Making the Argument for Intact Cord Resuscitation: A Case Report and Discussion
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
3.1. Lung Readiness and Preparation for the First Breath
3.1.1. Lung Growth and Development in the Fetus
3.1.2. Anatomy of the Respiratory Tract and Blood Supply
3.1.3. Switching from Fetal to Neonatal Respiratory Function
3.1.4. Breathing after Birth
3.2. Circulation
3.2.1. Contents of Cord Blood
3.2.2. Placental Transfusion Enables the Return of Blood Volume
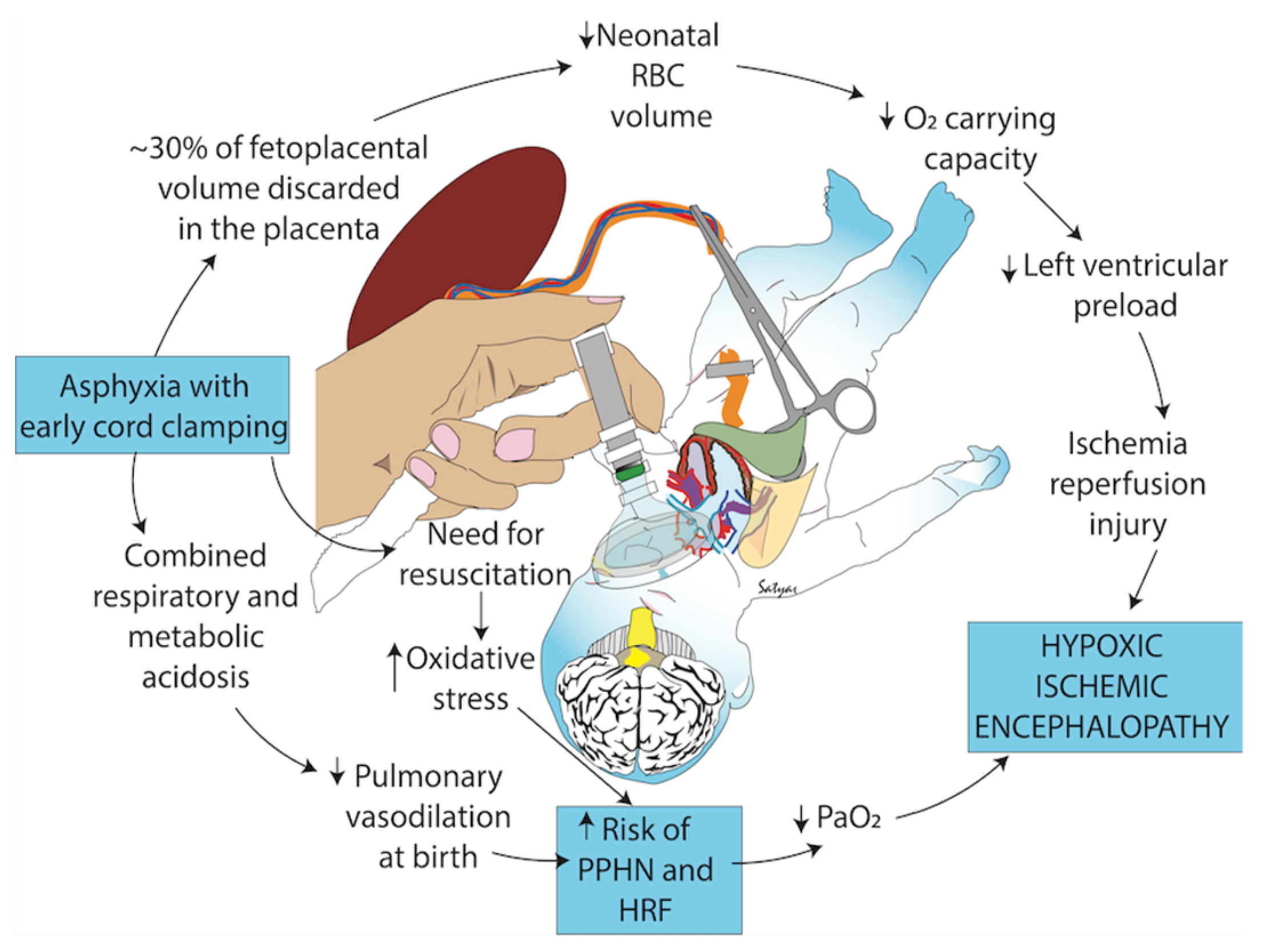
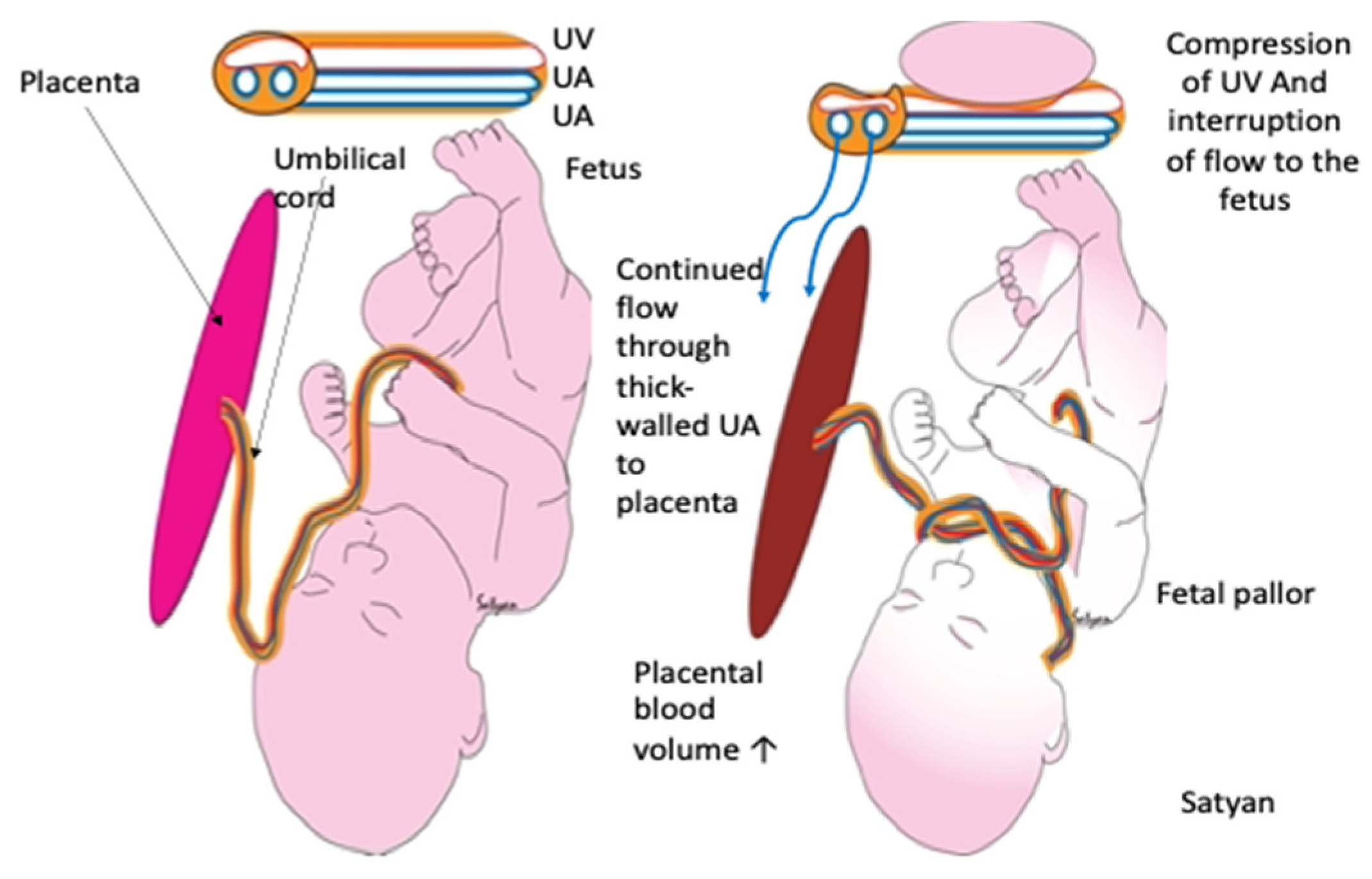
3.2.3. Effect of Occlusion of the Umbilical Cord
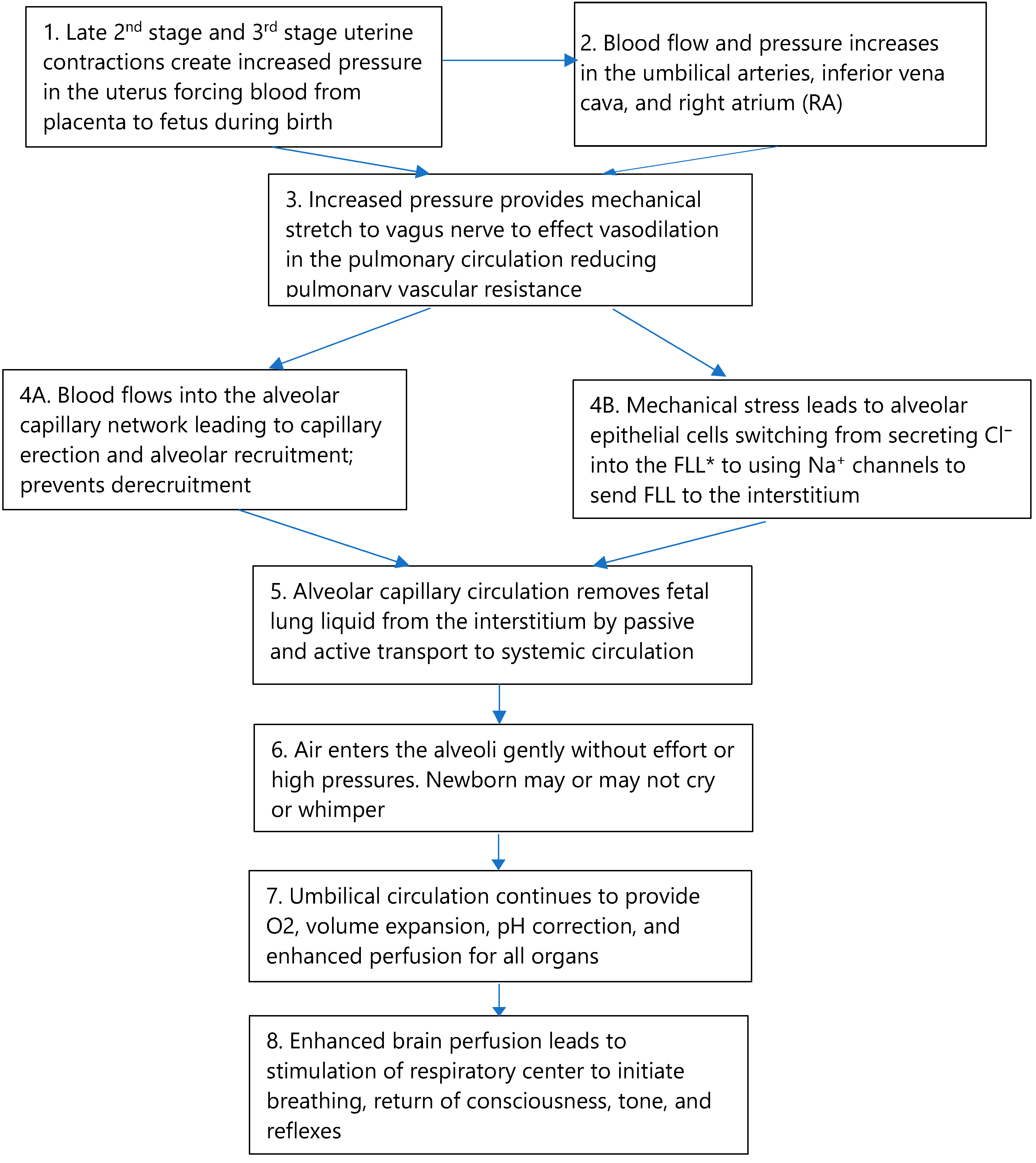
3.3. Full Perfusion, the Vagus Nerve, and Pulmonary Vascular Resistance
3.3.1. The Vagal Stimulation Hypothesis
3.3.2. The Autonomic Nervous System
3.4. Effects of Enhanced Perfusion on Consciousness, Breathing, Tone, and Reflexes
3.5. One Caveat: A Note of Caution
4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aziz, K.; Lee, H.C.; Escobedo, M.B.; Hoover, A.V.; Kamath-Rayne, B.D.; Kapadia, V.S.; Magid, D.J.; Niermeyer, S.; Schmölzer, G.M.; Szyld, E.; et al. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2021, 147, e2020038505E. [Google Scholar] [CrossRef] [PubMed]
- Katheria, A.C.; Rich, W.D.; Bava, S.; Lakshminrusimha, S. Placental Transfusion for Asphyxiated Infants. Front. Pediatr. 2019, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Rivera, B.K.; Haque, U.; Bridge, J.A.; Smith, C.V.; Hutchon, D.J.R.; Mercer, J.S. Placental Transfusion Strategies in Very Preterm Neonates. Obstet. Gynecol. 2014, 124, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, M.; Osborn, D.A.; Askie, L.; Seidler, A.L.; Hunter, K.; Lui, K.; Simes, J.; Tarnow-Mordi, W. Delayed vs early umbilical cord clamping for preterm infants: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2018, 218, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rabe, H.; Gyte, G.M.; Díaz-Rossello, J.L.; Duley, L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst. Rev. 2019, 9, CD003248. [Google Scholar] [CrossRef]
- Robledo, K.P.; O Tarnow-Mordi, W.; Rieger, I.; Suresh, P.; Martin, A.; Yeung, C.; Ghadge, A.; Liley, H.G.; Osborn, D.; Morris, J.; et al. Effects of delayed versus immediate umbilical cord clamping in reducing death or major disability at 2 years corrected age among very preterm infants (APTS): A multicentre, randomised clinical trial. Lancet Child Adolesc. Health 2022, 6, 150–157. [Google Scholar] [CrossRef]
- Mercer, J.S.; Erickson-Owens, D.A.; Rabe, H. Placental transfusion: May the “force” be with the baby. J. Perinatol. 2021, 41, 1495–1504. [Google Scholar] [CrossRef]
- Bhatt, S.; Polglase, G.; Wallace, E.; Pas, A.T.; Hooper, S.B. Ventilation before Umbilical Cord Clamping Improves the Physiological Transition at Birth. Front. Pediatr. 2014, 2, 113. [Google Scholar] [CrossRef] [Green Version]
- Kc, A.; Singhal, N.; Gautam, J.; Rana, N.; Andersson, O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth—Randomized clinical trial. Matern. Health Neonatol. Perinatol. 2019, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Yao, A. Lind, Distribution of blood between infant and placenta after birth. Lancet 1969, 2, 871–873. [Google Scholar] [CrossRef]
- Chaudhury, S.; Saqibuddin, J.; Birkett, R.; Falcon-Girard, K.; Kraus, M.; Ernst, L.M.; Grobman, W.; Mestan, K.K. Variations in Umbilical Cord Hematopoietic and Mesenchymal Stem Cells with Bronchopulmonary Dysplasia. Front. Pediatr. 2019, 7, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badurdeen, S.; Roberts, C.; Blank, D.; Miller, S.; Stojanovska, V.; Davis, P.; Hooper, S.; Polglase, G. Haemodynamic Instability and Brain Injury in Neonates Exposed to Hypoxia–Ischaemia. Brain Sci. 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katheria, A.; Hosono, S.; El-Naggar, W. A new wrinkle: Umbilical cord management (how, when, who). Semin. Fetal Neonatal Med. 2018, 23, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Andersson, O.; Rana, N.; Ewald, U.; Målqvist, M.; Stripple, G.; Basnet, O.; Subedi, K.; Kc, A. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 minutes of birth (Nepcord III)—A randomized clinical trial. Matern. Health Neonatol. Perinatol. 2019, 5, 15. [Google Scholar] [CrossRef]
- Isacson, M.; Gurung, R.; Basnet, O.; Andersson, O.; Kc, A. Neurodevelopmental outcomes of a randomised trial of intact cord resuscitation. Acta Paediatr. 2021, 110, 465–472. [Google Scholar] [CrossRef]
- Polglase, G.R.; Schmölzer, G.M.; Roberts, C.; Blank, D.A.; Badurdeen, S.; Crossley, K.J.; Miller, S.L.; Stojanovska, V.; Galinsky, R.; Kluckow, M.; et al. Cardiopulmonary Resuscitation of Asystolic Newborn Lambs Prior to Umbilical Cord Clamping; the Timing of Cord Clamping Matters! Front. Physiol. 2020, 11, 902. [Google Scholar] [CrossRef]
- Mercer, J.; Erickson-Owens, D.; Skovgaard, R. Fetal to Neonatal Transition: First, Do No Harm. In Normal Birth, 2nd ed.; Churchill Livingstone Elsevier: Edinburgh, Scotland, 2008; pp. 149–174. [Google Scholar]
- Fulton, C.; Stoll, K.; Thordarson, D. Bedside resuscitation of newborns with an intact umbilical cord: Experiences of midwives from British Columbia. Midwifery 2016, 34, 42–46. [Google Scholar] [CrossRef]
- Downey, C.L.; Bewley, S. Historical perspectives on umbilical cord clamping and neonatal transition. J. R. Soc. Med. 2012, 105, 325–329. [Google Scholar] [CrossRef]
- Plosa, E.; Guttentag, S.H. Lung Development. In Avery’s Diseases of the Newborn, 10th ed.; Elsevie: Philadephia, PA, USA, 2018; pp. 586–599. [Google Scholar]
- Hooper, S.B.; Harding, R. Harding, Fetal lung liquid: A major determinant of the growth and functional development of the fetal lung. Clin. Exp. Pharmacol. Physiol. 1995, 22, 235–247. [Google Scholar] [CrossRef]
- Bland, R.D. Lung Fluid Balance During Development. NeoReviews 2005, 6, e255–e267. [Google Scholar] [CrossRef]
- Lumb, A.; Slinger, P. Hypoxic Pulmonary Vasoconstriction. Anesthesiology 2015, 122, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Horowitz, J.C.; Naba, A.; Ambalavanan, N.; Atabai, K.; Balestrini, J.; Bitterman, P.B.; Corley, R.A.; Ding, B.-S.; Engler, A.J.; et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018, 73, 77–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaykka, S. Capillary erection and the structural appearance of fetal and neonatl lungs. Acta Paediatr. 1958, 47, 484–500. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.S.; Skovgaard, R.L. Neonatal Transitional Physiology. J. Périnat. Neonatal Nurs. 2002, 15, 56–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
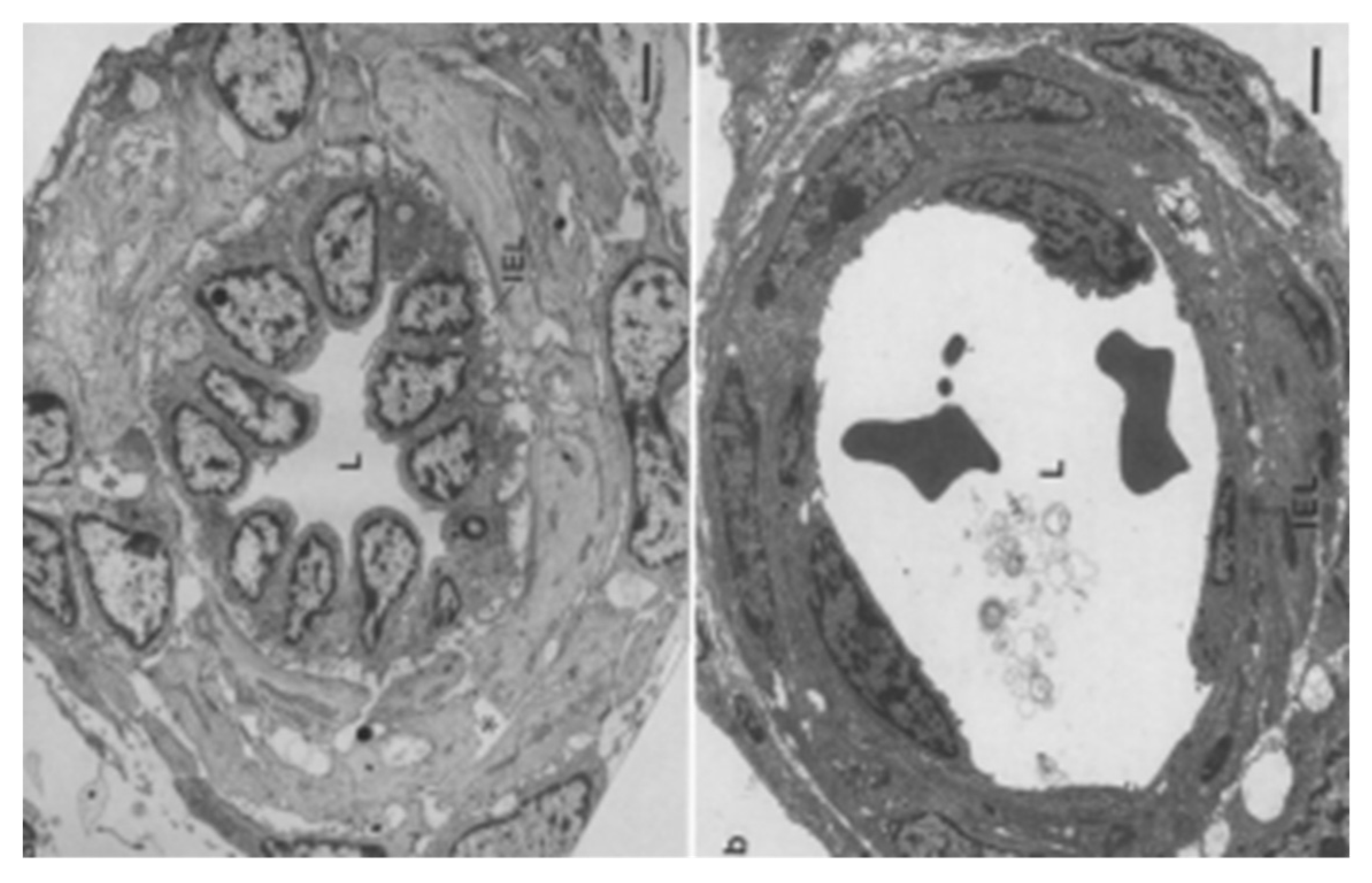
- Haworth, S.G.; Hall, S.M.; Chew, M.; Allen, K. Thinning of fetal pulmonary arterial wall and postnatal remodeling: Ultrastructural studies on the respiratory unit arteries of the pig. Virchows Arch A 1987, 411, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Raj, J.U. Regulation of the Pulmonary Circulation in the Fetus and Newborn. Physiol. Rev. 2010, 90, 1291–1335. [Google Scholar] [CrossRef] [Green Version]
- Perks, A.M.; Cassin, S. The rate of production of lung liquid in fetal goats, and the effect of expansion of the lungs. J. Dev. Physiol. 1985, 7, 149–160. [Google Scholar]
- Lorenz, L.; Axnick, J.; Buschmann, T.; Henning, C.; Urner, S.; Fang, S.; Nurmi, H.; Eichhorst, N.; Holtmeier, R.; Bódis, K.; et al. Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival. Nature 2018, 562, 128–132. [Google Scholar] [CrossRef]
- Hall, S.M.; Haworth, S.G. Normal adaptation of pulmonary arterial intima to extrauterine life in the pig: Ultrastructural studies. J. Pathol. 1986, 149, 55–66. [Google Scholar] [CrossRef]
- Giovannini, N.; Crippa, B.L.; Denaro, E.; Raffaeli, G.; Cortesi, V.; Consonni, D.; Cetera, G.E.; Parazzini, F.; Ferrazzi, E.; Mosca, F.; et al. The effect of delayed umbilical cord clamping on cord blood gas analysis in vaginal and caesarean-delivered term newborns without fetal distress: A prospective observational study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 405–413. [Google Scholar] [CrossRef]
- Wiberg, N.; Källén, K.; Olofsson, P. Delayed umbilical cord clamping at birth has effects on arterial and venous blood gases and lactate concentrations. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Lara-Cantón, I.; Badurdeen, S.; Dekker, J.; Davis, P.; Roberts, C.; Pas, A.T.; Vento, M. Oxygen saturation and heart rate in healthy term and late preterm infants with delayed cord clamping. Pediatr. Res. 2022; epub ahead of print. [Google Scholar] [CrossRef]
- Spiers, A.; Legendre, G.; Biquard, F.; Descamps, P.; Corroenne, R. Ex Utero Intrapartum Technique (EXIT): Indications, procedure methods and materno-fetal complications – A literature review. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102252. [Google Scholar] [CrossRef]
- Hirose, S.; Sydorak, R.M.; Tsao, K.; Cauldwell, C.B.; Newman, K.D.; Mychaliska, G.; Albanese, C.T.; Lee, H.; Farmer, D. Spectrum of intrapartum management strategies for giant fetal cervical teratoma. J. Pediatr. Surg. 2003, 38, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.L.; Lamberska, T.; Martherus, T.; Dekker, J.; Böhringer, S.; Hooper, S.B.; Plavka, R.; Pas, A.B.T. The effect of a face mask for respiratory support on breathing in preterm infants at birth. Resuscitation 2019, 144, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuypers, K.; Martherus, T.; Lamberska, T.; Dekker, J.; Hooper, S.B.; Pas, A.B.T. Reflexes that impact spontaneous breathing of preterm infants at birth: A narrative review. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 675–679. [Google Scholar] [CrossRef]
- Gaertner, V.D.; Rüegger, C.M.; O’Currain, E.; Kamlin, C.O.F.; Hooper, S.B.; Davis, P.G.; Springer, L. Physiological responses to facemask application in newborns immediately after birth. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 381–385. [Google Scholar] [CrossRef]
- Dekker, J.; van Kaam, A.H.; Roehr, C.C.; Flemmer, A.W.; Foglia, E.E.; Hooper, S.B.; Pas, A.B.T. Stimulating and maintaining spontaneous breathing during transition of preterm infants. Pediatr. Res. 2021, 90, 722–730. [Google Scholar] [CrossRef]
- Panneton, W.M.; Gan, Q. The Mammalian Diving Response: Inroads to Its Neural Control. Front. Neurosci. 2020, 14, 524. [Google Scholar] [CrossRef]
- Brouwer, E.; Pas, A.B.T.; Polglase, G.R.; McGillick, E.; Böhringer, S.; Crossley, K.J.; Rodgers, K.; Blank, D.; Yamaoka, S.; Gill, A.W.; et al. Effect of spontaneous breathing on umbilical venous blood flow and placental transfusion during delayed cord clamping in preterm lambs. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 26–32. [Google Scholar] [CrossRef]
- Nair, J.; Davidson, L.; Gugino, S.; Koenigsknecht, C.; Helman, J.; Nielsen, L.; Sankaran, D.; Agrawal, V.; Chandrasekharan, P.; Rawat, M.; et al. Sustained Inflation Reduces Pulmonary Blood Flow during Resuscitation with an Intact Cord. Children 2021, 8, 353. [Google Scholar] [CrossRef]
- Kirpalani, H.; Ratcliffe, S.; Keszler, M.; Davis, P.G.; Foglia, E.E.; Pas, A.T.; Fernando, M.; Chaudhary, A.; Localio, R.; Van Kaam, A.H.; et al. Effect of Sustained Inflations vs Intermittent Positive Pressure Ventilation on Bronchopulmonary Dysplasia or Death Among Extremely Preterm Infants. JAMA 2019, 321, 1165–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claassen, C.C.; Strand, M.L. Understanding the Risks and Benefits of Delivery Room CPAP for Term Infants. Pediatrics 2019, 144, e20191720. [Google Scholar] [CrossRef] [PubMed]
- Avery, M.E.; Frank, N.R.; Gribetz, I. The inflationary force produced by pulmonary vascular distension in excised lungs. J. Clin. Investig. 1959, 38, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Katheria, A.C.; Brown, M.K.; Faksh, A.; Hassen, K.O.; Rich, W.; Lazarus, D.; Steen, J.; Daneshmand, S.S.; Finer, N.N. Delayed Cord Clamping in Newborns Born at Term at Risk for Resuscitation: A Feasibility Randomized Clinical Trial. J. Pediatr. 2017, 187, 313–317.e1. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Nevill, E. The Assisted Breathing before Cord Clamping (ABC) Study Protocol. Children 2021, 8, 336. [Google Scholar] [CrossRef]
- Dewey, K.G.; Chaparro, C.M. Session 4: Mineral metabolism and body composition Iron status of breast-fed infants. Proc. Nutr. Soc. 2007, 66, 412–422. [Google Scholar] [CrossRef]
- Andersson, O.; Mercer, J.S. Cord Management of the Term Newborn. Clin. Perinatol. 2021, 48, 447–470. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Lawton, C.; Acosta, S.; Watson, N.; Gonzales-Portillo, C.; Diamandis, T.; Tajiri, N.; Kaneko, Y.; Sanberg, P.R. Enhancing endogenous stem cells in the newborn via delayed umbilical cord clamping. Neural Regen. Res. 2015, 10, 1359–1362. [Google Scholar] [CrossRef]
- González-Orozco, J.C.; Camacho-Arroyo, I. Progesterone Actions During Central Nervous System Development. Front. Neurosci. 2019, 13, 503. [Google Scholar] [CrossRef] [Green Version]
- Sippell, W.G.; Becker, H.; Versmold, H.T.; Bidlingmaier, F.; Knorr, D. Longitudinal Studies of Plasma Aldosterone, Corticosterone, Deoxycorticosterone, Progesterone, 17-Hydroxyprogesterone, Cortisol, and Cortisone Determined Simultaneously in Mother and Child at Birth and during the Early Neonatal Period. I. Spontaneous Delivery. J. Clin. Endocrinol. Metab. 1978, 46, 971–985. [Google Scholar] [CrossRef]
- Disdier, C.; Zhang, J.; Fukunaga, Y.; Lim, Y.; Qiu, J.; Santoso, A.; Stonestreet, B.S. Alterations in inter-alpha inhibitor protein expression after hypoxic-ischemic brain injury in neonatal rats. Int. J. Dev. Neurosci. 2018, 65, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Arcilla, R.A.; Oh, W.; Lind, J.; Gessner, I.H. Pulmonary Arterial Pressures of Newborn Infants Born with Early and Late Clamping of the Cord. Acta Paediatr. 1966, 55, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Caldeyro, R.; Alvarez, H.; Reynolds, S.R.M. Reynolds, A better understanding of uterine contractility through simultaneous recording with an internal and a seven channel external method. Obstet. Gynecol. Surv. 1950, 91, 641–650. [Google Scholar] [CrossRef]
- Hutchon, D.; Pratesi, S.; Katheria, A. How to Provide Motherside Neonatal Resuscitation with Intact Placental Circulation? Children 2021, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Lear, C.A.; Galinsky, R.; Wassink, G.; Yamaguchi, K.; Davidson, J.; Westgate, J.A.; Bennet, L.; Gunn, A.J. The myths and physiology surrounding intrapartum decelerations: The critical role of the peripheral chemoreflex. J. Physiol. 2016, 594, 4711–4725. [Google Scholar] [CrossRef]
- Boere, I.; Roest, A.A.W.; Wallace, E.; Harkel, A.D.J.T.; Haak, M.C.; Morley, C.; Hooper, S.B.; Pas, A.T. Umbilical blood flow patterns directly after birth before delayed cord clamping. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F121–F125. [Google Scholar] [CrossRef] [Green Version]
- Mercer, J.; Erickson-Owens, D.; Skovgaard, R. Cardiac asystole at birth: Is hypovolemic shock the cause? Med. Hypotheses 2009, 72, 458–463. [Google Scholar] [CrossRef]
- Menticoglou, S.; Schneider, C. Resuscitating the Baby after Shoulder Dystocia. Case Rep. Obstet. Gynecol. 2016, 2016, 8674167. [Google Scholar] [CrossRef] [Green Version]
- Ancora, G.; Meloni, C.; Soffritti, S.; Sandri, F.; Ferretti, E. Intrapartum Asphyxiated Newborns Without Fetal Heart Rate and Cord Blood Gases Abnormalities: Two Case Reports of Shoulder Dystocia to Reflect Upon. Front. Pediatr. 2020, 8, 570332. [Google Scholar] [CrossRef]
- Polglase, G.; Kluckow, M.; Gill, A.; Hooper, S. Hemodynamics of the Circulatory Transition. In Hypoxic Respiratory Failure in the Newborn: From Origins to Clinical Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 57–60. [Google Scholar]
- Lang, J.A.R.; Pearson, J.T.; Binder-Heschl, C.; Wallace, M.J.; Siew, M.L.; Kitchen, M.; Pas, A.T.; Lewis, R.A.; Polglase, G.R.; Shirai, M.; et al. Vagal denervation inhibits the increase in pulmonary blood flow during partial lung aeration at birth. J. Physiol. 2017, 595, 1593–1606. [Google Scholar] [CrossRef] [Green Version]
- Segar, J.L. Ontogeny of the arterial and cardiopulmonary baroreflex during fetal and postnatal life. Am. J. Physiol. Content 1997, 273, R457–R471. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, S.; Gilbert, G.; Cardouat, G.; Baudrimont, I.; Freund-Michel, V.; Guibert, C.; Marthan, R.; Vacher, P.; Quignard, J.-F.; Ducret, T. Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension. Biomolecules 2021, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.C.; Sinoway, L.I. Autonomic Control of the Heart. In Primer on Autonomic Nervous System, 3rd ed.; Elsivier: New York, NY, USA, 2012; pp. 177–180. [Google Scholar]
- Mulkey, S.B.; Plessis, A.D. The Critical Role of the Central Autonomic Nervous System in Fetal-Neonatal Transition. Semin. Pediatr. Neurol. 2018, 28, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. Cardiac vagal tone: A neurophysiological mechanism that evolved in mammals to dampen threat reactions and promote sociality. World Psychiatry 2021, 20, 296–298. [Google Scholar] [CrossRef]
- McIntyre, S.; Nelson, K.; Mulkey, S.; Lechpammer, M.; Molloy, E.; Badawi, N. Neonatal encephalopathy: Focus on epidemiology and underexplored aspects of etiology. Semin. Fetal Neonatal Med. 2021, 26, 101265. [Google Scholar] [CrossRef]
- Brace, R.A. Fetal blood volume responses to acute fetal hypoxia. Am. J. Obstet. Gynecol. 1986, 155, 889–893. [Google Scholar] [CrossRef]

| Component | Lung Fluid | Interstitial Fluid | Plasma | Amniotic Fluid |
|---|---|---|---|---|
| pH | 6.27 | 7.31 | 7.34 | 7.02 |
| Bicarbonate * | 3 | 25 | 24 | 19 |
| Protein (g/dL) | 0.03 | 3.27 | 4.09 | 0.10 |
| Sodium * | 150 | 147 | 150 | 113 |
| Potassium * | 6.3 | 4.8 | 4.8 | 7.6 |
| Chloride * | 157 | 107 | 107 | 87 |
| Factors/Messengers | Role |
|---|---|
| Angiopoietin | Vascular growth factor |
| Granulocyte-colony stimulating factor (G-CSF) | Stimulates bone marrow to make granulocytes and stem cells to release them |
| Bone morphogenic protein-9 (BMP-9) | Regulates iron metabolism; role in memory, learning, attention; bone formation |
| Endoglin (ENG) | Transmembrane glycoprotein in endothelial cells; growth factor; role in angiogenesis |
| Endothelian-1 (ET-1) | Peptide with key role in vascular homeostasis; vasoconstriction |
| Epidermal Growth Factor (EGF) | Transmembrane protein binding; cellular proliferation, differentiation, and survival |
| Interleukin-8 (IL-8) | Increases angiogenesis and phagocytosis; causes WBCs to migrate to site of injury; chemotaxis |
| Hepatic Growth Factor (HGF) | Morphogenic factor; paracrine cell growth motility |
| Heparin Binding EGF-like GF (HBEGF | Glycoprotein; role in heart development and function; would healing |
| Placental Growth Factor (PGF) | Role in angiogenesis and vasculogenesis |
| VEGF-A | Acts on ECs, increases vascular permeability, angio- and vasculogenesis, EC cell growth, cell migration; decreases apoptosis |
| Intra-Alpha inhibitor protein (IAIP) * | Provides anti-inflammatory neuroprotection; reduces production of reactive oxygen species * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercer, J.; Erickson-Owens, D.; Rabe, H.; Jefferson, K.; Andersson, O. Making the Argument for Intact Cord Resuscitation: A Case Report and Discussion. Children 2022, 9, 517. https://doi.org/10.3390/children9040517
Mercer J, Erickson-Owens D, Rabe H, Jefferson K, Andersson O. Making the Argument for Intact Cord Resuscitation: A Case Report and Discussion. Children. 2022; 9(4):517. https://doi.org/10.3390/children9040517
Chicago/Turabian StyleMercer, Judith, Debra Erickson-Owens, Heike Rabe, Karen Jefferson, and Ola Andersson. 2022. "Making the Argument for Intact Cord Resuscitation: A Case Report and Discussion" Children 9, no. 4: 517. https://doi.org/10.3390/children9040517
APA StyleMercer, J., Erickson-Owens, D., Rabe, H., Jefferson, K., & Andersson, O. (2022). Making the Argument for Intact Cord Resuscitation: A Case Report and Discussion. Children, 9(4), 517. https://doi.org/10.3390/children9040517






