Effect of Massage with Oil Balanced in Essential Fatty Acids on Development and Lipid Parameters in Very Premature Neonates: A Randomized, Controlled Study
Abstract
:1. Introduction
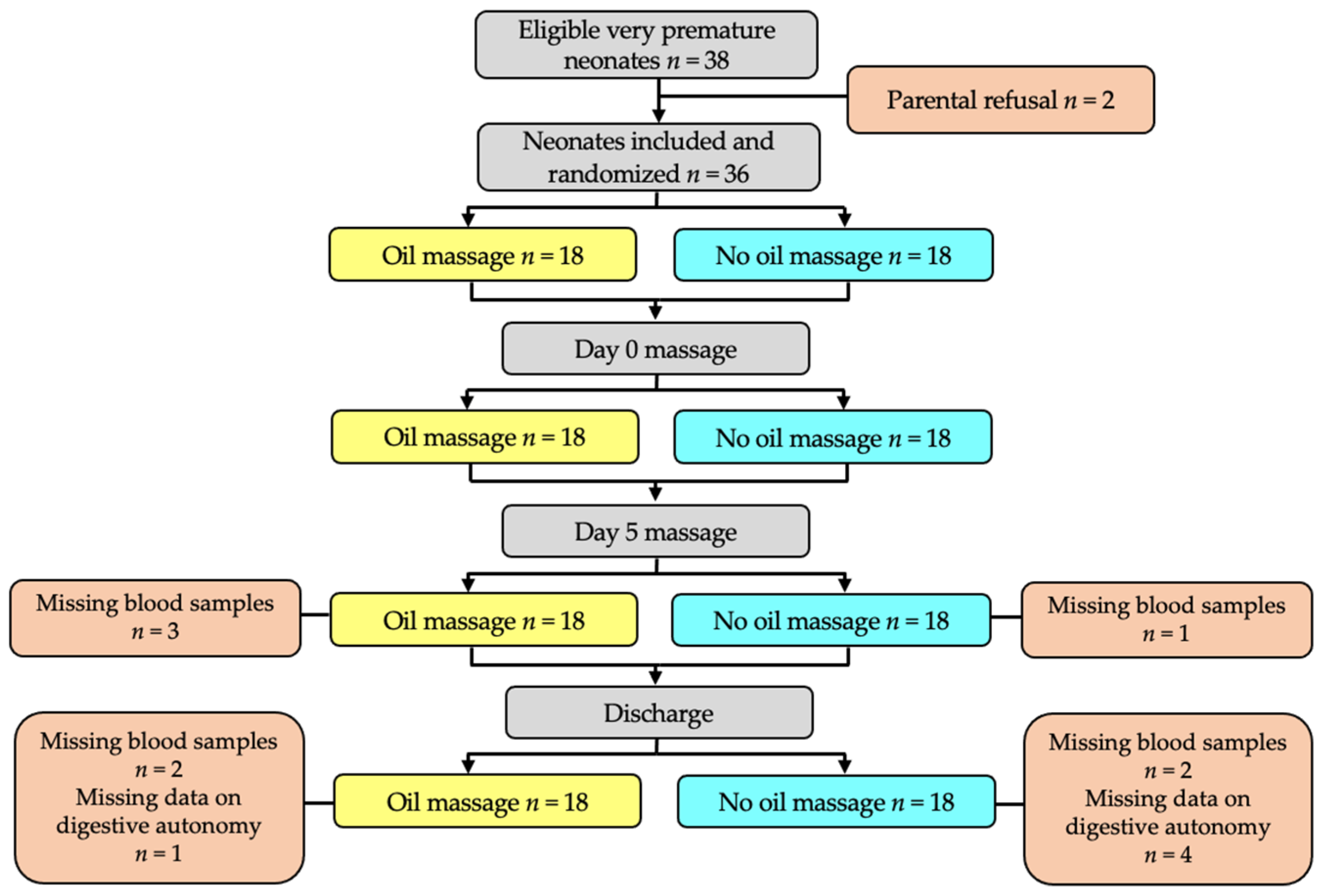
2. Subjects and Methods
2.1. Ethical Approval
2.2. Design
2.3. Studied Population
2.4. Massage Intervention
2.5. Measurements
2.6. Blood Collection and Lipid Analysis
2.7. Statistical Analyzes
3. Results
3.1. Characteristics of the Two Groups after Randomization
3.2. Effect of Oil Massage on Neonate Development
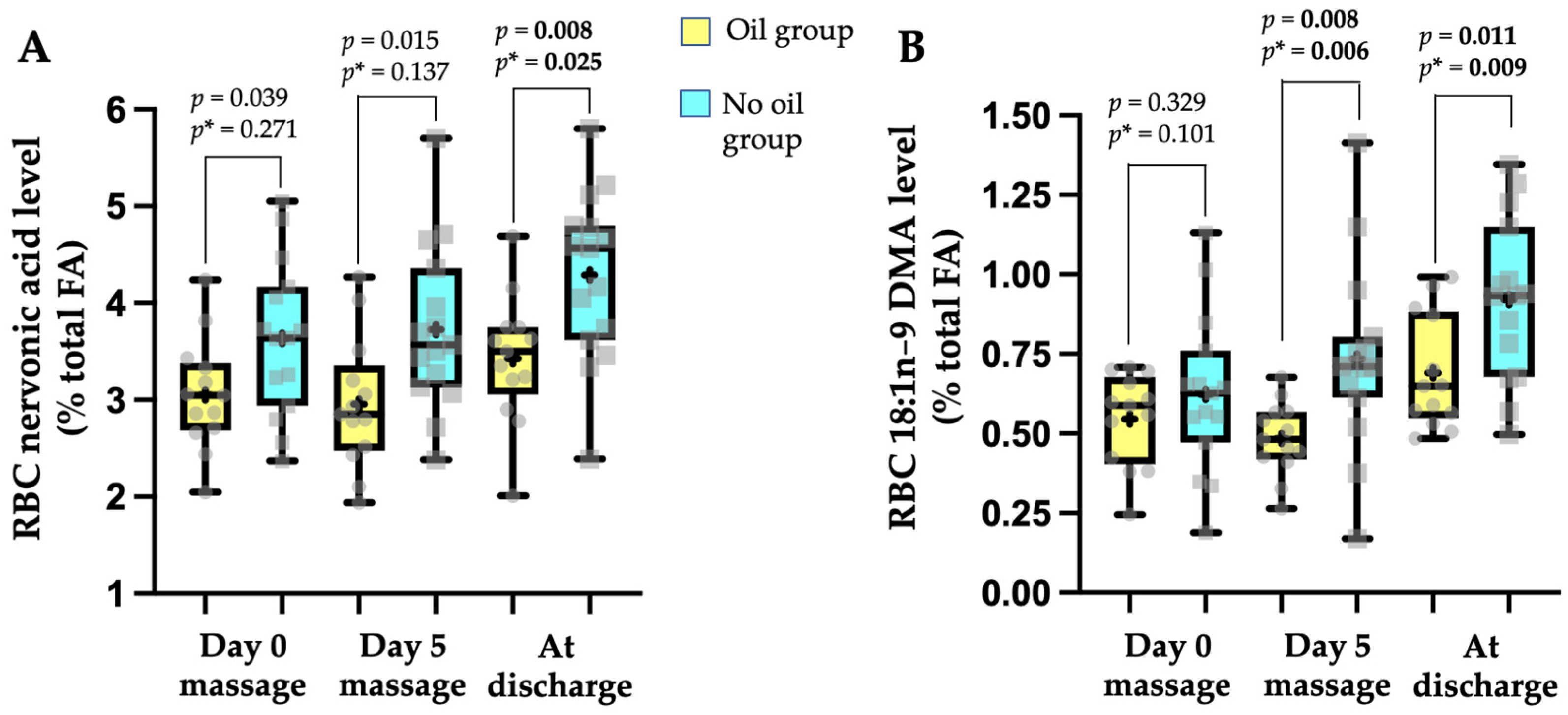
3.3. Effect of Oil Massage on Plasma Lipid Parameters and Fatty Acid Biological Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howson, C.P.; Kinney, M.V.; Lawn, J.E. Born Too Soon: The Global Action Report on Preterm Birth; March of Dimes, PMNCH, Save the Children, Eds.; World Health Organization: New York, NY, USA, 2012; Available online: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241503433/en/ (accessed on 24 March 2022).
- Dehan, M. Epidemiology and ethics of the very premature infant. Arch. Pediatr. 2002, 4, 423–426. [Google Scholar] [CrossRef]
- Vickers, A.; Ohlsson, A.; Lacy, J.B.; Horsley, A. Massage for promoting growth and development of preterm and/or low birth-weight infants. Cochrane Database Syst. Rev. 2004, 2, CD000390. [Google Scholar] [CrossRef]
- Niemi, A.K. Review of Randomized Controlled Trials of Massage in Preterm Infants. Children 2017, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Field, T. Pediatric massage therapy research: A narrative review. Children 2019, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Badr, L.K.; Abdallah, B.; Kahale, L. A meta-analysis of preterm infant massage: An ancient practice with contemporary applications. MCN Am. J. Matern. Nurs. 2015, 40, 344–358. [Google Scholar] [CrossRef]
- Li, X.; Zhong, Q.; Tang, L. A meta-analysis of the efficacy and safety of using oil massage to promote infant growth. J. Pediatric Nurs. 2016, 31, e313–e322. [Google Scholar] [CrossRef]
- Solanki, K.; Matnani, M.; Kale, M.; Joshi, K.; Bavdekar, A.; Bhave, S.; Pandit, A. Transcutaneous absorption of topically massaged oil in neonates. Indian Pediatrics 2005, 42, 998–1005. [Google Scholar]
- Arora, J.; Kumar, A.; Ramji, S. Effect of oil massage on growth and neurobehavior in very low birth weight premature neonates. Indian Pediatrics 2005, 42, 1092–1100. [Google Scholar]
- Ognean, M.L.; Ognean, M. The best vegetable oil for preterm and term infant massage. J. Pediatrului 2017, 20, 9–17. [Google Scholar]
- Vaivre–Douret, L.; Oriot, D.; Blossier, P.; Py, A.; Kasolter-Péré, M.; Zwang, J. The effect of multimodal stimulation and cutaneous application of vegetable oils on development in preterm infants: A randomized controlled trial. Child Care Health Dev. 2008, 35, 96–105. [Google Scholar] [CrossRef]
- Garcia, C.; Duan, R.D.; Brévaut-Malaty, V.; Gire, C.; Millet, V.; Simeoni, U.; Bernard, M.; Armand, M. Bioactive compounds in human milk and intestinal health and maturity in preterm newborn: An overview. Cell. Mol. Biol. 2013, 59, 108–131. [Google Scholar]
- Innis, S.M. Essential fatty acids in growth and development. Prog. Lipid Res. 1991, 30, 39–103. [Google Scholar] [CrossRef]
- Hamosh, M.; Henderson, T.R.; Kemper, M.A.; Orr, N.M.; Gil, A.; Hamosh, P. Long-chain polyunsaturated fatty acids (LC-PUFA) during early development. In Bioactive Components of Human Milk; Newburg, D., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; pp. 397–401. [Google Scholar]
- Carlson, S.E. Docosahexaenoic acid and arachidonic acid in infant development. Semin. Neonatol. 2001, 6, 437–449. [Google Scholar] [CrossRef]
- Sankaranarayanan, K.; Mondkar, J.A.; Chauhan, M.M.; Mascarenhas, B.M.; Mainkar, A.R.; Salvi, R.Y. Oil massage in neonates: An open randomized controlled study of coconut versus mineral oil. Pediatrics 2005, 42, 877–884. [Google Scholar]
- Masood, A.; Stark, K.D.; Salem, N., Jr. A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J. Lipid Res. 2005, 46, 2299–2305. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Bernard, J.Y.; Forhan, A.; Heude, B.; Charles, M.A. Maternal nutritional determinants of colostrum fatty acids in the EDEN mother-child cohort. Clin. Nutr. 2018, 37, 2127–2136. [Google Scholar] [CrossRef]
- Garcia, C.; Millet, V.; Coste, T.C.; Mimoun, M.; Ridet, A.; Antona, C.; Simeoni, U.; Armand, M. French mother’s milk deficient in DHA contains phospholipid species of potential interest for infant development. J. Pediatric Gastroenterol. Nutr. 2011, 53, 206–212. [Google Scholar] [CrossRef]
- Labadaridis, I.; Moraitou, M.; Theodoraki, M.; Triantafyllidis, G.; Sarafidou, J.; Michelakakis, H. Plasmalogen levels in full-term neonates. Acta Paediatr. 2009, 98, 640–642. [Google Scholar] [CrossRef]
- Fallah, R.; Akhavan Karbasi, S.; Golestan, M.; Fromandi, M. Sunflower oil versus no oil moderate pressure massage leads to greater increases in weight in preterm neonates who are low birth weight. Early Hum. Dev. 2013, 89, 769–772. [Google Scholar] [CrossRef]
- Saeidi, R.; Ghorbani, Z.; Moghadam, A.S. The effect of massage with medium-chain triglyceride oil on weight gain in premature neonates. Acta Med. Iran. 2015, 53, 134–138. [Google Scholar]
- Fenton, T.R.; Anderson, D.; Groh-Wargo, S.; Hoyos, A.; Ehrenkranz, R.A.; Senterre, T. An Attempt to Standardize the Calculation of Growth Velocity of Preterm Infants-Evaluation of Practical Bedside Methods. J. Pediatrics 2018, 196, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Patankar, S.; Chawla, C.; Taskar, T.; Prabhu, S.V. Oil application in preterm babies–A source of warmth and nutrition. Indian Pediatr. 1987, 24, 1111–1116. [Google Scholar] [PubMed]
- Rutter, N. Percutaneous drug absorption in the newborn: Hazards and uses. Clin. Perinatol. 1987, 14, 911–930. [Google Scholar] [CrossRef]
- Friedman, Z.; Shochat, S.J.; Maisels, M.J.; Marks, K.H.; Lamberth, E.L., Jr. Correction of essential fatty acid deficiency in newborn infants by cutaneous application of sunflower-seed oil. Pediatrics 1976, 58, 650–654. [Google Scholar] [CrossRef]
- Skolnik, P.; Eaglstein, W.H.; Ziboh, V.A. Human essential fatty acid deficiency: Treatment by topical application of linoleic acid. Arch. Dermatol. 1977, 113, 939–941. [Google Scholar] [CrossRef]
- Lee, E.J.; Gibson, R.A.; Simmer, K. Transcutaneous application of oil and prevention of essential fatty acid deficiency in preterm infants. Arch. Dis. Child. 1993, 68, 27–28. [Google Scholar] [CrossRef] [Green Version]
- Bougle, D.; Pepin, D.; Delhaye, M.; Chambaz, J.; Ricour, C. Plasma and erythrocyte essential fatty acids during total parenteral nutrition in infants: Effects of a cutaneous supply. J. Parenter. Enteral. Nutr. 1986, 10, 216–219. [Google Scholar] [CrossRef]
- Soriano, C.R.; Martinez, F.E.; Jorge, S.M. Cutaneous application of vegetable oil as a coadjuvant in the nutritional management of preterm infants. J. Pediatric Gastroenterol. Nutr. 2000, 31, 387–390. [Google Scholar] [CrossRef]
- Armand, M. Lipases and lipolysis in the human digestive tract: Where do we stand? Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 156–164. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Pontes, P.V.; Torres, A.G.; Trugo, N.M.F.; Fonseca, V.M.; Sichieri, R. n-6 and n-3 long-chain polyunsaturated fatty acids in the erythrocyte membrane of Brazilian preterm and term neonate and their mothers at delivery. Prostaglandins Leukot. Essent. Fat Acids 2006, 74, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Labadaridis, I.; Moraitou, M.; Theodoraki, M.; Dimitriou, E.; Sarafidou, J.; Michelakakis, H. Linoleic and arachidonic acid iin perinatal asphyxia and prematurity. J. Matern.-Fetal Neonatal Med. 2007, 20, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Khatun, N.; Islam, K.; Das, K. Outcome of coconut oil massage in newborns. J. Neonatal Nurs. 2022, 28, 107–112. [Google Scholar] [CrossRef]
- Jena, A.; Montoya, C.A.; Mullaney, J.A.; Dilger, R.N.; Young, W.; McNabb, W.C.; Roy, N.C. Gut-Brain axis in the early postnatal years of life: A developmental perspective. Front. Integr. Neurosci. 2020, 14, 44. [Google Scholar] [CrossRef]
- Patel, T.B.; Clark, J.B. Comparison of the development of the fatty acid content and composition of the brain of a precocial species (guinea pig) and a non-precocial species (rat). J. Neurochem. 1980, 35, 149–154. [Google Scholar] [CrossRef]
- Babin, F.; Sarda, P.; Limasset, B.; Descomps, B.; Rieu, D.; Mendy, F.; Crastes de Paulet, A. Nervonic acid in red blood cell sphingomyelin in premature infants: An index of myelin maturation? Lipids 1993, 8, 627–630. [Google Scholar] [CrossRef]
- Strandvik, B.; Ntoumani, E.; Lundqvist-Persson, C.; Sabel, K.G. Long-chain saturated and monounsaturated fatty acids associate with development of premature infants up to 18 months of age. Prostaglandins Leukot. Essent. Fat Acids 2016, 107, 43–49. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Wimmer, I.; Berger, J. Plasmalogens, platelet-activating factor and beyond–Ether lipids in signaling and neurodegeneration. Neurobiol. Dis. 2020, 145, 105061. [Google Scholar] [CrossRef]
- Moser, A.B.; Steinberg, S.J.; Watkins, P.A.; Moser, H.W.; Ramaswamy, K.; Siegmund, K.D.; Lee, D.R.; Ely, J.J.; Ryder, O.A.; Hacia, J.G. Human and great ape red blood cells differ in plasmalogen levels and composition. Lipids Health Dis. 2011, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Guan, Z.; Wang, Y.A.; Cairns, N.J.; Lantos, P.L.; Dallner, G.; Sindelar, P.J. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999, 58, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, J.; Smith, T.; Lamontagne-Proulx, J.; Bourque, M.; Al Sweidi, S.; Jayasinghe, D.; Ritchie, S.; Si Paolo, T.; Soulet, D. Neuroprotection and immunomodulation in the gut of parkinsonian mice with a plasmalogen precursor. Brain Res. 2019, 1725, 146460. [Google Scholar] [CrossRef] [PubMed]
- Ntoumani, E.; Strandvik, B.; Sabel, K.G. Nervonic acid is much lower in donor milk than in milk from mothers delivering premature infants–Of neglected importance? Prostaglandins Leukot. Essent. Fat Acids 2013, 89, 241–244. [Google Scholar] [CrossRef] [PubMed]
| Variables 1 | Oil Massage n = 18 | No Oil Massage n = 18 | p |
|---|---|---|---|
| Antenatal | |||
| Corticosteroid treatment, n (%) | 18 (100) | 18 (100) | 1.000 |
| Premature rupture of membrane, n (%) | 6 (33.3) | 6 (33.3) | 1.000 |
| Premature labor, n (%) | 6 (33.3) | 8 (44.4) | 0.494 |
| Chorioamniotitis, n (%) | 3 (16.7) | 3 (16.7) | 0.603 |
| Vascular pathology, n (%) | 8 (44.4) | 8 (44.4) | 1.000 |
| Intrauterine growth restriction, n (%) | 7 (38.9) | 5 (27.8) | 0.480 |
| Fetal cardiac rhythm abnormality, n (%) | 7 (38.9) | 7 (38.9) | 1.000 |
| Singleton fetus, n (%) | 11 (61.1) | 12 (66.7) | 1.000 |
| Twin fetus, n (%) | 4 (22.2) | 3 (16.7) | 1.000 |
| Triples fetus, n (%) | 3 (16.7) | 3 (16.7) | 1.000 |
| At birth | |||
| Gestational age (GA), weeks | 28.9 ± 1.6 | 29.8 ± 1.1 | 0.039 |
| Female gender, n (%) | 11 (61.1) | 10 (55.6) | 0.735 |
| Apgar at 5 min | 8.6 ± 1.3 | 8.1 ± 1.6 | 0.415 |
| Birth weight, g | 1050 ± 24 | 1309 ± 288 | 0.012 |
| Small for GA, n (%) | 3 (16.7) | 2 (11.1) | 1.000 |
| Head circumference, cm | 25.4 ± 1.9 | 27.7 ± 1.6 | 0.001 |
| Length, cm | 35.3 ± 2.6 | 37.6 ± 2.3 | 0.027 |
| Postnatal | |||
| Exogenous surfactant therapy, n (%) | 11 (61.1) | 10 (55.6) | 0.735 |
| Respiratory distress syndrome, n (%) | 17 (94.4) | 17 (94.4) | 1.000 |
| Bronchopulmonary dysplasia 28 days, n (%) | 10 (55.6) | 4 (22.2) | 0.040 |
| Patent ductus arteriosus >7 days, n (%) | 7 (38.9) | 2 (11.1) | 0.121 |
| Necrotizing enterocolitis, n (%) | 2 (11.1) | 0 (0) | 0.486 |
| Number of nosocomial infections | 0.126 | ||
| None nosocomial infection, n (%) | 7 (38.9) | 12 (66.7) | |
| One nosocomial infection, n (%) | 8 (44.4) | 4 (22.2) | |
| Two nosocomial infections, n (%) | 3 (16.7) | 2 (11.1) | |
| At inclusion day 0 massage | |||
| Corrected GA, weeks | 35.2 ± 1.3 | 34.3 ± 1.0 | 0.044 |
| Age, day | 44 ± 16 | 31 ± 11 | 0.018 |
| Weight, g | 1725 ± 307 | 1867 ± 372 | 0.221 |
| Between Day 0 and after 5-Day Massage | Between the End of Massage and Discharge | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables 1 | n Total | Oil Massage | No Oil Massage | Group Difference (95% IC) | p | Oil Massage | No Oil Massage | Group Difference (95% IC) | p |
| Absolute weight gain, g | 36 | 128 ± 77 | 110 ± 46 | −18.44 (−61.62;24.73) | 0.391 *0.221 | 703 ± 343 | 659 ± 451 | −43.61 (−315.10;227.87) | 0.746 *0.739 |
| Weight gain velocity, g/kg/day | 36 | 12.3 ± 7.3 | 9.8 ± 4.1 | −2.53 (−6.55;1.48) | 0.209 *0.108 | 16.7 ± 7.3 | 16.9 ± 5.6 | 0.15 (−4.33;4.55) | 0.945 *0.636 |
| Variables 1 | Oil Massage n = 18 | No Oil Massage n = 18 | p |
|---|---|---|---|
| Enteral autonomy corrected GA, weeks | 37.5 ± 1.1 2 | 36.9 ± 0.7 2 | 0.126/*0.658 |
| Enteral autonomy, day | 61.5 ± 16.7 | 49.5 ± 10.2 3 | 0.027/*0.626 |
| Duration of hospital stay, day | 23.6 ± 9.7 | 20.4 ± 15.0 | 0.136/*0.738 |
| Corrected GA at discharge, weeks | 39.2 ± 1.6 | 37.9 ± 2.5 | 0.015/*0.646 |
| Age at discharge, day | 72 ± 20 | 57 ± 18 | 0.020/*0.816 |
| Head circumference at discharge, cm | 33.1 ± 2.0 | 33.0 ± 2.0 | 0.987/*0.956 |
| Length at discharge, cm | 44.6 ± 2.7 | 44.9 ± 2.9 | 0.744/*0.808 |
| Day 0 | Day 5 | At Discharge | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables 1 | Total n | Oil Massage | No Oil Massage | Group Difference (95% IC) | p | Oil Massage | No oil Massage | Group Difference (95% IC) | p | Oil Massage | No Oil Massage | Group Difference (95% IC) | p |
| Lipid parameters g/L | |||||||||||||
| Triglycerides | 28 | 0.84 ± 0.43 | 0.69 ± 0.27 | −0.15 (−0.43;0.12) | 0.263/*0.284 | 0.93 ± 0.25 | 0.80 ± 0.39 | −0.13 (−0.39;0.13) | 0.321/*0.519 | 0.80 ± 0.19 | 0.70 ± 0.25 | −0.10 (−0.28;0.07) | 0.228/*0.464 |
| Total cholesterol | 28 | 1.11 ± 0.37 | 1.06 ± 0.26 | −0.06 (−0.30;0.19) | 0.644/*0.448 | 1.12 ± 0.26 | 1.07 ± 0.18 | −0.04 (−0.22;0.13) | 0.612/*0.439 | 1.02 ± 0.20 | 1.05 ± 0.18 | 0.03 (−0.12;0.18) | 0.661/*0.575 |
| PUFA % total FA | |||||||||||||
| PUFA n-6 | |||||||||||||
| LA | 28 | 16.03 ± 4.73 | 18.41 ± 4.24 | 2.38 (−1.11;5.86) | 0.173/*0.210 | 18.37 ± 5.08 | 18.97 ± 4.95 | 0.60 (−3.30;4.51) | 0.753/*0.991 | 20.09 ± 4.72 | 22.04 ± 2.78 | 1.95 (−1.01;4.91) | 0.187/*0.488 |
| ARA | 28 | 4.90 ± 1.31 | 5.49 ± 0.69 | 0.59 (−0.21;1.38) | 0.140/*0.193 | 4.84 ± 1.04 | 5.74 ± 1.18 | 0.90 (0.03;1.78) | 0.043/*0.072 | 5.90 ± 1.69 | 6.24 ± 1.11 | 0.38 (−0,72;1.48) | 0.485/*0.736 |
| PUFA n-3 | |||||||||||||
| ALA | 28 | 0.29 ± 0.16 | 0.40 ± 0.17 | 0.11 (−0.02;0.24) | 0.088/*0.039 | 0.41 ± 0.23 | 0.40 ± 0.20 | −0.006 (−0.17;0.16) | 0.939/*0.642 | 0.52 ± 0.28 | 0.57 ± 0.21 | 0.05 (−0.15;0.24) | 0.627/*0.929 |
| DHA | 28 | 1.17 ± 0.32 | 1.33 ± 0.23 | 0.16 (−0.05;0.37) | 0.134/*0.044 | 1.21 ± 0.38 | 1.37 ± 0.34 | 0.1 (−0.12;0.44) | 0.248/*0.103 | 1.64 ± 0.59 | 1.82 ± 0.44 | 0.17 (−0.23;0.58) | 0.381/*0.102 |
| PUFA g/L | |||||||||||||
| PUFA n-6 | |||||||||||||
| LA | 28 | 0.549 ± 0.227 | 0.575 ± 0.187 | 0.03 (−0.14;0.19) | 0.747/*0.753 | 0.676 ± 0.294 | 0.634 ± 0.238 | −0.04 (−0.25;0.16) | 0.680/*0.526 | 0.646 ± 0.184 | 0.671 ± 0.151 | 0.03 (−0.10;0.16) | 0.686/*0.980 |
| ARA | 28 | 0.164 ± 0.050 | 0.175 ± 0.058 | 0.01 (−0.03;0.05) | 0.604/*0.581 | 0.172 ± 0.051 | 0.191 ± 0.053 | 0.02 (−0.02;0.06) | 0.341/*0.488 | 0.184 ± 0.043 | 0.190 ± 0.045 | 0.01 (−0.03;0.04) | 0.731/*0.892 |
| PUFA n-3 | |||||||||||||
| ALA | 28 | 0.010 ± 0.006 | 0.012 ± 0.006 | 0.002 (−0.002;0.01) | 0.310/*0.108 | 0.016 ± 0.012 | 0.013 ± 0.009 | −0.002 (−0.01;0.01) | 0.603/*0.886 | 0.017 ± 0.011 | 0.018 ± 0.009 | 0.001 (−0.007;0.01) | 0.886/*0.896 |
| DHA | 28 | 0.039 ± 0.011 | 0.041 ± 0.010 | 0.002 (−0.006;0.01) | 0.588/*0.249 | 0.043 ± 0.013 | 0.046 ± 0.016 | 0.003 (−0.008;0.01) | 0.570/*0.402 | 0.053 ± 0.012 | 0.056 ± 0.018 | 0.003 (−0.009;0.01) | 0.640/*0.298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbi, A.; Armand, M.; Beltran-Anzola, A.-A.; Sarté, C.; Brévaut-Malaty, V.; Tosello, B.; Gire, C. Effect of Massage with Oil Balanced in Essential Fatty Acids on Development and Lipid Parameters in Very Premature Neonates: A Randomized, Controlled Study. Children 2022, 9, 463. https://doi.org/10.3390/children9040463
Garbi A, Armand M, Beltran-Anzola A-A, Sarté C, Brévaut-Malaty V, Tosello B, Gire C. Effect of Massage with Oil Balanced in Essential Fatty Acids on Development and Lipid Parameters in Very Premature Neonates: A Randomized, Controlled Study. Children. 2022; 9(4):463. https://doi.org/10.3390/children9040463
Chicago/Turabian StyleGarbi, Aurélie, Martine Armand, Any-Alejandra Beltran-Anzola, Catherine Sarté, Véronique Brévaut-Malaty, Barthélémy Tosello, and Catherine Gire. 2022. "Effect of Massage with Oil Balanced in Essential Fatty Acids on Development and Lipid Parameters in Very Premature Neonates: A Randomized, Controlled Study" Children 9, no. 4: 463. https://doi.org/10.3390/children9040463
APA StyleGarbi, A., Armand, M., Beltran-Anzola, A.-A., Sarté, C., Brévaut-Malaty, V., Tosello, B., & Gire, C. (2022). Effect of Massage with Oil Balanced in Essential Fatty Acids on Development and Lipid Parameters in Very Premature Neonates: A Randomized, Controlled Study. Children, 9(4), 463. https://doi.org/10.3390/children9040463






