Influence of Maternal Region of Birth on Placental Pathology of Babies Born Small
Abstract
:1. Introduction
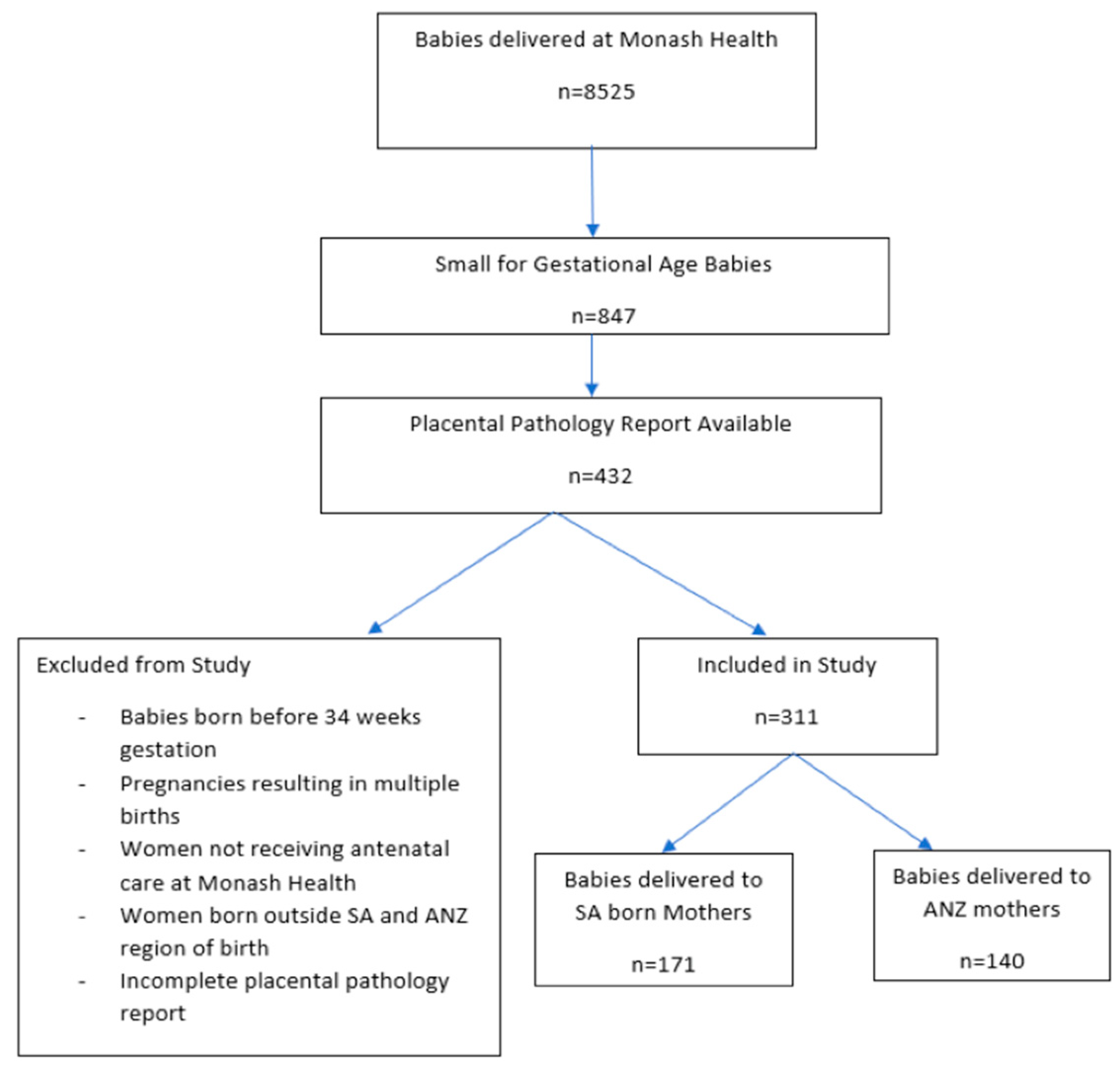
2. Methods
Ethics Approval
3. Results
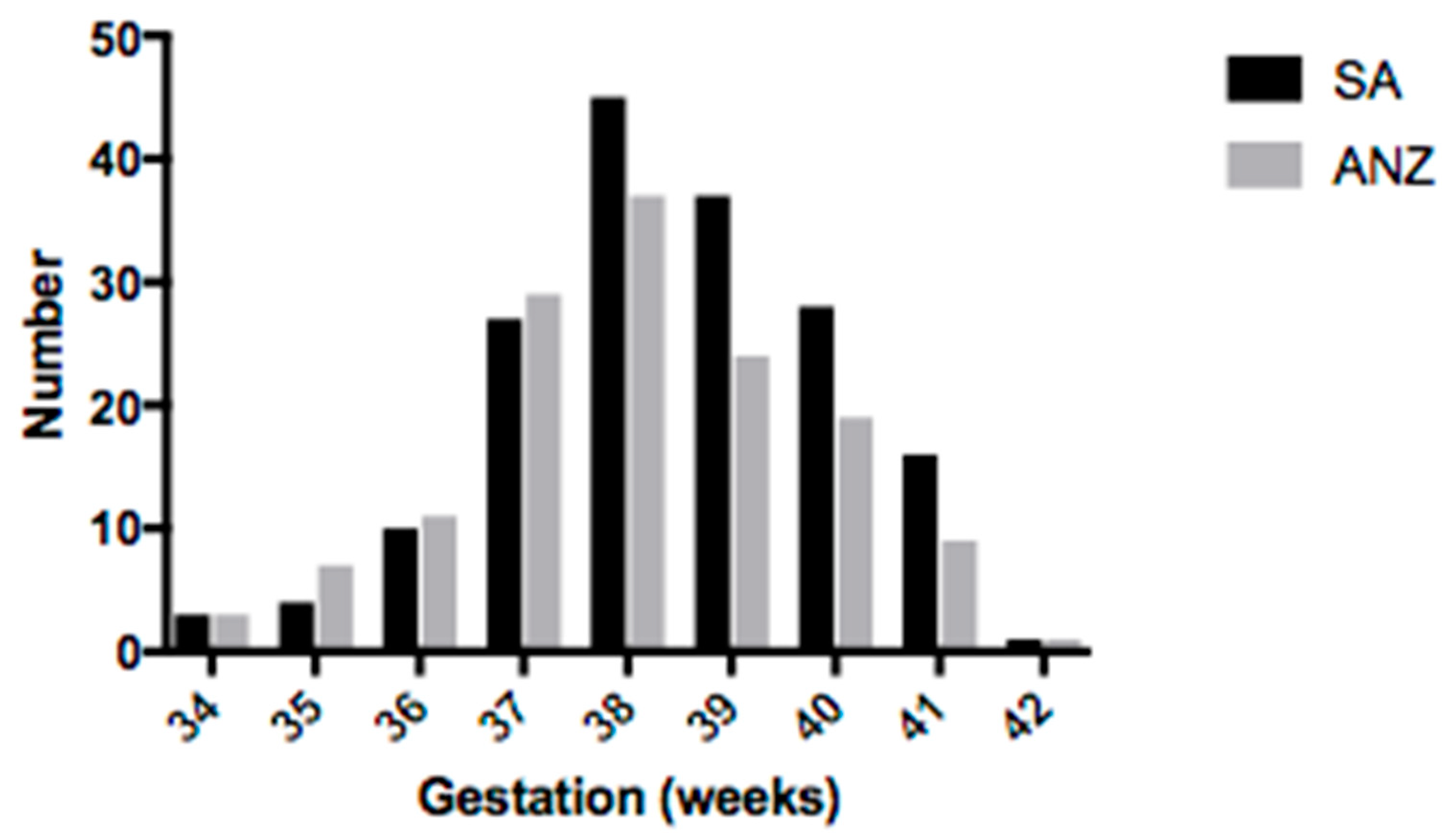
3.1. Demographic Information
3.2. Placental Outcomes
3.3. Selected Neonatal Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCowan, L.; Horgan, R.P. Risk factors for small for gestational age infants. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Re-striction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.; Wallace, E.M.; Mockler, J.C.; Stewart, L.; Knight, M.; Hodges, R.; Skinner, S.; Davies-Tuck, M. Maternal Asian ethnicity and obstetric intrapartum intervention: A retrospective cohort study. BMC Pregnancy Childbirth 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audette, M.C.; Levytska, K.; Lye, S.J.; Melamed, N.; Kingdom, J.C. Parental ethnicity and placental maternal vascular malperfusion pathology in healthy nulliparous women. Placenta 2018, 66, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, J.; Tang, X. Analysis of perinatal risk factors for small-for-gestational-age and appropriate-for-gestational-age late-term infants. Exp. Ther. Med. 2020, 19, 1719–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, N.E.; Davies-Tuck, M.; Malhotra, A. Influence of maternal region of birth on neonatal outcomes of babies born small. Acta Paediatr. 2021, 110, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Resnik, R. Intrauterine growth restriction. Obstet. Gynecol. 2002, 99, 490–496. [Google Scholar] [PubMed]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Scifres, C.M.; Nelson, D.M. Intrauterine growth restriction, human placental development and trophoblast cell death. J. Physiol. 2009, 587, 3453–3458. [Google Scholar] [CrossRef]
- Margetts, B.M.; Yusof, S.M.; Al Dallal, Z.; A Jackson, A. Persistence of lower birth weight in second generation South Asian babies born in the United Kingdom. J. Epidemiol. Community Health 2002, 56, 684–687. [Google Scholar] [CrossRef] [Green Version]
- Division UNS. Composition of Macro Geographical (Continental) Regions, Geographical Sub-Regions, and Selected Economic and Other Groupings; United Nations Statistics Division (UNSD): New York, NY, USA, 2008. [Google Scholar]
- Dobbins, T.A.; Sullivan, E.A.; Roberts, C.L.; Simpson, J.M. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med. J. Aust. 2012, 197, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balchin, I.; Whittaker, J.; Patel, R.R.; Lamont, R.F.; Steer, P.J. Racial variation in the association between gestational age and perinatal mortality: Prospective study. BMJ 2007, 334, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravelli, A.C.; Schaaf, J.M.; Eskes, M.; Abu-Hanna, A.; De Miranda, E.; Mol, B.W.J. Ethnic disparities in perinatal mortality at 40 and 41 weeks of gestation. J. Perinat. Med. 2013, 41, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Davies-Tuck, M.L.; Davey, M.A.; Wallace, E.M. Maternal region of birth and stillbirth in Victoria, Australia 2000–2011: A retro-spective cohort study of Victorian perinatal data. PLoS ONE 2017, 12, e0178727. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, V.; Jagtap, V.S.; Sarathi, V.; Lila, A.R.; Kamalanathan, S.; Bandgar, T.R.; Menon, P.S.; Shah, N.S. Prevalence and Impact of Thyroid Disorders on Maternal Outcome in Asian-Indian Pregnant Women. J. Thyroid Res. 2011, 2011, 429097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, T.-J.; Tsai, L.-Y.; Chu, L.-C.; Yeh, S.-J.; Leung, C.; Chen, C.-Y.; Chou, H.-C.; Tsao, P.-N.; Chen, P.-C.; Hsieh, W.-S. Parental Smoking During Pregnancy and Its Association with Low Birth Weight, Small for Gestational Age, and Preterm Birth Offspring: A Birth Cohort Study. Pediatr. Neonatol. 2014, 55, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, A.G.; Narula, S.; Malhotra, A.; Fernando, S.; Wallace, E.; Davies-Tuck, M. The influence of maternal ethnicity on neonatal res-piratory outcome. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.; Santana, P.; Cruickshank, J.K.; Boroujerdi, M. Birth Weights of Black African Babies of Migrant and Nonmigrant Mothers Compared with Those of Babies of European Mothers in Portugal. Ann. Epidemiol. 2006, 16, 572–579. [Google Scholar] [CrossRef]
- Ruan, S.; E Abdel-Latif, M.; Bajuk, B.; Lui, K.; Oei, J.L. On behalf of the NSW and the ACT Neonatal Intensive Care Units (NICUs) Group The associations between ethnicity and outcomes of infants in neonatal intensive care units. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F133–F138. [Google Scholar] [CrossRef]
- Chisholm, K.; Folkins, A.K. Placental and Clinical Characteristics of Term Small-for-Gestational-Age Neonates: A Case-Control Study. Pediatr. Dev. Pathol. 2016, 19, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Salafia, C.M.; Vintzileos, A.M.; Silberman, L.; Bantham, K.F.; Vogel, C.A. Placental Pathology of Idiopathic Intrauterine Growth Retardation at Term. Am. J. Perinatol. 1992, 9, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Morales-Roselló, J.; Buongiorno, S.; Loscalzo, G.; Scarinci, E.; Roca, L.G.; Martínez, A.J.C.; Rosati, P.; Lanzone, A.; Marín, A.P. Birth-weight differences at term are explained by placental dysfunction and not by maternal ethnicity. Study in newborns of first generation immigrants. J. Matern. Neonatal Med. 2020, 2020, 1755651. [Google Scholar] [CrossRef] [PubMed]
- Barron, S.L. Birthweight and ethnicity. Br. J. Obstet. Gynaecol. 1983, 90, 289–290. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Zhang, H.; Klebanoff, M.; Yang, Z.; Zhang, J. Racial disparity in placental pathology in the collaborative per-inatal project. Int. J. Clin. Exp. Pathol. 2015, 8, 15042–15054. [Google Scholar]
- Assibey-Mensah, V.; Parks, W.T.; Gernand, A.D.; Catov, J.M. Race and risk of maternal vascular malperfusion lesions in the placenta. Placenta 2018, 69, 102–108. [Google Scholar] [CrossRef]
- Pinar, H.; Goldenberg, R.L.; Koch, M.A.; Heim-Hall, J.; Hawkins, H.K.; Shehata, B.; Abramowsky, C.; Parker, C.B.; Dudley, D.J.; Silver, R.M.; et al. Placental Findings in Singleton Stillbirths. Obstet. Gynecol. 2014, 123, 325–336. [Google Scholar] [CrossRef] [Green Version]

| SA Infants (n = 171) | ANZ Infants (n = 140) | p Value | |
|---|---|---|---|
| Maternal age (years) | 31.16 (4.1) | 29.04 (6.1) | 0.0003 |
| Body mass index (BMI) | 24.27 (4.1) | 25.73 (6.6) | 0.01 |
| Alcohol use in pregnancy | 2 (1) | 7 (4) | 0.04 |
| Smoking | 1 (1) | 64 (46) | 0.0001 |
| Maternal thyroid disease | 18 (11) | 9 (6) | 0.04 |
| Pre-existing hypertension | 3 (2) | 2 (1) | 0.90 |
| Gestational diabetes—insulin dependent | 16 (9) | 2 (1) | 0.004 |
| Maternal vitamin D deficiency | 69 (40) | 44 (31) | 0.05 |
| Nulliparous | 88 (51) | 79 (56) | 0.78 |
| Maternal diabetes mellitus | 4 (2) | 3 (2) | 0.93 |
| Maternal polycystic ovarian disease | 9 (5) | 3 (2) | 0.22 |
| Maternal anaemia | 13 (8) | 11 (8) | 0.92 |
| Family history of diabetes mellitus/hypertension | 100 (65) | 82 (66) | 0.41 |
| Spontaneous preterm labour (34–36 weeks) | 17 (10) | 21 (15) | 0.26 |
| Prolonged pregnancy > 41 weeks | 14 (8) | 10 (7) | 0.71 |
| Suspected FGR | 70 (41) | 58 (41) | 0.56 |
| Suspected fetal compromise | 40 (23) | 32 (23) | 0.64 |
| Pre-eclampsia | 6 (3) | 6 (4) | 1.00 |
| Antepartum haemorrhage | 6 (4) | 5 (4) | 0.95 |
| SA Infants (n = 171) | ANZ Infants (n = 140) | p Value | |
|---|---|---|---|
| Placenta weight, gms | 376 (72) | 383 (77) | 0.45 |
| Umbilical cord length, mm | 329 (105.7) | 320 (108) | 0.43 |
| Membranes complete | 20 (19.4) | 22 (23.7) | 0.47 |
| Maternal surface abnormalities | 36 (21) | 35(25) | 0.41 |
| 19 (11) | 15 (11) | |
| 1 (1) | 1 (1) | |
| 1 (1) | 3 (2) | |
| 0 (0) | 5 (4) | |
| Maternal vascular malperfusion | 139 (81.3) | 121 (86.4) | 0.28 |
| 62 (36) | 52 (38) | |
| 28 (18) | 29 (22) | |
| 38 (24) | 28 (22) | |
| 5 (3) | 3 (2) | |
| 4 (2) | 4 (3) | |
| 2 (1) | 3 (2) | |
| 0 (0) | 0 (0) | |
| 0 (0) | 0 (0) | |
| Fetal vascular malperfusion | 114 (66.7) | 80 (57.1) | 0.09 |
| 45 (26.3) | 26 (18.6) | |
| 6 (4) | 10 (8) | |
| 5 (3) | 6 (4) | |
| 27 (16) | 24 (17) | |
| 11 (6) | 4 (3) | |
| 20 (12) | 10 (7) | |
| Delayed villous maturation | 2 (1) | 3 (2) | 0.66 |
| Ascending uterine infection | 73 (43) | 55 (39) | 0.60 |
| 5 (3) | 5 (4) | |
| 38 (22) | 30 (21) | |
| 15 (9) | 10 (7) | |
| 15 (9) | 10 (7) | |
| 0 (0) | 0 (0) | |
| Villitis of unknown etiology | 26 (15) | 15 (11) | 0.83 |
| Significant pathology identified | 138 (81) | 113 (81) | 1.00 |
| SA Infants (n = 171) | ANZ Infants (n = 140) | p Value | |
|---|---|---|---|
| Birth weight, gms | 2582 (316) | 2497 (351) | 0.02 |
| Gestation, wks | 38.7 (1.6) | 38.3 (1.7) | 0.05 |
| Placental weight, gms | 376 (72) | 383 (77) | 0.45 |
| Feto-placental ratio | 7.0 (1.1) | 6.6 (1.3) | 0.13 |
| SCN/NICU admission | 60 (35) | 57 (41) | 0.05 |
| Respiratory support | 28 (16.4) | 25 (18) | 1.0 |
| Congenital abnormality | 4 (2.3) | 6 (4.3) | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, M.; Choudhary, N.; Kumar, B.; Juchkov, N.; Shearer, K.; Ellery, S.J.; Davies-Tuck, M.; Malhotra, A. Influence of Maternal Region of Birth on Placental Pathology of Babies Born Small. Children 2022, 9, 388. https://doi.org/10.3390/children9030388
Fernando M, Choudhary N, Kumar B, Juchkov N, Shearer K, Ellery SJ, Davies-Tuck M, Malhotra A. Influence of Maternal Region of Birth on Placental Pathology of Babies Born Small. Children. 2022; 9(3):388. https://doi.org/10.3390/children9030388
Chicago/Turabian StyleFernando, Mindi, Nalin Choudhary, Beena Kumar, Natasha Juchkov, Kathryn Shearer, Stacey J. Ellery, Miranda Davies-Tuck, and Atul Malhotra. 2022. "Influence of Maternal Region of Birth on Placental Pathology of Babies Born Small" Children 9, no. 3: 388. https://doi.org/10.3390/children9030388
APA StyleFernando, M., Choudhary, N., Kumar, B., Juchkov, N., Shearer, K., Ellery, S. J., Davies-Tuck, M., & Malhotra, A. (2022). Influence of Maternal Region of Birth on Placental Pathology of Babies Born Small. Children, 9(3), 388. https://doi.org/10.3390/children9030388






