefits and risks associated with different uses of the COVID-19 vaccine Vaxzevria: A modelling study, France, May to September 2021. Eurosurveillance 2021, 26, 2100533. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; Mcmahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef]
- González, S.; Olszevicki, S.; Salazar, M.; Calabria, A.; Regairaz, L.; Marín, L.; Campos, P.; Varela, T.; Martínez, V.V.G.; Ceriani, L.; et al. Effectiveness of the first component of Gam-COVID-Vac (Sputnik V) on reduction of SARS-CoV-2 confirmed infections, hospitalisations and mortality in patients aged 60-79: A retrospective cohort study in Argentina. EClinicalMedicine 2021, 40, 101126. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G.; et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Reg. Health Eur. 2021, 11, 100241. [Google Scholar] [CrossRef]
- Hernández-Bello, J.; Morales-Núñez, J.J.; Machado-Sulbarán, A.C.; Díaz-Pérez, S.A.; Torres-Hernández, P.C.; Balcázar-Félix, P.; Gutiérrez-Brito, J.A.; Lomelí-Nieto, J.A.; Muñoz-Valle, J.F. Neutralizing Antibodies against SARS-CoV-2, Anti-Ad5 Antibodies, and Reactogenicity in Response to Ad5-nCoV (CanSino Biologics) Vaccine in Individuals with and without Prior SARS-CoV-2. Vaccines 2021, 9, 1047. [Google Scholar] [CrossRef]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Zakarya, K.; Kutumbetov, L.; Orynbayev, M.; Abduraimov, Y.; Sultankulova, K.; Kassenov, M.; Sarsenbayeva, G.; Kulmagambetov, I.; Davlyatshin, T.; Sergeeva, M.; et al. Safety and immunogenicity of a QazCovid-in® inactivated whole-virion vaccine against COVID-19 in healthy adults: A single-centre, randomised, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. EClinicalMedicine 2021, 39, 101078. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-D.; Li, Q.-H. SARS-CoV-2: Vaccines in the pandemic era. Mil. Med Res. 2021, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, A.; Aalizadeh, R.; Aminianfar, H.; Kianmehr, Z.; Teimoori, A.; Azimi, E.; Emamipour, N.; Eghtedardoost, M.; Siavashi, V.; Jamshidi, H.; et al. Safety and potency of BIV1-CovIran inactivated vaccine candidate for SARS-CoV-2: A preclinical study. Rev. Med Virol. 2021, e2305. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Aguilar-Guerra, T.L.; Díaz, E.M.F.; Gorry, C. Cuba’s National Regulatory Authority & COVID-19: Olga Lidia Jacobo-Casanueva MS Director, Center for State Control of Medicines and Medical Devices (CECMED). MEDICC Rev. 2021, 23, 3–4. [Google Scholar] [CrossRef]
- Brusa, M.; Barilan, Y.M. Voluntary COVID-19 vaccination of children: A social responsibility. J. Med. Ethic 2021, 47, 543–546. [Google Scholar] [CrossRef]
- Armitage, R. Online ‘anti-vax’ campaigns and COVID-19: Censorship is not the solution. Public Health 2021, 190, e29–e30. [Google Scholar] [CrossRef] [PubMed]
- Kamidani, S.; Rostad, C.A.; Anderson, E.J. COVID-19 vaccine development: A pediatric perspective. Curr. Opin. Pediatr. 2021, 33, 144–151. [Google Scholar] [CrossRef]
- Bansal, U.; Ghate, S.; Bhattacharya, P.; Thapar, R.K.; Gupta, P.; Pradhan, P.; Bersain, R.; Mishra, D. Research Letters. Indian Pediatr. 2020, 57, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Munro, A.P.S.; Smith, G.D.; Pollock, A.M. Closing schools is not evidence based and harms children. BMJ 2021, 372, n52. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.; Gorwood, P. The consequences of the COVID-19 pandemic on mental health and implications for clinical practice. Eur. Psychiatry 2020, 63, e32. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Lima, C.K.T.; Carvalho, P.M.D.M.; Lima, I.D.A.A.S.; Nunes, J.V.A.D.O.; Saraiva, J.S.; de Souza, R.I.; da Silva, C.G.L.; Neto, M.L.R. The emotional impact of Coronavirus 2019-nCoV (new Coronavirus disease). Psychiatry Res. 2020, 287, 112915. [Google Scholar] [CrossRef] [PubMed]
- Hanna, F.; Barbui, C.; Dua, T.; Lora, A.; Altena, M.V.R.; Saxena, S. Global mental health: How are we doing? World Psychiatry 2018, 17, 367–368. [Google Scholar] [CrossRef] [Green Version]
- Giallonardo, V.; Sampogna, G.; Del Vecchio, V.; Luciano, M.; Albert, U.; Carmassi, C.; Carrà, G.; Cirulli, F.; Dell’Osso, B.; Nanni, M.G.; et al. The Impact of Quarantine and Physical Distancing Following COVID-19 on Mental Health: Study Protocol of a Multicentric Italian Population Trial. Front. Psychiatry 2020, 11, 533. [Google Scholar] [CrossRef]
- Shigemura, J.; Ursano, R.J.; Morganstein, J.C.; Kurosawa, M.; Benedek, D.M. Public responses to the novel 2019 coronavirus (2019-nCoV) in Japan: Mental health consequences and target populations. Psychiatry Clin. Neurosci. 2020, 74, 281–282. [Google Scholar] [CrossRef]
- Park, S.-C.; Park, Y.C. Mental Health Care Measures in Response to the 2019 Novel Coronavirus Outbreak in Korea. Psychiatry Investig. 2020, 17, 85–86. [Google Scholar] [CrossRef] [Green Version]
- Nowland, R.; Necka, E.; Cacioppo, J.T. Loneliness and Social Internet Use: Pathways to Reconnection in a Digital World? Perspect. Psychol. Sci. 2018, 13, 70–87. [Google Scholar] [CrossRef] [Green Version]
- Masedu, F.; Mazza, M.; Di Giovanni, C.; Calvarese, A.; Tiberti, S.; Sconci, V.; Valenti, M. Facebook, Quality of Life, and Mental Health Outcomes in Post-Disaster Urban Environments: The Ll’aquila Earthquake Experience. Front. Public Health 2014, 2, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.L.H.; Ramsay, M.E.; Ladhani, S.N. Should children be vaccinated against COVID-19 now? Arch. Dis. Child. 2021, 106, 1147–1148. [Google Scholar] [CrossRef] [PubMed]
- Children Are Getting Long COVID and Being Left with Lasting Problems | New Scientist. Available online: https://www.newscientist.com/article/mg24933233-600-children-are-getting-long-covid-and-being-left-with-lasting-problems/ (accessed on 1 December 2021).
| Comirnaty (BioNTech/Pfizer) | Spikevax (Moderna) | Vaxzevria (Formerly AstraZeneca) | Janssen (Johnson & Johnson) | |
|---|---|---|---|---|
| Minimum age | ≥12 years | ≥18 years | ||
| Method of administration | 2 administrations at least 3 weeks apart | 2 administrations at least 4 weeks apart | 2 administrations at 4/12 weeks apart | Single administration |
| Mechanism of action | Molecule of messenger RNA, contained in lipid vesicles, which fuse with human cells | Recombinant vector, based on chimpanzee adenovirus (ChAdOx1) | Recombinant vector, based on type 26 human adenovirus | |
| → Coding of the spike glycoprotein (S) of SARS-CoV-2, which induces, in the host, an immune response against the SARS-CoV-2 virus. | ||||
| Vaccine | Producer | Active Principle |
|---|---|---|
| Sputnik V | Gameleya Research Institute of Epidemiology (Russia) | Viral Vector |
| Sputnik V Light | ||
| Convidencia (Ad5-nCoV) | CanSino Biologics (China) | |
| BBIBP-CorV | Sinopharm (China) | Inactive virus |
| CoronaVac | Sinovac (China) | |
| Covaxin (BBV152) | Bharat Biotech + Indian Councid of Medical Research | |
| QazVac | Research Institute for Biological Safety Problems in Kazakhstan | |
| IMBCAMS COVID-19 vaccine | Institute of Medical Biology of the Chinese Academy of Medical Sciences (IMBCAMS) | |
| COVID-19 Inactivated Vaccin (COVIran Barekat) | Shifa Pharmed Industrial Group in Iran | |
| EpiVacCorona | VECTOR center of Virology (Russia) | Peptide subunit |
| RBD-dimer (ZF2001) | Anhui Zhifei Longcom (Cina) | |
| Abdala | Center for Genetic Engineering and Biotechnology in Cuba |
| Court of Milan 1 |
| Topic → Refusal of the mother to have her 11-year-old daughter undergo the vaccine, molecular swabs for the diagnosis of COVID-19 and antigen tests, as well as the use of a mask, which is considered “harmful”.Outcome → Decree of 2–13 September 2021 (procedure inscribed under n.ro 6014/2021 R.G.) which authorizes the father to provide autonomously, without the consent of the mother, thus limiting the maternal parental responsibility in relation to all issues related to mandatory or optional vaccinations, swabs, the possible administration of the COVID-19 vaccine, and the use of the mask, attributing it exclusively to the father. |
| Court of MONZA 2 |
| Theme → Unjustified refusal opposed by the father; willingness in favor of vaccination expressed by the 15-year-old and 6-month-old child.Outcome → Decree of 22 July 2021 which authorized the administration of the COVID-19 vaccine to the minor, giving the mother the right to accompany the child to a vaccination center and sign the relative informed consent, even in the absence the consent of the other parent. |
| Court of BRINDISI |
| Topic → Father is anti-vax for people younger than 14 years old due to being “fragile”Outcome → Authorized administration as “in vaccination, since it is impossible to seek zero risk, a risk-benefit assessment must be carried out”; in this specific case, the benefits associated with the administration of the COVID-19 vaccine outweighed the risks associated with the vaccination itself. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrone, M.; Luca, B.P.D.; Stellacci, A.; Buongiorno, L.; Caricato, P.; Cazzato, G.; Ferorelli, D.; Solarino, B.; Stefanizzi, P.; Tafuri, S.; et al. COVID-19 Vaccination in Italian Children: The Limits of Parental Rights. Children 2022, 9, 625. https://doi.org/10.3390/children9050625
Marrone M, Luca BPD, Stellacci A, Buongiorno L, Caricato P, Cazzato G, Ferorelli D, Solarino B, Stefanizzi P, Tafuri S, et al. COVID-19 Vaccination in Italian Children: The Limits of Parental Rights. Children. 2022; 9(5):625. https://doi.org/10.3390/children9050625
Chicago/Turabian StyleMarrone, Maricla, Benedetta Pia De Luca, Alessandra Stellacci, Luigi Buongiorno, Pierluigi Caricato, Gerardo Cazzato, Davide Ferorelli, Biagio Solarino, Pasquale Stefanizzi, Silvio Tafuri, and et al. 2022. "COVID-19 Vaccination in Italian Children: The Limits of Parental Rights" Children 9, no. 5: 625. https://doi.org/10.3390/children9050625
APA StyleMarrone, M., Luca, B. P. D., Stellacci, A., Buongiorno, L., Caricato, P., Cazzato, G., Ferorelli, D., Solarino, B., Stefanizzi, P., Tafuri, S., Gorini, E., Landro, M. d., Dell’Erba, A., & Laforgia, N. (2022). COVID-19 Vaccination in Italian Children: The Limits of Parental Rights. Children, 9(5), 625. https://doi.org/10.3390/children9050625










