Abstract
Several factors affect drug delivery from dry powder inhalers (DPIs). Some are related to patient’s physiological characteristics, while others depend on DPIs’ technical aspects. The patient’s inspiratory airflow rate (IAR) affects the pressure drop and the turbulence needed to disaggregate the powder inside a DPI. The present study investigated whether lung function limitations occurring in asthmatic adolescents affect their IAR when inhaling through a DPI simulator. Eighteen consecutive adolescents with asthma were recruited, and IAR was randomly assessed at low-, mid-, and high-resistance regimens. A multiple logistic model was developed to evaluate the association of patients’ lung function characteristics and devices’ resistance with the probability to achieve the expected IAR (E-IAR). The mean value of E-IAR achieved seemed to be sex- and age-independent. Low- and high-resistance regimens were less likely to consent the E-IAR level (odds ratio [OR] = 0.035 and OR = 0.004, respectively). Only the basal residual volume and the inspiratory resistance, but not the Forced Expiratory Volume in 1 s (FEV1), seemed to affect the extent of IAR in asthmatic adolescents (OR = 1.131 and OR = 0.290, respectively). The results suggest that the assessment of current lung function is crucial for choosing the proper DPI for asthmatic adolescents.
1. Introduction
Inhalation is the most suitable and convenient route for delivering active drugs to patients suffering from airway obstruction. Respiratory drugs target the airways directly by this route, thus minimizing the drug dose and allowing a quicker onset of action [1,2].
The inhalation technology was greatly improved over the last years, particularly with the aim to improve its therapeutic effectiveness in more “difficult” and/or in “less compliant” patients who need long-term respiratory treatments, such as teenagers suffering from bronchial asthma.
The effectiveness of any inhalation therapy for bronchial asthma depends on several factors [3,4,5,6]. Dry powder inhalers (DPIs) require a faster and deeper inhalation than Metered-Dose Inhalers and Soft Mist Inhalers. However, they are the most prescribed devices for daily and long-term asthma treatments [7,8]. Each DPI is characterized by its own intrinsic resistance. This resistance depends on the original engineering of each DPI and determines the pressure drop induced by patients’ inspiratory airflow rate (IAR) across the DPI itself [9]. IAR is the only active force causing the pressure drop and consequent turbulence inside the DPI that are required for disaggregating the powder to inhale.
It was recently shown that subjects’ airflow limitations might variably affect the expected IAR across DPIs in adults and that different lung function predictors can be usefully employed, particularly in patients with asthma and other obstructive respiratory disorders [10,11]. This evidence is still missing for asthma adolescents, who usually do not comply with the inhalation procedures required for an effective asthma management.
The present study investigated whether some parameters of lung function may predict the IAR of adolescents suffering from asthma when inhaling at different regimens of resistance.
2. Materials and Methods
A sample of asthma adolescents with normal cognition and dexterity referring to the CEMS Lung Unit (Verona, Italy) was consecutively recruited between June and September 2020. The lung function parameters assessed by Plethysmography Platinum DX Elite, MedGraphics, USA were: Forced Expiratory Volume in 1 s (FEV1), Inspiratory Capacity (IC), Forced Inspiratory Volume (FIV), Forced Inspiratory Flow (FIF), Total Lung Capacity (TLC), Maximal Expiratory Flow at 25% of lung filling (MEF25), Residual Volume (RV), Inspiratory Resistance (IRaw), and Expiratory Resistance (ERaw). FEV1, IC, TLC, and RV were expressed both in L and in % predicted, FIV and IRaw in L/s, FIF and MEF25 in both L/s and % predicted.
The In-Check DIAL G16 (Clement Clarke International Ltd., Harlow, UK) was used for measuring IAR at three different resistance regimens, using a validated DPIs simulator capable to reproduce the patterns of intrinsic resistance that are peculiar for several DPIs. The In-Check DIAL G16 allows forced IARs ranging from 15 to 120 L/min, that correspond to the IAR levels of the majority of inhaling devices [9,10,12]. It should be taken into account that an inspiratory pressure drop of 4 kPa across the device corresponds to an inspiratory flow resistance (IFR) <0.02 kPa0.5min/L and requires an IAR > 100 L/min. The IFR increases to 0.020–0.040 kPa0.5min/L in the mid-resistance regimen and requires an IAR of 50–100 L/min. Finally, the high-resistance regimen produces an IFR > 0.040 kPa0.5min/L and requires an IAR < 50 L/min [3,9,13,14,15,16,17,18]. Values of IAR assessed in the present study were compared to these reference values.
At recruitment, two expert technicians trained all subjects in the use of the In-Check DIAL simulator. Patients were randomly tested using the three different resistance regimens. Each patient performed three sequential attempts, and only the best IAR was collected for calculations (inter-measure variability ≤5%). Subjects who produced the expected IAR (E-IAR) value for each regimen [3,9,13,14,15,16,17,18] were also recorded.
Data produced by adolescents with asthma were also compared to those previously obtained from a cohort of adult asthmatics [10].
Before the investigation started, the adolescents’ parents gave their informed consent to the use of the collected information for purposes of research.
Data are reported as means ± standard error (SE). Only the “sex” variable is reported as absolute and relative frequency. The non-parametric Wilcoxon test was used to compare adolescents and adult controls in terms of age, BMI, and lung function (the exact Fisher test was used for sex).
For each lung function parameter, we investigated the association with the probability to achieve the E-IAR (with the three simulated resistance regimens) by using univariate logistic models. The strength of this association was measured in terms of odds ratio (OR): OR > 1 (resp. OR < 1) and suggested a positive (resp., negative) association with the tested variable, i.e., the probability to achieve the E-IAR increased (resp., decreases) as the variable’s value increased. All variables associated with the outcome (preliminary fixed at p-value < 0.25) were included in a multiple regression model. Finally, the best subset of predictors was selected by a backward-stepwise selection.
Finally, a sample of adult controls enrolled in our previous study [10] was used to investigate if adolescents could be associated with different inspiratory flows or with a higher probability to achieve the expected IAR. The comparison was performed using a generalized linear model (Gamma family) for inspiratory flow and a logistic regression for the probability to produce the E-IAR value.
Stata Statistical Software (Release 15) was used for all statistical calculations.
3. Results
Eighteen asthmatic adolescents were tested. The baseline characteristics of the sample are summarized in Table 1. The resulting lung function was compatible with bronchial asthma and (with the obvious exception of age distribution), comparable to that of a control group of adults enrolled in a previous study [10] (Table 1).

Table 1.
Mean ± standard error of lung function parameters measured in the sample of adolescents and in the adult controls (absolute and relative frequency were used for the variable “sex”).
Adolescents’ IAR appeared to be inversely related to the increase of the resistance regimen (p < 0.001) (Table 2). The inspiratory airflows obtained in adolescents were higher than those observed in the adult controls. Such difference became progressively more evident when increasing the resistance regimen.

Table 2.
Mean inspiratory flow (L/min) ± standard error measured at the three resistance regimens.
The results of univariate and multivariate regressions are reported in Table 3. In general, resistance regimens were highly associated with the probability to achieve the E-IAR (Table 3). In particular, when forced inspiration was performed at low- and high-resistance regimens, adolescents with asthma were less likely to reach their E-IAR compared to when they inhaled at mid-resistance: OR Low vs. mid = 0.035 (95% CI 0.001 to 0.84) and OR High vs. mid = 0.004 (95% CI < 0.001 to 0.42, respectively). These data could suggest that low-resistance DPIs are less likely to consent reaching the optimal IAR in asthmatic adolescents because the required value of IAR > 100 L/min is too high, and only some asthmatic adolescents are capable to reach this threshold (Table 3). Moreover, high-resistance DPIs seemed to be less likely to allow the optimal IARs due to the too high intrinsic resistance that has to be overcome when inhaling, while mid-resistance DPIs were the most suitable and reliable from this point of view.

Table 3.
Results of univariate and multivariate logistic regression (after stepwise selection).
In the multivariate analysis, the only lung function parameters that contributed to the prediction of IAR were RV % predicted (OR = 1.131, 95% CI 1.03 to 1.25) and IRaw (OR = 0.29, 95% CI 0.09 to 0.92), whereas FEV1 was not useful (both in absolute and in % predicted values) (Table 3).
Finally, the proportion of asthmatic adolescents who reached their E-IAR value is reported in Figure 1 for the three simulated regimens. The probability to achieve the E-IAR in asthmatic adolescents was comparable to that of adults at low- and mid-resistance regimens, while it was lower at a high-resistance regimen (OR = 0.16, 95% CI 0.03 to 0.90).
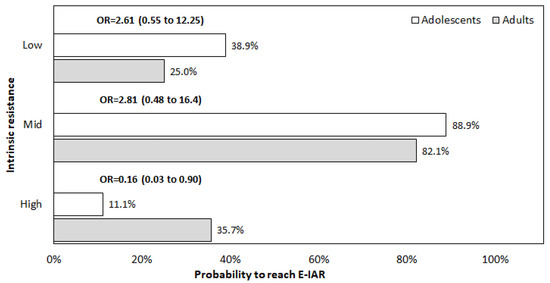
Figure 1.
Proportion of subjects who reached their E-IAR value at the three simulated regimens. E-IAR: expected inspiratory airflow rate; OR: odds ratio adjusted for RV and IRaw.
4. Discussion
The delivery of respiratory drugs is substantially improved by the use of DPIs. In fact, DPIs do not contain propellants, require simplified procedures for inhalation; improve patients’ adherence to treatments, minimize the variability of the emitted dose, favor drug(s) deposition within the airways, reduce the occurrence of side effects (both local and systemic), and contribute to improve the therapeutic outcomes [4,19,20].
Despite these consolidated advantages, choosing the best DPI is still a critical challenge in real life [21], as the DPIs presently available perform differently in terms of inhalation and deposition patterns, according to their engineering differences [3,22,23]. Several experimental and in vitro studies extensively investigated the relative contribution of different factors, even if the available data are frequently only partially reproducible in real-life conditions [24,25,26].
The drivers affecting the de-aggregation and the aerosolization of dry powders to be inhaled through DPIs are the pressure drop occurring during maximal inspiratory maneuvers and the flow rate and flow acceleration generated through the device [9,27,28,29,30,31]. The interactions between volumes, flow rates, changes in pressure drops, and DPI technical peculiarities are really complex, even though it has been suggested that larger pressure drops and higher flow rates and inhaled volumes usually correspond to more effective particle dispersion and aerosolization and to a larger amount of drug(s) impacting the airways for all DPIs. [25,32,33]. DPIs can then be ranked as low-resistance (<5 Mbar0.5L/min−1, i.e., Brezhaler), mid-resistance (5–10 Mbar0.5L/min−1, i.e., Accuhaler, Diskhaler, Ellipta, Genuair; Spiromax, Clickhaler, Turbohaler, Easyhaler, Twisthaler, Nexthaler), and high-resistance inhalers (>10 Mbar0.5L/min−1, i.e., Handihaler) [12,13].
In the case of low-resistance DPIs, the inspiratory pressures needed for the effective inhalation through the DPI are suggested to work as a factor limiting the patient’s capability to generate the E-IAR level [34], even if in some studies [3,35,36], but not in others [10,30,33], patient’s age and gender were described as the unique variables able to influence IAR through DPIs.
The patient’s respiratory disorder and the corresponding lung function limitations were considered trivial factors in this regard, though airway and parenchymal structures can be variably and peculiarly compromised, and their mechanical performance limited [37]. It should be emphasized that the patient’s IAR is the only force generated during inhalation. This drive is required (1) to produce the necessary pressure drop, (2) to elicit turbulence inside the DPI, and (3) to produce the disaggregation, the micro-dispersion and, finally, the delivery of the powdered drug [1,9,32]. However, even if specific data are very few, it has been shown that the IAR achievable through a DPI is related to the square root of the occurring pressure drop and that the dose of the inhaled drug targeting the airways is directly related to the IAR increase [6,32,38].
The role of a subject’s lung function, though minimized in some studies (particularly, in bronchial asthma) should be much more valued in our opinion, as different degrees of flow limitation can variably correspond to effects on patients’ inspiratory/expiratory performances. Unfortunately, the relationships linking pressure drops, IAR, and DPI resistance have not been frequently studied in asthma patients, likely because a complete plethysmography assessment of lung function parameters does not represent a usual procedure in clinical practice, particularly for asthmatic adolescents. Nevertheless, each lung function parameter provides, even if to a variable extent, a physiological sign, and the occurrence of peculiar respiratory limitations could be able to affect the effective use of DPIs. We emphasize that a DPI choice based only on FEV1 values, even if simple to obtain, failed to predict the effective IAR through DPIs, as FEV1 is characterized by a too low specificity [39,40,41]. It is presumable that other more appropriate parameters should be carefully assessed in order to unveil more specific lung function limitations affecting IAR when inhaling throughout DPIs at different regimens of intrinsic resistance.
The data of the present investigation suggest that the extent of IAR through DPIs in asthmatic adolescents could be affected by the subjects’ pattern of airflow limitation. In other words, changes in airflow limitation may variably affect the inspiratory airflow rate required to overcome the proper resistance of different DPIs, and, consequently, to assure the effective delivery of the powdered drug(s) within the airways. Our results also showed that only mid-resistance DPIs consented to achieve the most convenient IAR with the highest frequency in asthmatic adolescents. In other words, the majority of asthmatic adolescents can achieve their E-IAR only by inhaling through a mid-resistance DPI, regardless of age, sex, and BMI. As already observed in adult asthmatics [10], similar performances cannot be obtained when inhaling through low- and high- resistance DPIs (Figure 1).
In addition to DPI resistance, RV and IRaw were the only predictors of E-IAR in asthmatic adolescents, that is, in those individuals characterized by a not negligible reduction in their airway patency. The variable proportion of young patients who achieved their E-IAR seemed to mirror the effect of their respiratory condition.
Some limitation likely biased the present investigation. Data were obtained from a monocentric sample of patients, with a limited size. However, the results are consistent with those of a previous analysis focused on the adult population [10], and an extension of the present analysis, aimed to increase the sample size, is already in progress. The measurements of DPIs’ resistance were carried out by the In-Check DIAL G16, used as a simulator, and the results were assumed to be related to DPIs’ intrinsic resistance.
The points of strength of this study are: (1) the adolescents’ performance in terms of E-IAR was investigated in real life for the first time, (2) possible predictors of the proper IAR were explored for the first time in asthmatic adolescents using a quite exhaustive set of lung function parameters, (3) proper statistical models were used.
5. Conclusions
The assessment of DPIs is still a challenge in respiratory medicine, particularly in asthmatic adolescents, i.e., those individuals who are the least compliant with any regular and long-term inhalation treatment. In general, the engineering peculiarities of DPI and their intrinsic resistive regimens can variably affect the extent of patients’ IAR. Lung function further contributes to affect IAR per se, even in asthma adolescents. Only some lung function parameters, such as RV and IRaw but not FEV1, seem to predict the E-IAR through DPIs in asthmatic adolescents.
The analytic assessment of lung function is strongly suggested for asthmatic adolescents, with the aim to more effectively personalize the choice of DPI.
Author Contributions
R.W.D.N. and P.T.: Conceptualization and validation; M.P.: statistical models and software; R.W.D.N. and P.T.: resources; R.W.D.N. and M.P.: writing original draft preparation; P.T.: review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethical and Scientific Commission of the National Centre for Respiratory Pharmacoeconomics and Pharmacoepidemiology approved the study on 10 June 2020 (code: D/R/02/2020).
Informed Consent Statement
The parents of the enrolled adolescents provided the informed consent to the study.
Data Availability Statement
Authors do not wish to share their data without their permission.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Virchow, J.C. Guidelines versus clinical practice—Which therapy and which device. Respir. Med. 2004, 98 (Suppl. B), S28–S34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Virchow, J.C.; Crompton, G.K.; Dal Negro, R.W.; Pedersen, S.; Magnan, A.; Seidemberg, J.; Barnes, P.J. Importance of inhaler devices in the management of airway diseases. Respir. Med. 2008, 102, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Weers, J.G.; Dhand, R. The Confusing World of Dry Powder Inhalers: It Is All About Inspiratory Pressures, Not Inspiratory Flow Rates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 1–11. [Google Scholar] [CrossRef]
- Wieshammer, S.; Dreyhaupt, J. Dry powder inhalers: Which factors determine the frequency of handling errors? Respiration 2008, 75, 18–25. [Google Scholar] [CrossRef]
- Newman, S.P.; Busse, W.W. Evolution of dry powder inhaler design, formulation, and performance. Respir. Med. 2002, 96, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.R.; Fogarty, C.M.; Peckitt, C.; Lassen, C.; Jadayel, D.; Dedericha, J. Delivery characteristics and patients’ handling of two single-dose dry powder inhalers used in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 353–356. [Google Scholar]
- Sanchis, J.; Corrigan, C.; Levy, M.L.; Viejo, J.L. Inhaler devices-From theory to practice. Respir. Med. 2013, 107, 495–502. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Turco, P.; Povero, M. Patients’ Usability of seven most used Dry-Powder Inhalers in COPD. Multidiscip. Respir. Med. 2019, 14, 30. [Google Scholar] [CrossRef]
- Kruger, P.; Ehrlein, Z.M.; Greguletz, R. Inspiratory flow resistance of marketed dry powder inhalers. Eur. Respir. J. 2014, 44 (Suppl. 58), 4635. [Google Scholar]
- Dal Negro, R.W.; Turco, P.; Povero, M. The contribution of patients’ lung function to the inspiratory airflow rate achievable through a DPIs’ simulator reproducing different intrinsic resistance rates. Multidiscip. Respir. Med. 2021, 16, 752. [Google Scholar] [CrossRef]
- Capstick, T.G.D.; Clifton, I.J. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Exp. Rev. Respir. Med. 2012, 6, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.J. Guiding Inspiratory Flow: Development of the In-Check DIAL G16, a Tool for Improving Inhaler Technique. Pulm. Med. 2017, 2017, 1495867. [Google Scholar] [CrossRef] [PubMed]
- Berkenfeld, K.; Lamprecht, A.; McConville, J.T. Devices for dry powder drug delivery to the lung, AAPS Pharm. Sci. Tech. 2015, 16, 479–490. [Google Scholar]
- Dederichs, J.; Singh, D.; Pavkov, R. Inspiratory flow profiles generated by patients with COPD through the Breezhaler inhaler and other marketed dry powder inhalers. Am. J. Respir. Crit. Care Med. 2015, 191, A5793. [Google Scholar]
- Canonica, G.W.; Arp, J.; Keegstra, J.R.; Chrystyn, H. Spiromax, a new dry powder inhaler: Dose consistency under simulated real-world conditions. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, S.I.; Assi, K.H.; Chrystyn, H. Aerodynamic dose emission characteristics of dry powder inhalers using an Andersen Cascade Impactor with a mixing inlet: The influence of flow and volume. Intern. J. Pharm. 2013, 455, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Frijlink, H.W.; De Boer, A.H. Dry powder inhalers for pulmonary drug delivery. Exp. Op. Drug Del. 2004, 1, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Lexmond, A.J.; Kruizinga, T.J.; Hagedoorn, P.; Rottier, B.L.; Frijlink, H.W.; De Boer, A.H. Effect of inhaler design variables on paediatric use of dry powder inhalers. PLoS ONE 2014, 9, e99304. [Google Scholar] [CrossRef]
- Crompton, G.K. Problems patients have using pressurized aerosol inhalers. Eur. J Resp. Dis. 1982, 63 (Suppl. 119), 101–104. [Google Scholar]
- Brocklebank, D.; Ram, F.; Wright, J.; Barry, P.; Cates, C.; Davies, L.; Douglas, G.; Muers, M.; Smith, D.; White, J. Comparison of effectiveness of inhaler devices in asthma and chronic obstructive airway disease: A systematic review of the literature. Health Technol. Asses. 2001, 5, 1–149. [Google Scholar] [CrossRef]
- Thomas, M.; Williams, A.E. Are outcomes the same with all dry powder inhalers? Int. J. Clin. Pract. Suppl. 2005, 149, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, P.; Taylor, A.; Zanen, P.; Chrysyn, H. Can patients use all dry powder inhalers equally well? Int. J. Clin. Pract. Suppl. 2005, 149, 13–18. [Google Scholar] [CrossRef]
- Suarez-Barcelo, M.; Micca, J.L.; Clackum, S.; Ferguson, G.T. Chronic obstructive pulmonary disease in long-term care setting: Current practices, challenges, and unmet needs. Curr. Opin. Pulm. Med. 2017, 23 (Suppl. 1), S1–S28. [Google Scholar] [CrossRef]
- Ung, K.T.; Rao, N.; Weers, J.G.; Clark, A.R.; Chan, H.K. In vitro assessment of dose delivery performance of dry powders for inhalation. Aerosol Sci. Technol. 2014, 48, 1099–1110. [Google Scholar] [CrossRef]
- Ung, K.T.; Chan, H.K. Effects of ramp-up of inspired airflow on in vitro aerosol dose delivery performance of certain dry powder inhalers. Eur. J Pharm. Sci. 2016, 84, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Arp, I.; Chambers, F.; Copley, M.; Glaab, V.; Hammond, M.; Solomon, D.; Bradford, K.; Russell, T.; Sizer, Y.; et al. Investigation of Dry Powder Inhaler (DPI) Resistance and Aerosol Dispersion Timing on Emitted Aerosol Aerodynamic Particle Sizing by Multistage Cascade Impactor when Sampled Volume Is Reduced from Compendial Value of 4 L. AAPS Pharm. Sci. Tech. 2014, 15, 1126–1137. [Google Scholar] [CrossRef][Green Version]
- Haidl, P.; Heindl, S.; Siemon, K.; Bernacka, M.; Cloes, R.M. Inhalation device requirements for patients’ inhalation maneuvers. Respir. Med. 2016, 118, 65–75. [Google Scholar] [CrossRef]
- Buttini, F.; Brambilla, G.; Copelli, D.; Sisti, V.; Balducci, A.G.; Bettini, R.; Pasquali, I. Effect of flow rate on in vitro aerodynamic performance of Nexthaler in comparison with Diskus and Turbohaler dry powder inhalers. J. Aerosol Med. Pulm. Drug Del. 2016, 29, 167–178. [Google Scholar] [CrossRef]
- Dal Negro, R.W. Dry powder inhalers and the right things to remember: A concept review. Multidiscip. Respir. Med. 2015, 10, 13. [Google Scholar] [CrossRef]
- Laube, B.L.; Janssens, H.M.; De Jongh, F.H.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef]
- Pedersen, S.; Hansen, O.R.; Fuglsang, G. Influence of inspiratory flow rate upon the effect of a Turbuhaler. Arch. Dis. Child. 1990, 65, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Weers, J.; Clark, A. The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm. Res. 2017, 34, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Azouz, W.; Chetcuti, P.; Hosker, H.S.; Saralaya, D.; Stephenson, J.; Chrystyn, H. The inhalation characteristics of patients when they use different dry powder inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Altman, P.; Wehbe, L.; Dederichs, J.; Guerin, T.; Ament, B.; Cardenas Moronta, M.; Pino, A.V.; Goyal, P. Comparison of peak inspiratory flow rate via the Breezhaler®, Ellipta® and HandiHaler® dry powder inhalers in patients with moderate to very severe COPD: A randomized cross-over trial. BMC Pulm. Med. 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R. The role of inspiratory pressures in determining the flow rate through dry powder inhalers: A review. Curr. Pharm. Design. 2015, 21, 3973–3983. [Google Scholar] [CrossRef]
- Malmberg, L.P.; Rytilä, P.; Happonen, P.; Haahtela, T. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: Age and gender rather than severity matters. Int. J. Chronic Obstr. Pulm. Dis. 2010, 5, 257–262. [Google Scholar] [CrossRef]
- Cook, C.D.; Mead, J.; Orzalesi, M.M. Static volume/pressure characteristics of the respiratory system during maximal efforts. J. Appl. Physiol. 1964, 19, 1016–1022. [Google Scholar] [CrossRef]
- Clark, A.R.; Hollingworth, A.M. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers—Implications for in vitro testing. J. Aerosol. Med. 1993, 6, 99–110. [Google Scholar] [CrossRef]
- Mahler, D.A.; Waterman, L.A.; Gifford, A.H. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus. J. Aerosol. Med. Pulm. Drug Deliv. 2013, 26, 174–179. [Google Scholar] [CrossRef]
- Mahler, D.A.; Waterman, L.A.; Ward, J.; Gifford, A.H. Comparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J. Aerosol. Med. Pulm. Drug Deliv. 2014, 27, 103–109. [Google Scholar] [CrossRef]
- Janssens, W.; VandenBrande, P.; Hardeman, E.; De Langhe, E.; Philips, T.; Troosters, T.; Decramer, M. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur. Respir J. 2008, 31, 78–83. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).