Retropharyngeal, Parapharyngeal and Peritonsillar Abscesses
Abstract
:1. Background
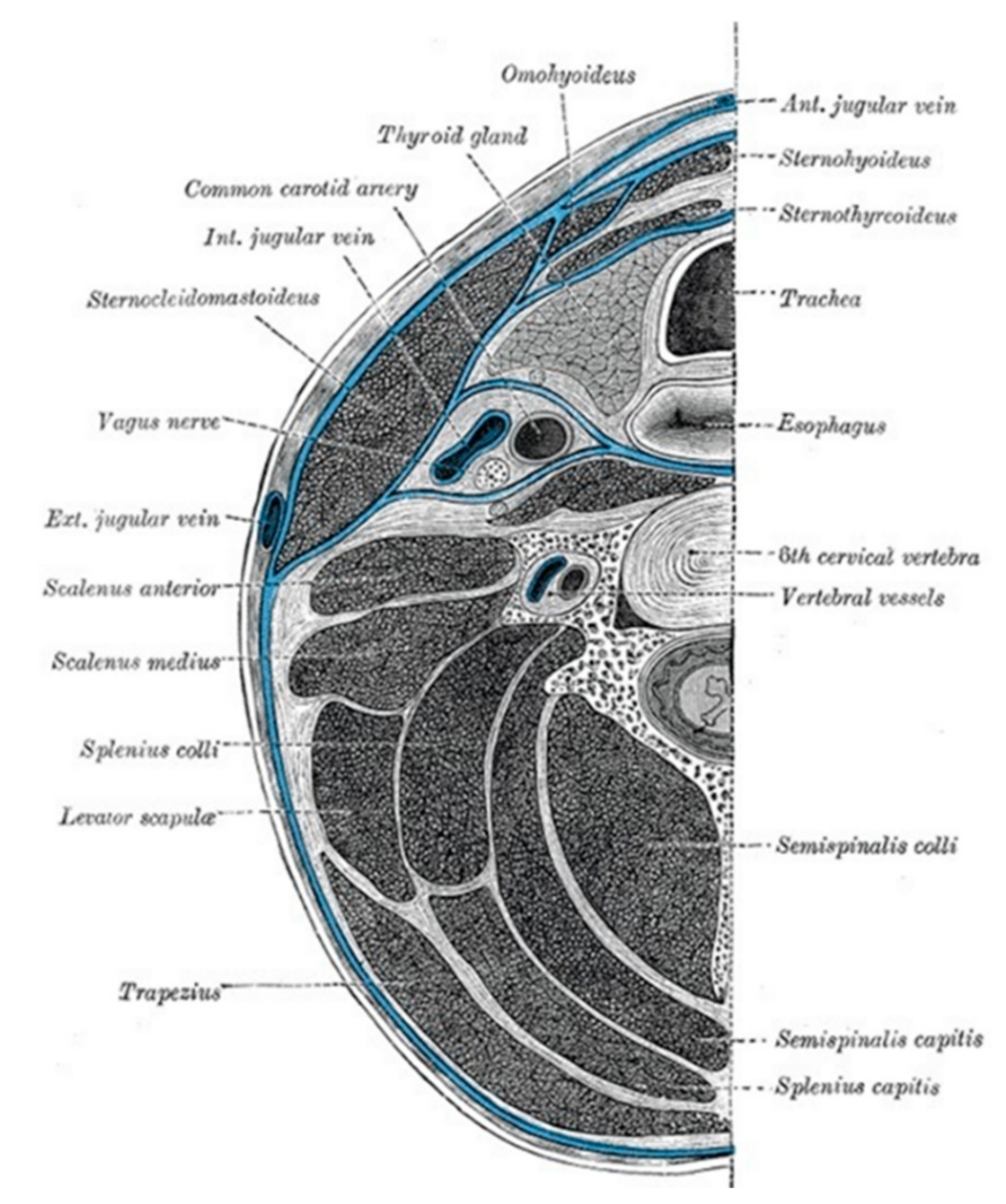
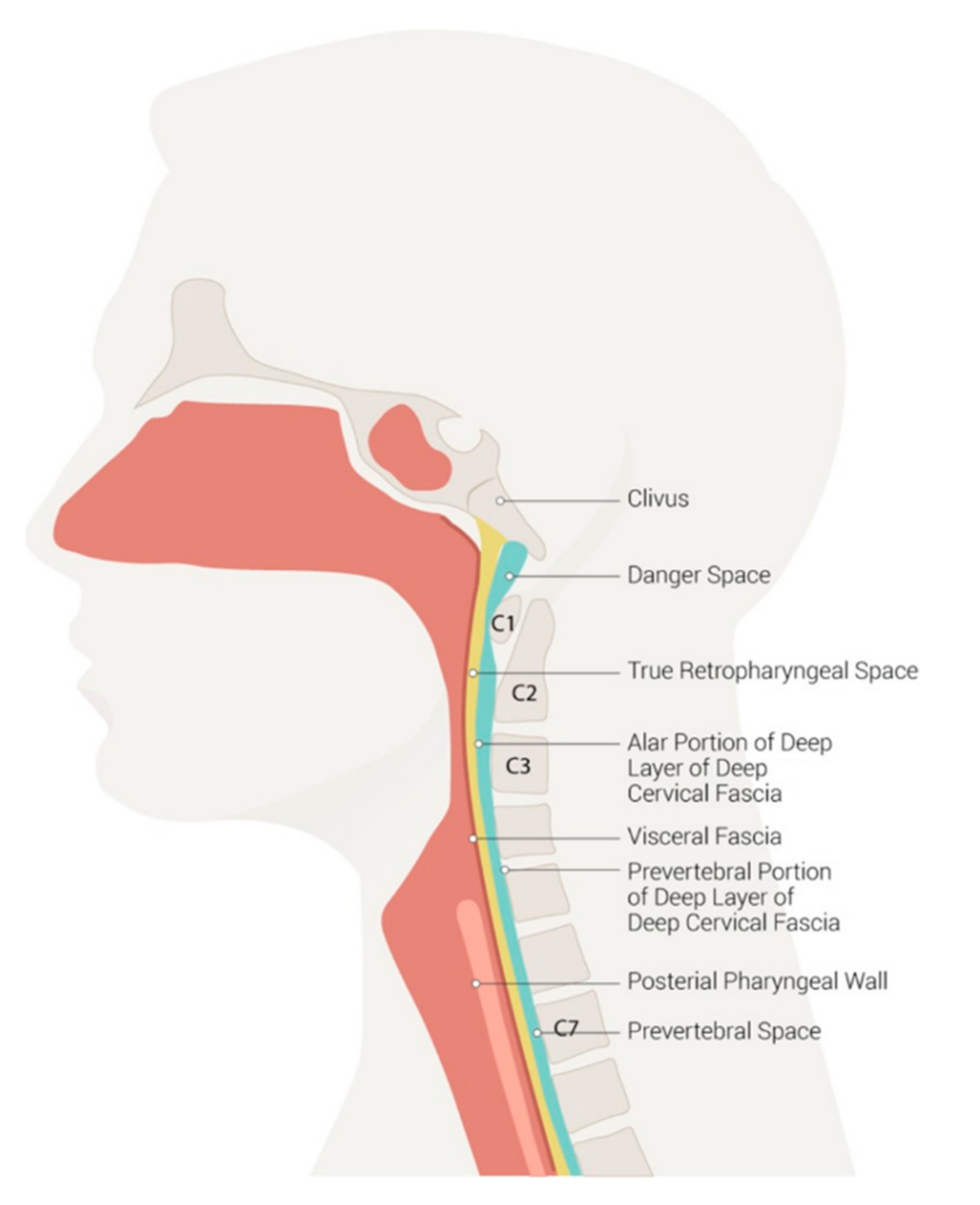
2. Neck Anatomy and Deep Infection Development
3. Epidemiology, Etiology and Clinical Manifestations
3.1. Epidemiology
3.2. Etiology
3.3. Clinical Manifestations
4. Diagnosis
4.1. Radiological Imaging
4.2. Microbiology Tests
4.3. Laboratory Tests
5. Treatment
5.1. Medical Treatment
5.2. Surgical Approach
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McDowell, R.H.; Hyser, M.J. Neck Abscess. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Almuqamam, M.; Gonzalez, F.J.; Kondamudi, N.P. Deep Neck Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Marra, S.; Hotaling, A.J. Deep Neck Infections. Am. J. Otolaryngol. 1996, 17, 287–298. [Google Scholar] [CrossRef]
- Li, R.M.; Kiemeney, M. Infections of the Neck. Emerg. Med. Clin. N. Am. 2019, 37, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, M. Ear, Nose, Throat: Beyond Pharyngitis: Retropharyngeal Abscess, Peritonsillar Abscess, Epiglottitis, Bacterial Tracheitis, and Postoperative Tonsillectomy. Emerg. Med. Clin. N. Am. 2021, 39, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, I.; Meher, R.; Raj, A.; Rajpurohit, P.; Prasad, P. Deep neck space abscesses in children below 5 years of age and their complications. Int. J. Pediatr. Otorhinolaryngol. 2018, 109, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, E.; Bianchini, S.; Nicoletti, L.; Monaco, S.; Carrara, E.; Opri, F.; Opri, R.; Caminiti, C.; Donà, D.; Giuffré, M.; et al. Antimicrobial Prophylaxis in Neonates and Children Undergoing Dental, Maxillo-Facial or Ear-Nose-Throat (ENT) Surgery: A RAND/UCLA Appropriateness Method Consensus Study. Antibiotics 2022, 11, 382. [Google Scholar] [CrossRef]
- Maharaj, S.; Mungul, S.; Ahmed, S. Deep Neck Space Infections: Changing Trends in Pediatric Versus Adult Patients. J. Oral Maxillofac. Surg. 2020, 78, 394–399. [Google Scholar] [CrossRef]
- Guidera, A.K.; Dawes, P.J.; Fong, A.; Stringer, M.D. Head and neck fascia and com-partments: No space for spaces. Head Neck 2014, 36, 1058–1068. [Google Scholar] [CrossRef]
- Skoulakis, C.E.; Papadakis, C.E.; Bizakis, J.G.; Nikolidakis, A.A.; Manios, A.G.; Helidonis, E.S. Abscess of the pharyngeal mucosal space—An unusual location. J. Otolaryngol. 2003, 32, 121–124. [Google Scholar] [CrossRef]
- Warshafsky, D.; Goldenberg, D.; Kanekar, S.G. Imaging Anatomy of Deep Neck Spaces. Otolaryngol. Clin. N. Am. 2012, 45, 1203–1221. [Google Scholar] [CrossRef]
- Bali, R.K.; Sharma, P.; Gaba, S.; Kaur, A.; Ghanghas, P. A review of complications of odontogenic infections. Natl. J. Maxillofac. Surg. 2015, 6, 136–143. [Google Scholar] [CrossRef]
- Kamalian, S.; Avery, L.; Lev, M.; Schaefer, P.; Curtin, H.; Kamalian, S. Nontraumatic Head and Neck Emergencies. RadioGraphics 2019, 39, 1808–1823. [Google Scholar] [CrossRef]
- Mnatsakanian, A.; Minutello, K.; Bordoni, B. Anatomy, Head and Neck, Retropharyngeal Space. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Johnson, R.F.; Stewart, M.G.; Wright, C.C. An evidence-based review of the treat-ment of peritonsillar abscess. Otolaryngol. Head Neck Surg. 2003, 128, 332–343. [Google Scholar] [CrossRef]
- Albertz, N.; Nazar, G. Peritonsillar abscess: Treatment with immediate tonsillectomy—10 years of experience. Acta Otolaryngol. 2012, 132, 1102–1107. [Google Scholar] [CrossRef]
- McClay, J.E.; Murray, A.D.; Booth, T. Intravenous Antibiotic Therapy for Deep Neck Abscesses Defined by Computed Tomography. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 1207–1212. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, C.E.; Deutsch, E.S.; Eppes, S.; Lawless, S.; Cook, S.; O’Reilly, R.C.; Reilly, J.S. Increased incidence of head and neck abscesses in children. Otolaryngol. Neck Surg. 2007, 136, 176–181. [Google Scholar] [CrossRef]
- Page, N.C.; Bauer, E.M.; Lieu, J.E. Clinical features and treatment of retropharyngeal abscess in children, Otolaryngol. Head Neck Surg. 2008, 138, 300–306. [Google Scholar] [CrossRef]
- Novis, S.J.; Pritchett cv Thorne, M.C.; Sun, G.H. Pediatric deep space neck infec-tions in U.S. children, 2000–2009. Int J Pediatr Otorhinolaryngol. 2014, 78, 832–836. [Google Scholar] [CrossRef]
- Paterson, G.K.; Harrison, E.; Holmes, M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014, 22, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Galli, L.; Venturini, E.; Bassi, A.; Gattinara, G.C.; Chiappini, E.; Defilippi, C.; Diociaiuti, A.; Esposito, S.; Garazzino, S.; Giannattasio, A.; et al. Common Community-acquired Bacterial Skin and Soft-tissue Infections in Children: An Intersociety Consensus on Impetigo, Abscess, and Cellulitis Treatment. Clin Ther. 2019, 41, 532–551.e7. [Google Scholar] [CrossRef]
- Raffaldi, I.; Le Serre, D.; Garazzino, S.; Scolfaro, C.; Bertaina, C.; Mignone, F.; Peradotto, F.; Tavormina, P.; Tovo, P.-A. Diagnosis and management of deep neck infections in children: The experience of an Italian paediatric centre. J. Infect. Chemother. 2015, 21, 110–113. [Google Scholar] [CrossRef]
- Chang, L.; Chi, H.; Chiu, N.-C.; Huang, F.-Y.; Lee, K.-S. Deep Neck Infections in Different Age Groups of Children. J. Microbiol. Immunol. Infect. 2010, 43, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Côrte, F.C.; Firmino-Machado, J.; Moura, C.P.; Spratley, J.; Santos, M. Acute pediatric neck infections: Outcomes in a seven-year series. Int. J. Pediatr. Otorhinolaryngol. 2017, 99, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Hidaka, H.; Ozawa, D.; Kakuta, R.; Nomura, K.; Yano, H.; Watanabe, K.-I.; Katori, Y. Clinical and bacteriological differences of deep neck infection in pediatric and adult patients: Review of 123 cases. Int. J. Pediatr. Otorhinolaryngol. 2017, 99, 95–99. [Google Scholar] [CrossRef]
- Klug, T.E.; Rusan, M.; Fuursted, K.; Ovesen, T. Peritonsillar Abscess: Complication of Acute Tonsillitis or Weber’s Glands Infection? Otolaryngol. Head Neck Surg. 2016, 155, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Coticchia, J.M.; Getnick, G.S.; Yun, R.D.; Arnold, J.E. Age-, Site-, and Time-Specific Differences in Pediatric Deep Neck Abscesses. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Haq, N.; Quezada, M.; Asmar, B.I. Retropharyngeal abscess in children: The rising incidence of methicillin-resistant Staphylococcus aureus. Pediatr. Infect. Dis. J. 2012, 31, 696–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulthard, M.; Isaacs, D. Retropharyngeal abscess. Arch. Dis. Child. 1991, 66, 1227–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, A.; Hansen, T.M.; Bank, S.; Kristensen, L.H.; Prag, J. Fusobacterium necrophorum tonsillitis: An important cause of tonsillitis in adolescents and young adults. Clin. Microbiol. Infect. 2015, 21, 266.e1–266.e3. [Google Scholar] [CrossRef] [Green Version]
- Flodström, A.; Hallander, H.O. Microbiological Aspects on Peritonsillar Abscesses. Scand. J. Infect. Dis. 1976, 8, 157–160. [Google Scholar] [CrossRef]
- Weldetsadik, A.Y.; Bedane, A.; Riedel, F. Retropharyngeal Tuberculous Abscess: A Rare Cause of Upper Airway Obstruction and Obstructive Sleep Apnea in Children: A Case Report. J. Trop. Pediatr. 2019, 65, 642–645. [Google Scholar] [CrossRef]
- Desai, L.; Shah, I.; Shaan, M. Retropharyngeal abscess as a paradoxical reaction in a child with multidrug-resistant tuberculosis. Paediatr. Int. Child. Health 2019, 39, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.P.; Hazboun, I.M.; Fernandes, F.L.; Bento, L.R.; Zappelini, C.E.M.; Chone, C.T.; Crespo, A.N. Deep neck abscesses: Study of 101 cases. Braz. J. Otorhinolaryngol. 2017, 83, 341–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorjón, P.S.; Pérez, P.B.; Martín, A.C.M.; Dios, J.C.P.; Alonso, S.E.; de la Cabanillas, M.I.C. Deep Neck Infection: Review of 286 Cases. Acta Otorrinolaringol. 2012, 63, 31–41. [Google Scholar] [CrossRef]
- Yang, W.; Hu, L.; Wang, Z.; Nie, G.; Li, X.; Lin, D.; Luo, J.; Qin, H.; Wu, J.; Wen, W.; et al. Deep neck infection: A review of 130 cases in southern China. Medicine 2015, 94, e944. [Google Scholar] [CrossRef]
- Celakovsky, P.; Kalfert, D.; Tucek, L.; Mejzlik, J.; Kotulek, M.; Vrbacky, A.; Matousek, P.; Stanikova, L.; Hoskova, T.; Pasz, A. Deep neck infections: Risk fac-tors for mediastinal extension. Eur. Arch. Otorhinolaryngol. 2014, 271, 1679–1683. [Google Scholar] [CrossRef]
- Conrad, D.E.; Parikh, S.R. Deep Neck Infections. Infect. Disord. Drug Targets 2012, 12, 286–290. [Google Scholar] [CrossRef]
- Adoviča, A.; Veidere, L.; Ronis, M.; Sumeraga, G. Deep neck infections: Review of 263 cases. Otolaryngol. Pol. 2017, 71, 37–42. [Google Scholar] [CrossRef]
- Favaretto, N.; Fasanaro, E.; Staffieri, A.; Marchese-Ragona, R.; Staffieri, C.; Giacomelli, L.; Stramare, R.; Ottaviano, G.; Marioni, G. Deep neck infections originating from the major salivary glands. Am. J. Otolaryngol. 2015, 36, 559–564. [Google Scholar] [CrossRef]
- Daramola, O.O.; Flanagan, C.E.; Maisel, R.H.; Odland, R.M. Diagnosis and treatment of deep neck space abscesses. Otolaryngol. Head Neck Surg. 2009, 141, 123–130. [Google Scholar] [CrossRef]
- Cmejrek, R.C.; Coticchia, J.M.; Arnold, J.E. Presentation, Diagnosis, and Management of Deep-Neck Abscesses in Infants. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 1361–1364. [Google Scholar] [CrossRef]
- Klug, T.E.; Greve, T.; Hentze, M. Complications of peritonsillar abscess. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 32. [Google Scholar] [CrossRef]
- Lee, J.-K.; Kim, H.-D.; Lim, S.-C. Predisposing Factors of Complicated Deep Neck Infection: An Analysis of 158 Cases. Yonsei Med J. 2007, 48, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Carbone, P.N.; Capra, G.G.; Brigger, M.T. Antibiotic therapy for pediatric deep neck abscesses: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1647–1653. [Google Scholar] [CrossRef]
- Menezes, A.S.; Ribeiro, D.C.; Guimarães, J.R.; Lima, A.F.; Dias, L. Management of pediatric peritonsillar and deep neck infections-cross-sectional retrospective analysis. World J. Otorhinolaryngol. Head Neck Surg. 2019, 5, 207–214. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Shah, J.R.; Gazali, N.; Rajan, P. Life-threatening para-pharyngeal and retropharyngeal abscess in an infant. BMJ Case Rep. 2017, 2017, bcr2017221269. [Google Scholar] [CrossRef]
- Bernardini, L.; Serra, L.; Calamelli, E.; Bottau, P.; Silvestrini, D.; Pession, A. Gli ascessi retrofaringei. Med. Bambino 2020, 39, 161–166. [Google Scholar]
- Dawes, L.C.; Bova, R.; Peter Carter, P. Retropharyngeal abscess in children. ANZ J. Surg. 2020, 72, 417–420. [Google Scholar] [CrossRef]
- Sankararaman, S.; Riel-Romero, R.M.S.; Gonzalez-Toledo, E. Brain abscess from a peritonsillar abscess in an immunocompetent child: A case report and review of the literature. Pediatr. Neurol. 2012, 47, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Pericleous, A.; Wilkinson, S.; Gerrish, A.; Daniel, M. Peritonsillar abscess in an infant with congenital bone marrow failure. Int. J. Pediatr. Otorhinolaryngol. 2019, 124, 200–202. [Google Scholar] [CrossRef]
- Oleske, J.M.; Starr, S.E.; Nahmias, A.J. Complications of Peritonsillar Abscess Due to Fusobacterium necrophorum. Pediatrics 1976, 57, 570–571. [Google Scholar] [CrossRef]
- Ravi, K.V.; Brooks, J.R. Peritonsillar abscess—An unusual presentation of Kawasaki disease. J. Laryngol. Otol. 1997, 111, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Isidori, C.; Sebastiani, L.; Esposito, S. A Case of Incomplete and Atypical Kawasaki Disease Presenting with Retropharyngeal Involvement. Int. J. Environ. Res. Public Health 2019, 16, 3262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, E.R.; John, S.D. Imaging of Pediatric Neck Masses. Radiol. Clin. N. Am. 2011, 49, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Backstrom, J. Comparison of the sensitivity of lateral neck radio-graphs and computed to mography scanning in pediatric deep-neck infections. Laryngoscope 1999, 109, 775–779. [Google Scholar] [CrossRef]
- Lawrence, R.; Bateman, N. Controversies in the management of deep neck space infection in children: An evidence-based review. Clin. Otolaryngol. 2017, 42, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Elden, L.M.; Grundfast, K.M.; Vezina, G. Accuracy and Usefulness of Radiographic Assessment of Cervical Neck Infections in Children. J. Otolaryngol. 2001, 30, 82–89. [Google Scholar] [CrossRef]
- Meyer, A.C.; Kimbrough, T.G.; Finkelstein, M.; Sidman, J.D. Symptom duration and CT findings in pediatric deep neck infection. Otolaryngol. Head Neck Surg. 2009, 140, 183–186. [Google Scholar] [CrossRef]
- Maroldi, R.; Farina, D.; Ravanelli, M.; Lombardi, D.; Nicolai, P. Emergency Imaging Assessment of Deep Neck Space Infections. Semin. Ultrasound CT MRI 2012, 33, 432–442. [Google Scholar] [CrossRef]
- Han, S.M.; Chae, H.S.; Lee, H.N.; Jeon, H.J.; Bong, J.P.; Kim, J.H. Computed tomography-guided navigation assisted drainage for inaccessible deep neck abscess: A case report. Medicine 2019, 98, e14674. [Google Scholar] [CrossRef]
- Miller, W.D.; Furst, I.M.; Sàndor, G.K.; Keller, M.A. A prospective, blinded compari-son of clinical examination and computed tomography in deep neck infections. Laryngoscope 1999, 109, 1873–1879. [Google Scholar] [CrossRef]
- Collins, B.; Stoner, J.A.; Digoy, G.P. Benefits of ultrasound vs. computed tomography in the diagnosis of pediatric lateral neck abscesses. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 423–426. [Google Scholar] [CrossRef]
- Mallorie, C.N.J.; Jones, S.D.; Drage, N.A.; Shepherd, J. The reliability of high resolu-tion ultrasound in the identification of pus collections in head and neck swellings. Int. J. Oral Maxillofac. Surg. 2012, 41, 252–255. [Google Scholar] [CrossRef]
- Araujo Filho, B.C.; Sakae, F.A.; Sennes, L.U.; Imamura, R.; de Menezes, M.R. Intraoral and transcutaneous cervical ultrasound in the differential diagnosis of peritonsillar cellulitis and abscesses. Braz. J. Otorhinolaryngol. 2006, 72, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Fordham, M.T.; Rock, A.N.; Bandarkar, A.; Preciado, D.; Levy, M.; Cohen, J.; Safdar, N.; Reilly, B.K. Transcervical ultrasonogra-phy in the diagnosis of pediatric peritonsillar abscess. Laryngoscope 2015, 125, 2799–2804. [Google Scholar] [CrossRef]
- Gonzalez-Beicos, A.; Nunez, D. Imaging of Acute Head and Neck Infections. Radiol. Clin. N. Am. 2012, 50, 73–83. [Google Scholar] [CrossRef]
- Nurminen, J.; Heikkinen, J.; Happonen, T.; Velhonoja, J.; Irjala, H.; Soukka, T.; Ivaska, L.; Mattila, K.; Hirvonen, J. Magnetic resonance imaging findings in pediatric neck infections—A comparison with adult patients. Pediatr. Radiol. 2022; in press. [Google Scholar] [CrossRef]
- Nurminen, J.; Heikkinen, J.; Velhonoja, J.; Happonen, T.; Nyman, M.; Irjala, H.; Soukka, T.; Mattila, K.; Hirvonen, J. Emergency neck MRI: Feasibility and diagnostic accuracy in cases of neck infection. Acta Radiol. 2021, 62, 735–742. [Google Scholar] [CrossRef]
- Heikkinen, J.; Nurminen, J.; Velhonoja, J.; Irjala, H.; Happonen, T.; Soukka, T.; Mattila, K.; Hirvonen, J. Clinical and prognostic significance of emergency MRI findings in neck infections. Eur. Radiol. 2022, 32, 1078–1086. [Google Scholar] [CrossRef]
- Conte, M.; Vinci, F.; Muzzi, E.; Canuto, A.; Barbi, E.; Cozzi, G. Magnetic resonance imaging accuracy before surgery in children with retropharyngeal abscesses. J. Paediatr. Child Health 2021, 58, 504–507. [Google Scholar] [CrossRef]
- Brook, I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal ab-scesses. J. Oral Maxillofac. Surg. 2004, 62, 1545–1550. [Google Scholar] [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schraff, S.; McGinn, J.D.; Derkay, C.S. Peritonsillar abscess in children: A 10-year review of diagnosis and management. Int. J. Pediatr. Otorhinolaryngol. 2001, 57, 213–218. [Google Scholar] [CrossRef]
- Westmore, G.A. Cervical abscess: A life-threatening complication of infectious mononucleosis. J. Laryngol. Otol. 1990, 104, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.K.; Brown, C.; Mills, N.; Spielmann, P.; Neeff, M. To drain or not to drain—Management of pediat-ric deep neck abscesses: A case-control study. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1810–1813. [Google Scholar] [CrossRef]
- Cheng, J.; Elden, L. Children with Deep Space Neck Infections: Our Experience with 178 Children and Proposed Management Strategy. Otolaryngol. Neck Surg. 2013, 149, P114–P115. [Google Scholar] [CrossRef]
- Stone, M.E.; Walner, D.L.; Koch, B.L.; Egelhoff, J.C.; Myer, C.M. Correlation between computed tomography and surgical findings in retropharyngeal inflamma-tory processes in children. Int. J. Pediatr. Otorhinolaryngol. 1999, 49, 121–125. [Google Scholar] [CrossRef]
- Song, M.; Demiray, U.; Adibelli, Z.H.; Adibelli, H. Bilateral deep neck space infec-tion in the paediatric age group: A case report and review of the literature. Acta Otorhinolaryngol. Ital. 2011, 31, 190. [Google Scholar]
- Castagnini, L.A.; Goyal, M.; Ongkasuwan, J. Tonsillitis and Peritonsillar Abscess. In Infectious Diseases in Pediatric Otolaryngology; Valdez, T., Vallejo, J., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Tagarro, A.; Otheo, E.; Baquero-Artigao, F.; Navarro, M.-L.; Velasco, R.; Ruiz, M.; Penín, M.; Moreno, D.; Rojo, P.; Madero, R.; et al. Dexamethasone for Parapneumonic Pleural Effusion: A Randomized, Double-Blind, Clinical Trial. J. Pediatr. 2017, 185, 117–123.e6. [Google Scholar] [CrossRef]
- Hur, K.; Zhou, S.; Kysh, L. Adjunct steroids in the treatment of peritonsillar ab-scess: A systematic review. Laryngoscope 2018, 128, 72–77. [Google Scholar] [CrossRef]
- Tansey, J.B.; Hamblin, J.; Mamidala, M.; Thompson, J.; Mclevy, J.; Wood, J.; Sheyn, A. Dexamethasone Use in the Treatment of Pediatric Deep Neck Space Infections. Ann. Otol. Rhinol. Laryngol. 2020, 129, 376–379. [Google Scholar] [CrossRef]
- Chang, B.A.; Thamboo, A.; Diamond, C.; Nunez, D.A. Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess. Cochrane Database Syst. Rev. 2016, 12, CD006287. [Google Scholar] [CrossRef]
- Herzon, F.S.; Martin, A.D. Medical and surgical treatment of peritonsillar, retro-pharyngeal, and parapharyngeal abscesses. Curr. Infect. Dis. Rep. 2006, 8, 196–202. [Google Scholar] [CrossRef]
- Tapiovaara, L.; Bäck, L.; Aro, K. Comparison of intubation and tracheotomy in patients with deep neck infection. Eur. Arch. Otorhinolaryngol. 2017, 274, 3767–3772. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, S.; De Guido, C.; Pappalardo, M.; Laudisio, S.; Meccariello, G.; Capoferri, G.; Rahman, S.; Vicini, C.; Principi, N. Retropharyngeal, Parapharyngeal and Peritonsillar Abscesses. Children 2022, 9, 618. https://doi.org/10.3390/children9050618
Esposito S, De Guido C, Pappalardo M, Laudisio S, Meccariello G, Capoferri G, Rahman S, Vicini C, Principi N. Retropharyngeal, Parapharyngeal and Peritonsillar Abscesses. Children. 2022; 9(5):618. https://doi.org/10.3390/children9050618
Chicago/Turabian StyleEsposito, Susanna, Claudia De Guido, Marco Pappalardo, Serena Laudisio, Giuseppe Meccariello, Gaia Capoferri, Sofia Rahman, Claudio Vicini, and Nicola Principi. 2022. "Retropharyngeal, Parapharyngeal and Peritonsillar Abscesses" Children 9, no. 5: 618. https://doi.org/10.3390/children9050618
APA StyleEsposito, S., De Guido, C., Pappalardo, M., Laudisio, S., Meccariello, G., Capoferri, G., Rahman, S., Vicini, C., & Principi, N. (2022). Retropharyngeal, Parapharyngeal and Peritonsillar Abscesses. Children, 9(5), 618. https://doi.org/10.3390/children9050618






