Lung Function Can Predict the Expected Inspiratory Airflow Rate through Dry Powder Inhalers in Asthmatic Adolescents
Abstract
1. Introduction
2. Materials and Methods
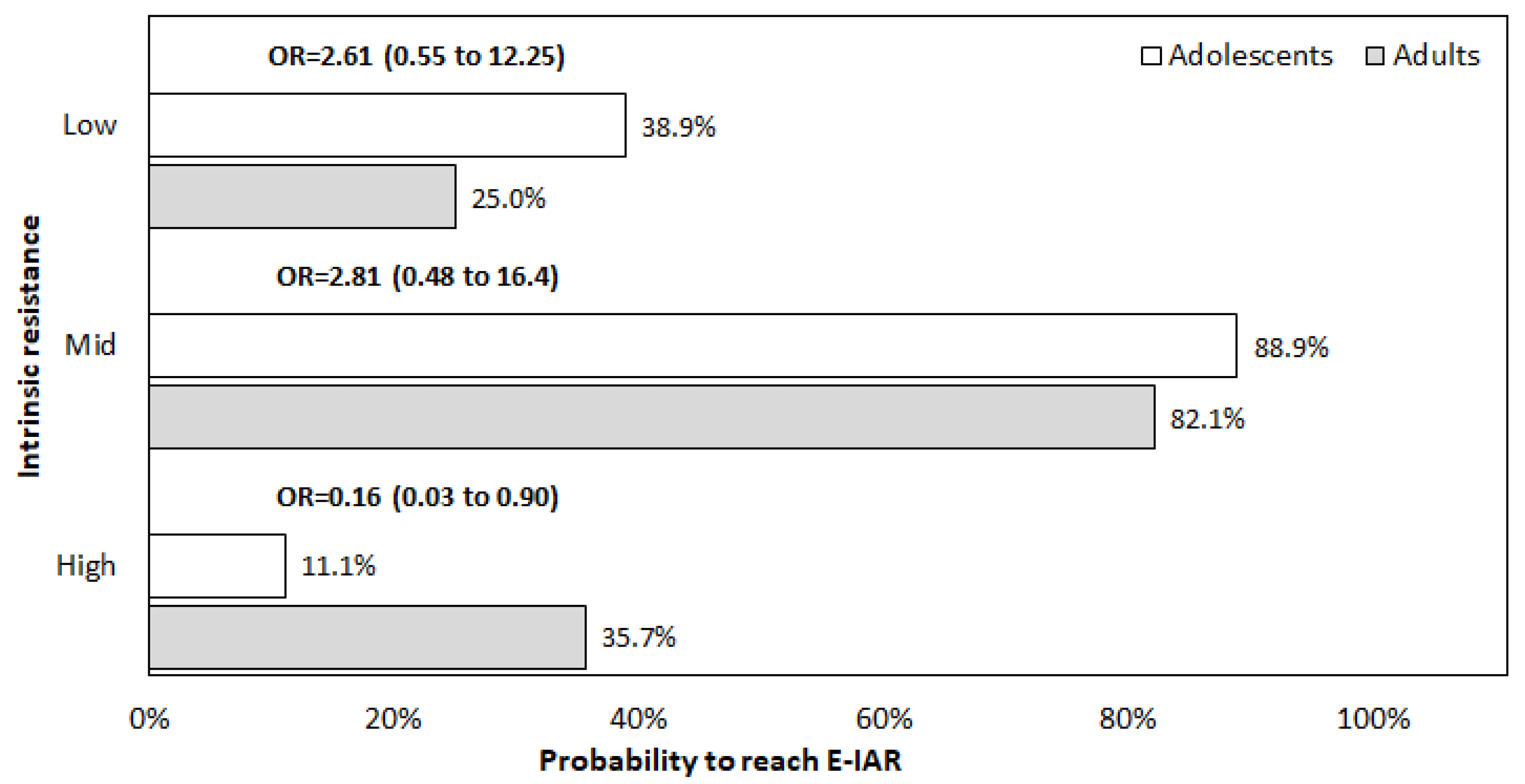
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virchow, J.C. Guidelines versus clinical practice—Which therapy and which device. Respir. Med. 2004, 98 (Suppl. B), S28–S34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Virchow, J.C.; Crompton, G.K.; Dal Negro, R.W.; Pedersen, S.; Magnan, A.; Seidemberg, J.; Barnes, P.J. Importance of inhaler devices in the management of airway diseases. Respir. Med. 2008, 102, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Weers, J.G.; Dhand, R. The Confusing World of Dry Powder Inhalers: It Is All About Inspiratory Pressures, Not Inspiratory Flow Rates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 1–11. [Google Scholar] [CrossRef]
- Wieshammer, S.; Dreyhaupt, J. Dry powder inhalers: Which factors determine the frequency of handling errors? Respiration 2008, 75, 18–25. [Google Scholar] [CrossRef]
- Newman, S.P.; Busse, W.W. Evolution of dry powder inhaler design, formulation, and performance. Respir. Med. 2002, 96, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.R.; Fogarty, C.M.; Peckitt, C.; Lassen, C.; Jadayel, D.; Dedericha, J. Delivery characteristics and patients’ handling of two single-dose dry powder inhalers used in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 353–356. [Google Scholar]
- Sanchis, J.; Corrigan, C.; Levy, M.L.; Viejo, J.L. Inhaler devices-From theory to practice. Respir. Med. 2013, 107, 495–502. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Turco, P.; Povero, M. Patients’ Usability of seven most used Dry-Powder Inhalers in COPD. Multidiscip. Respir. Med. 2019, 14, 30. [Google Scholar] [CrossRef]
- Kruger, P.; Ehrlein, Z.M.; Greguletz, R. Inspiratory flow resistance of marketed dry powder inhalers. Eur. Respir. J. 2014, 44 (Suppl. 58), 4635. [Google Scholar]
- Dal Negro, R.W.; Turco, P.; Povero, M. The contribution of patients’ lung function to the inspiratory airflow rate achievable through a DPIs’ simulator reproducing different intrinsic resistance rates. Multidiscip. Respir. Med. 2021, 16, 752. [Google Scholar] [CrossRef]
- Capstick, T.G.D.; Clifton, I.J. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Exp. Rev. Respir. Med. 2012, 6, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.J. Guiding Inspiratory Flow: Development of the In-Check DIAL G16, a Tool for Improving Inhaler Technique. Pulm. Med. 2017, 2017, 1495867. [Google Scholar] [CrossRef] [PubMed]
- Berkenfeld, K.; Lamprecht, A.; McConville, J.T. Devices for dry powder drug delivery to the lung, AAPS Pharm. Sci. Tech. 2015, 16, 479–490. [Google Scholar]
- Dederichs, J.; Singh, D.; Pavkov, R. Inspiratory flow profiles generated by patients with COPD through the Breezhaler inhaler and other marketed dry powder inhalers. Am. J. Respir. Crit. Care Med. 2015, 191, A5793. [Google Scholar]
- Canonica, G.W.; Arp, J.; Keegstra, J.R.; Chrystyn, H. Spiromax, a new dry powder inhaler: Dose consistency under simulated real-world conditions. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, S.I.; Assi, K.H.; Chrystyn, H. Aerodynamic dose emission characteristics of dry powder inhalers using an Andersen Cascade Impactor with a mixing inlet: The influence of flow and volume. Intern. J. Pharm. 2013, 455, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Frijlink, H.W.; De Boer, A.H. Dry powder inhalers for pulmonary drug delivery. Exp. Op. Drug Del. 2004, 1, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Lexmond, A.J.; Kruizinga, T.J.; Hagedoorn, P.; Rottier, B.L.; Frijlink, H.W.; De Boer, A.H. Effect of inhaler design variables on paediatric use of dry powder inhalers. PLoS ONE 2014, 9, e99304. [Google Scholar] [CrossRef]
- Crompton, G.K. Problems patients have using pressurized aerosol inhalers. Eur. J Resp. Dis. 1982, 63 (Suppl. 119), 101–104. [Google Scholar]
- Brocklebank, D.; Ram, F.; Wright, J.; Barry, P.; Cates, C.; Davies, L.; Douglas, G.; Muers, M.; Smith, D.; White, J. Comparison of effectiveness of inhaler devices in asthma and chronic obstructive airway disease: A systematic review of the literature. Health Technol. Asses. 2001, 5, 1–149. [Google Scholar] [CrossRef]
- Thomas, M.; Williams, A.E. Are outcomes the same with all dry powder inhalers? Int. J. Clin. Pract. Suppl. 2005, 149, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, P.; Taylor, A.; Zanen, P.; Chrysyn, H. Can patients use all dry powder inhalers equally well? Int. J. Clin. Pract. Suppl. 2005, 149, 13–18. [Google Scholar] [CrossRef]
- Suarez-Barcelo, M.; Micca, J.L.; Clackum, S.; Ferguson, G.T. Chronic obstructive pulmonary disease in long-term care setting: Current practices, challenges, and unmet needs. Curr. Opin. Pulm. Med. 2017, 23 (Suppl. 1), S1–S28. [Google Scholar] [CrossRef]
- Ung, K.T.; Rao, N.; Weers, J.G.; Clark, A.R.; Chan, H.K. In vitro assessment of dose delivery performance of dry powders for inhalation. Aerosol Sci. Technol. 2014, 48, 1099–1110. [Google Scholar] [CrossRef]
- Ung, K.T.; Chan, H.K. Effects of ramp-up of inspired airflow on in vitro aerosol dose delivery performance of certain dry powder inhalers. Eur. J Pharm. Sci. 2016, 84, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Arp, I.; Chambers, F.; Copley, M.; Glaab, V.; Hammond, M.; Solomon, D.; Bradford, K.; Russell, T.; Sizer, Y.; et al. Investigation of Dry Powder Inhaler (DPI) Resistance and Aerosol Dispersion Timing on Emitted Aerosol Aerodynamic Particle Sizing by Multistage Cascade Impactor when Sampled Volume Is Reduced from Compendial Value of 4 L. AAPS Pharm. Sci. Tech. 2014, 15, 1126–1137. [Google Scholar] [CrossRef][Green Version]
- Haidl, P.; Heindl, S.; Siemon, K.; Bernacka, M.; Cloes, R.M. Inhalation device requirements for patients’ inhalation maneuvers. Respir. Med. 2016, 118, 65–75. [Google Scholar] [CrossRef]
- Buttini, F.; Brambilla, G.; Copelli, D.; Sisti, V.; Balducci, A.G.; Bettini, R.; Pasquali, I. Effect of flow rate on in vitro aerodynamic performance of Nexthaler in comparison with Diskus and Turbohaler dry powder inhalers. J. Aerosol Med. Pulm. Drug Del. 2016, 29, 167–178. [Google Scholar] [CrossRef]
- Dal Negro, R.W. Dry powder inhalers and the right things to remember: A concept review. Multidiscip. Respir. Med. 2015, 10, 13. [Google Scholar] [CrossRef]
- Laube, B.L.; Janssens, H.M.; De Jongh, F.H.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef]
- Pedersen, S.; Hansen, O.R.; Fuglsang, G. Influence of inspiratory flow rate upon the effect of a Turbuhaler. Arch. Dis. Child. 1990, 65, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Weers, J.; Clark, A. The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm. Res. 2017, 34, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Azouz, W.; Chetcuti, P.; Hosker, H.S.; Saralaya, D.; Stephenson, J.; Chrystyn, H. The inhalation characteristics of patients when they use different dry powder inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Altman, P.; Wehbe, L.; Dederichs, J.; Guerin, T.; Ament, B.; Cardenas Moronta, M.; Pino, A.V.; Goyal, P. Comparison of peak inspiratory flow rate via the Breezhaler®, Ellipta® and HandiHaler® dry powder inhalers in patients with moderate to very severe COPD: A randomized cross-over trial. BMC Pulm. Med. 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R. The role of inspiratory pressures in determining the flow rate through dry powder inhalers: A review. Curr. Pharm. Design. 2015, 21, 3973–3983. [Google Scholar] [CrossRef]
- Malmberg, L.P.; Rytilä, P.; Happonen, P.; Haahtela, T. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: Age and gender rather than severity matters. Int. J. Chronic Obstr. Pulm. Dis. 2010, 5, 257–262. [Google Scholar] [CrossRef]
- Cook, C.D.; Mead, J.; Orzalesi, M.M. Static volume/pressure characteristics of the respiratory system during maximal efforts. J. Appl. Physiol. 1964, 19, 1016–1022. [Google Scholar] [CrossRef]
- Clark, A.R.; Hollingworth, A.M. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers—Implications for in vitro testing. J. Aerosol. Med. 1993, 6, 99–110. [Google Scholar] [CrossRef]
- Mahler, D.A.; Waterman, L.A.; Gifford, A.H. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus. J. Aerosol. Med. Pulm. Drug Deliv. 2013, 26, 174–179. [Google Scholar] [CrossRef]
- Mahler, D.A.; Waterman, L.A.; Ward, J.; Gifford, A.H. Comparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J. Aerosol. Med. Pulm. Drug Deliv. 2014, 27, 103–109. [Google Scholar] [CrossRef]
- Janssens, W.; VandenBrande, P.; Hardeman, E.; De Langhe, E.; Philips, T.; Troosters, T.; Decramer, M. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur. Respir J. 2008, 31, 78–83. [Google Scholar] [CrossRef] [PubMed]
| Variables | Adolescents | Retrospective Adult Controls | p-Value |
|---|---|---|---|
| N | 18 | 28 | |
| Sex (% female) | 10 (55.6%) | 17 (60.7%) | 0.4820 |
| Age | 16.9 ± 0.39 | 52.1 ± 2.89 | <0.0001 |
| BMI | 22.1 ± 0.44 | 25.9 ± 1.15 | 0.0229 |
| FEV1 (L) | 3.1 ± 0.07 | 2.8 ± 0.16 | 0.1315 |
| FEV1 (% pred) | 97.6 ± 1.32 | 93.5 ± 2.91 | 0.3216 |
| IC (L) | 3.1 ± 0.10 | 2.9 ± 0.14 | 0.3368 |
| IC (% pred) | 104.3 ± 1.76 | 107.4 ± 4.12 | 0.9312 |
| FIV (L) | 3.4 ± 0.21 | 3.3 ± 0.19 | 0.8728 |
| FIF max (L/s) | 4.9 ± 0.45 | 4.9 ± 0.36 | >0.9999 |
| FIF max (% pred) | 84.1 ± 4.48 | 79.9 ± 4.82 | 0.6845 |
| MEF25 (L/s) | 1.9 ± 0.12 | 1.4 ± 0.14 | 0.0164 |
| MEF25% (% pred) | 95.2 ± 3.59 | 81.4 ± 6.22 | 0.0165 |
| TLC (L) | 5.8 ± 0.23 | 5.6 ± 0.17 | 0.3976 |
| TLC (% pred) | 98.6 ± 2.36 | 95.7 ± 2.93 | 0.3358 |
| RV (L) | 1.5 ± 0.08 | 1.8 ± 0.14 | 0.0425 |
| RV (% pred) | 88.8 ± 2.82 | 91.1 ± 6.59 | 0.4867 |
| IRaw (L) | 1.8 ± 0.18 | 3.1 ± 0.57 | 0.0717 |
| DPI Resistance | Adolescents | Retrospective Adult Controls | Mean Difference (95% CI) |
|---|---|---|---|
| Low | 90.56 ± 3.75 | 86.96 ± 3.87 | 3.59 (−6.84 to 14.02) |
| Mid | 76.67 ± 3.38 | 70.89 ± 3.66 | 5.77 (−3.78 to 15.33) |
| High | 64.44 ± 2.77 | 53.21 ± 3.37 | 11.23 (2.81 to 19.65) |
| Non parametric test for trend | p < 0.001 | p < 0.001 |
| Variables | Univariate Model OR (95% CI) | Multivariate Model OR (95% CI) |
|---|---|---|
| Sex (male) | 1.204 (0.67 to 2.16) | |
| Age (years) | 1 (0.98 to 1.02) | |
| BMI | 1.01 (0.96 to 1.06) | |
| FEV1 (L) | 0.969 (0.71 to 1.32) | |
| FEV1 (%) | 1.002 (0.98 to 1.02) | |
| IC (L) | 1.006 (0.69 to 1.46) | |
| IC (%) | 1.003 (0.99 to 1.02) | |
| FIV (L) | 1.024 (0.71 to 1.47) | |
| FIF (L/s) | 0.98 (0.83 to 1.15) | |
| FIF (%) | 1.002 (0.99 to 1.02) | |
| MEF25 (L/s) | 1.026 (0.75 to 1.41) | |
| MEF25 (%) | 0.996 (0.99 to 1) | |
| TLC (L) | 1.147 (0.86 to 1.53) | |
| TLC (%) | 1.011 (0.99 to 1.03) | |
| RV (L) | 1.222 (0.85 to 1.76) | |
| RV (%) | 1.006 (1 to 1.01) | 1.131 (1.03 to 1.25) |
| IRaw (L) | 1.027 (0.97 to 1.09) | 0.290 (0.09 to 0.92) |
| DPI Resistance | ||
| Low vs. mid | 0.086 (0.02 to 0.36) | 0.035 (0.001 to 0.84) |
| High vs. mid | 0.116 (0.03 to 0.41) | 0.004 (<0.001 to 0.42) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Negro, R.W.; Turco, P.; Povero, M. Lung Function Can Predict the Expected Inspiratory Airflow Rate through Dry Powder Inhalers in Asthmatic Adolescents. Children 2022, 9, 377. https://doi.org/10.3390/children9030377
Dal Negro RW, Turco P, Povero M. Lung Function Can Predict the Expected Inspiratory Airflow Rate through Dry Powder Inhalers in Asthmatic Adolescents. Children. 2022; 9(3):377. https://doi.org/10.3390/children9030377
Chicago/Turabian StyleDal Negro, Roberto Walter, Paola Turco, and Massimiliano Povero. 2022. "Lung Function Can Predict the Expected Inspiratory Airflow Rate through Dry Powder Inhalers in Asthmatic Adolescents" Children 9, no. 3: 377. https://doi.org/10.3390/children9030377
APA StyleDal Negro, R. W., Turco, P., & Povero, M. (2022). Lung Function Can Predict the Expected Inspiratory Airflow Rate through Dry Powder Inhalers in Asthmatic Adolescents. Children, 9(3), 377. https://doi.org/10.3390/children9030377






