Maturation of Arousals during Day and Night in Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Questionnaires
2.2. Polysomnograms
2.3. Psychometric Assessment
2.4. Ethics
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | Active Sleep |
| AuBE | Autonomic Baby Evaluation study |
| M0 | Term (usually 2 days of life) |
| M6 | 6 months |
| NREM | Non-Rapid-Eye-Movement Sleep |
| QS | Quiet Sleep |
| REM | Rapid Eye Movement Sleep |
| SE | Sleep Efficiency |
| T | Term Infants |
| P | Preterm Infants |
| TST | Total Sleep Time |
| SIDS | Sudden Infant Death Syndrome |
| WPPSI-III | Wechsler Preschool and Primary Scale of Intelligence |
References
- Phillipson, E.A.; Sullivan, C.E. Arousal: The forgotten response to respiratory stimuli. Am. Rev. Respir. Dis. 1978, 118, 807–809. [Google Scholar] [PubMed]
- Kahn, A.; Groswasser, J.; Rebuffat, E.; Sottiaux, M.; Blum, D.; Foerster, M.; Franco, P.; Bochner, A.; Alexander, M.; Bachy, A.; et al. Sleep and cardiorespiratory characteristics of infant victims of sudden death: A prospective case-control study. Sleep 1992, 15, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schechtman, V.L.; Harper, R.M.; Wilson, A.J.; Southall, D.P. Sleep state organization in normal infants and victims of the sudden infant death syndrome. Pediatrics 1992, 89, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Kahn, A.; Sawaguchi, T.; Groswasser, J.; Franco, P.; Scaillet, S.; Kelmanson, I.; Dan, B. Sudden Infant deaths: From epidemiology to physiology. Forensic Sci. Int. 2002, 130, 8–20. [Google Scholar] [CrossRef]
- Filiano, J.J.; Kinney, H.C. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: The triple-risk model. Biol. Neonate 1994, 65, 194–197. [Google Scholar] [CrossRef]
- Smith, G.C.; White, I.R. Predicting the risk for sudden infant death syndrome from obstetric characteristics: A retrospective cohort study of 505,011 live births. Pediatrics 2006, 117, 60–66. [Google Scholar] [CrossRef]
- Getahun, D.; Amre, D.; Rhoads, G.; Demissie, K. Maternal and obstetric risk factors for Sudden Infant Death Syndrome in the United States. Obstet. Gynecol. 2004, 103, 646–652. [Google Scholar] [CrossRef]
- Carpenter, R.; Irgens, L.; Blair, P.; England, P.; Fleming, P.; Huber, J.; Jorch, G.; Schreuder, P. Sudden unexplained infant death in 20 regions in Europe: Case control study. Lancet 2004, 363, 185–191. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Hillman, L.S. Epidemiology of the sudden infant death syndrome: Maternal, neonatal, and postnatal risk factors. In Clinics in Perinatology: Apnea and SIDS; Hunt, C.E., Ed.; WB Saunders: Philadelphia, PA, USA, 1992; pp. 717–738. [Google Scholar]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 forselected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [Green Version]
- Blair, P.S.; Sidebotham, P.; Berry, P.J.; Evans, M.; Fleming, P.J. Major epidemiological changes in sudden infant death syndrome: A 20-year population-based study in the UK. Lancet 2006, 367, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Malloy, M.H. Prematurity and sudden infant death syndrome: United States 2005–2007. J. Perinatol. 2013, 33, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachtenberg, F.L.; Haas, E.A.; Kinney, H.C.; Stanley, C.; Krous, H.F. Risk factor changes for sudden infant death syndrome after initiation of Back-to-Sleep campaign. Pediatrics 2012, 129, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Challamel, M.J.; Franco, P.; Hardy, M. Le Sommeil de L’enfant; Masson: Paris, France, 2009. [Google Scholar]
- Shimada, M.; Takahashi, K.; Segawa, M.; Higurashi, M.; Samejim, M.; Horiuchi, K. Emerging and entraining patterns of the sleep-wake rhythm in preterm and term infants. Brain Dev. 1999, 21, 468–473. [Google Scholar] [CrossRef]
- Rivkees, S.A. Developing circadian rhythmicity in infants. Pediatrics 2003, 112, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Jenni, O.G.; Deboer, T.; Achermann, P. Development of the 24-h rest-activity pattern in human infants. Infant Behav. Dev. 2006, 29, 143–152. [Google Scholar] [CrossRef]
- Louis, J.; Cannard, C.; Bastuji, H.; Challamel, M.J. Sleep ontogenesis revisited: A longitudinal 24-hour home polygraphic study on 15 normal infants during the first two years of life. Sleep 1997, 20, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Rivkees, S.A.; Mayes, L.; Jacobs, H.; Gross, I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics 2004, 113, 833–839. [Google Scholar] [CrossRef]
- Horne, R.S.C.; Bandopadhayay, P.; Vitkovic, J.; Cranage, S.M.; Adamson, T.M. Effects of Age and Sleeping Position on Arousal from Sleep in Preterm Infants. Sleep 2002, 25, 746–750. [Google Scholar] [CrossRef] [Green Version]
- Navelet, Y.; Payan, C.; Guilhaume, A.; Benoit, O. Nocturnal sleep organization in infants “at risk” for sudden infant death syndrome. Pediatr. Res. 1984, 18, 654–657. [Google Scholar] [CrossRef] [Green Version]
- Coons, M.A.; Guilleminault, C. Development of sleep-wake patterns and non-rapid eye movement sleep stages during the first six months of life in normal infants. Pediatrics 1981, 69, 793–798. [Google Scholar] [CrossRef]
- Vecchierini-Blineau, M.F.; Nogues, B.; Louvet, S.; Desfontaines, O. Maturation of generalized motility, spontaneous during sleep, from birth at term to the age of 6 months. Neurophysiol. Clin. 1994, 24, 141–154. [Google Scholar] [CrossRef]
- Horne, R.S.; Andrew, S.; Mitchell, K.; Sly, D.J.; Cranage, S.M.; Chau, B.; Adamson, T. Apnoea of prematurity and arousal from sleep. Early Hum. Dev. 2001, 61, 119–133. [Google Scholar] [CrossRef]
- Verbeek, M.M.A.; Richardson, H.L.; Parslow, P.M.; Walker, A.M.; Harding, R.; Horne, R.S.C. Arousal and ventilatory responses to mild hypoxia in sleeping preterm infants. J. Sleep Res. 2008, 17, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.Y.; Horne, R.S.; Hauck, F.R. Sudden infant death syndrome. Lancet 2007, 370, 1578–1587. [Google Scholar] [CrossRef] [Green Version]
- Gillioen, B.; Plancoulaine, S.; Montemitro, E.; Flori, S.; Lin, J.-S.; Guyon, A.; Stagnara, C.; Bat-Pitault, F.; Patural, H.; Gustin, M.-P.; et al. Maturation of arousals during day and night in infants with non-smoking and smoking mothers. Early Hum. Dev. 2017, 115, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Patural, H.; Pichot, P.; Franco, P.; Pladys, P.; Beuchee, A.; Montemitro, E.; Porcher-Guinet, V.; Gillioen, B.; Dauphinot, V.; Rapin, S.; et al. Autonomic Nervous System: A Biomarker of Neurodevelopmental Comportment—The AuBE study. J. Clin. Trials 2014, 4, 176–183. [Google Scholar]
- Wechsler, D. The Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III); The Psychological Corporation: San Antonio, TX, USA, 2002. [Google Scholar]
- Hoppenbrouwers, T.; Hodgman, J.E.; Rybine, D.; Fabrikant, G.; Corwin, M.; Crowell, D.; Weese-Mayer, D.E. CHIME Study Group, Sleep architecture in term and preterm infants beyond the neonatal period: The influence of gestational age, steroids, and ventilatory support. Sleep 2005, 28, 1428–1436. [Google Scholar] [CrossRef] [Green Version]
- Spangler, G. The emergence of adrenocortical circadian function in newborns and infants and its relationship to sleep, feeding and maternal adrenocortical activity. Early Hum. Dev. 1991, 25, 197–208. [Google Scholar] [CrossRef]
- Freudigman, K.; Thoman, E.B. Ultradian and diurnal cyclicity in the sleep states of newborn infants during the first two postnatal days. Early Hum. Dev. 1994, 38, 67–80. [Google Scholar] [CrossRef]
- Parmelee, A.H.; Schulz, H.R.; Disbrow, M.A. Sleep patterns of the newborn. J. Pediatr. 1961, 58, 241–250. [Google Scholar] [CrossRef]
- Franco, P.; Seret, N.; Van Hees, J.N.; Scaillet, S.; Vermeulen, F.; Groswasser, J.; Kahn, A. Decreased arousals among healthy infants after short-term sleep deprivation. Pediatrics 2004, 114, 192–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliff, H.S.; Gallardo, K.A. The effect of nicotine on developing brain catecholamine systems. Front. Biosci. 1999, 4, 883–897. [Google Scholar] [CrossRef] [Green Version]
- Franco, P.; Kato, I.; Richardson, H.L.; Yang, J.S.; Montemitro, E.; Horne, R.S. Arousal from sleep mechanisms in infants. Sleep Med. 2010, 11, 603–614. [Google Scholar] [CrossRef]
- Kahn, A.; Groswasser, J.; Sottiaux, M.; Kelmanson, I.; Franco, P.; Rebuffat, E.; Dramaix, M.; Wayenberg, J.L. Prenatal Exposure to Cigarettes in Infants with Obstructive Sleep Apneas. Pediatrics 1994, 93, 778–783. [Google Scholar] [CrossRef]
- Aserinski, E.; Kleitman, N. Regularly occurring periods of eye mobility and concomitant phenomena during sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef] [Green Version]
- Douglas, N.J. Control of ventilation. In Principles and Practice of Sleep Medicine; Kryger, M.H., Roth, T., Dement, W.C., Eds.; WB Saunders: Philadelphia, PA, USA, 1994; pp. 204–211. [Google Scholar]
- Glotzbach, S.T.; Heller, H.C. Temperature Regulation. In Principles and Practice of Sleep Medicine; Kryger, M.H., Roth, T., Dement, W.C., Eds.; WB Saunders: Philadelphia, PA, USA, 1994; pp. 260–275. [Google Scholar]
- Parrino, L.; Ferri, R.; Bruni, O.; Terzano, M.G. Cyclic alternating pattern (CAP): The marker of sleep instability. Sleep Med. Rev. 2012, 16, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Miano, S.; Castaldo, R.; Ferri, R.; Peraita-Adrados, R.; Paolino, M.C.; Montesano, M.; Villa, M.P. Sleep cyclic alternating pattern analysis in infants with apparent life-threatening events: A daytime polysomnographic study. Clin. Neurophysiol. 2012, 123, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Montemitro, E.; Scaillet, S.; Groswasser, J.; Kato, I.; Lin, J.S.; Villa, M.P. Fewer spontaneous arousals in infants with apparent life-threatening event. Sleep 2011, 34, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Richardson, H.L.; Walker, A.M.; Horne, R.S.C. Sleep position alters arousal processes maximally at the high-risk age for sudden infant death syndrome. J. Sleep Res. 2008, 17, 450–457. [Google Scholar] [CrossRef]
- Kato, I.; Scaillet, S.; Groswasser, J.; Montemitro, E.; Togari, H.; Lin, J.-S.; Kahn, A.; Franco, P. Spontaneous Arousability in Prone and Supine Position in Healthy Infants. Sleep 2006, 29, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilleminault, C.; Huang, Y.-S. From oral facial dysfunction to dysmorphism and the onset of pediatric OSA. Sleep Med. Rev. 2017, 40, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Patural, H.; Barthelemy, J.C.; Pichot, V.; Mazzocchi, C.; Teyssier, G.; Damon, G.; Roche, F. Birth prematurity determines prolonged autonomic nervous system immaturity. Clin. Auton. Res. 2004, 14, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.S.; Witcombe, N.B.; Yiallourou, S.R.; Scaillet, S.; Thiriez, G.; Franco, P. Cardiovascular control during sleep in infants: Implications for Sudden Infant Death Syndrome. Sleep Med. 2010, 11, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Thiriez, G.; Tournoud, M.; Wermenbol, V.; Vermeylen, D.; Ecochard, R.; Iwaz, J.; Lin, J.S.; Franco, P. Decreased spontaneous arousability in preterm newborns with impaired neurological outcome. J. Sleep Res. 2012, 21, 552–560. [Google Scholar] [CrossRef] [PubMed]

| Preterm Infants | Term Infants | p | |
|---|---|---|---|
| N | 12 | 21 | |
| Male sex, n (%) | 8 (67%) | 7 (33%) | 0.08 |
| Gestational age, weeks, mean ± SD | 35.1 ± 2.1 | 39.8 ± 0.8 | <10−4 |
| Corrected age at M0, weeks, mean ± SD | 37.2 ± 1.6 | 40.1 ± 1.2 | <10−4 |
| Corrected age at M6, weeks, mean ± SD | 66.4 ± 7.0 | 70.6 ± 12.0 | <10−4 |
| Birth weight, g, mean ± SD | 2033 ± 598 | 3319 ± 308 | <10−4 |
| Immaturity, n (%) | 3 (25%) | 0 (0%) | 0.04 |
| Smoking during pregnancy, n (%) | 3 (25%) | 0 (0%) | 0.04 |
| Maternal age during pregnancy, years, mean ± SD | 32.1 ± 5.3 | 32.4 ± 4.0 | 0.81 |
| Verbal IQ at 3 years, mean ± SD | 109 ± 17 | 103 ± 17 | 0.19 |
| Performance IQ at 3 years, mean ± SD | 87 ± 13 | 86 ± 11 | 0.60 |
| Total IQ at 3 years, mean ± SD | 98 ± 15 | 93 ± 14 | 0.20 |
| NIGHT SLEEP | ||||||||
| At M0 | At M6 | |||||||
| mean ± SD | All | Preterm | Term | p value | All | Preterm | Term | p value |
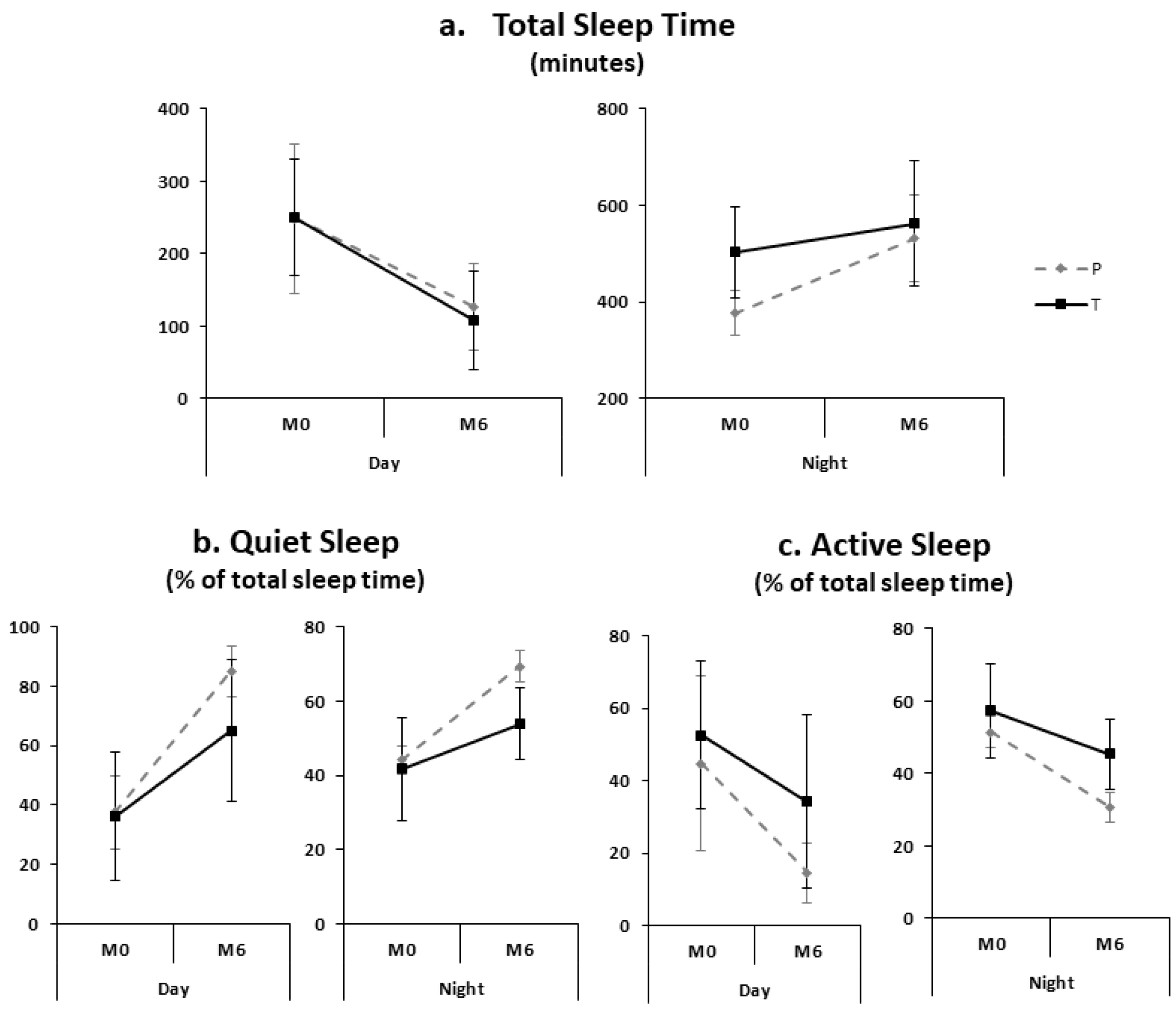
| TR (min) | 728 ± 94 | 710 ± 20 | 738 ± 116 | 0.07 | 692 ± 124 | 720 ± 1.2 | 676 ± 155 | 0.82 |
| TST (min) | 457 ± 101 | 377 ± 47 | 503 ± 95.4 | 0.0005 | 552 ± 118 | 532 ± 90 | 563 ± 131 | 0.12 |
| Sleep efficiency (%) | 64.4 ± 12.3 | 53.2 ± 6.8 | 70.8 ± 9.9 | <10−4 | 82.9 ± 11.5 | 74.0 ± 12.6 | 88.1 ± 6.9 | 0.002 |
| Quiet sleep (% of TST) | 42.6 ± 11.2 | 44.1 ± 3.8 | 41.7 ± 13.8 | 0.50 | 59.5 ± 11.1 | 69.3 ± 4.2 | 53.9 ± 9.8 | <10−4 |
| Active sleep (% of TST) | 55.2 ± 10.8 | 51.5 ± 4.4 | 57.3 ± 12.8 | 0.38 | 10.0 ± 10.7 | 30.7 ± 4.2 | 45.4 ± 9.6 | <10−4 |
| Arousal index (/h of TST) | 12.3 ± 7.2 | 8.6 ± 7.9 | 14.5 ± 5.9 | 0.005 | 13.7 ± 6.7 | 10.1 ± 4.0 | 15.8 ± 7.4 | 0.02 |
| Arousal in quiet sleep (/h of TST) | 12.8 ± 18.9 | 24.3 ± 25.3 | 6.3 ± 9.8 | 0.0005 | 10.3 ± 8.3 | 18.8 ± 7.3 | 5.5 ± 3.4 | <10−4 |
| Arousal in active sleep (/h of TST) | 18.3 ± 9.6 | 11.0 ± 10.1 | 22.4 ± 6.6 | 0.002 | 24.3 ± 11.5 | 18.6 ± 10.7 | 27.5 ± 10.9 | 0.03 |
| DAYTIME SLEEP | ||||||||
| At M0 | At M6 | |||||||
| mean ± SD | All | Preterm | Term | p value | All | Preterm | Term | p value |
| TR (min) | 483 ± 140 | 500 ± 174 | 474 ± 120 | 0.39 | 419 ± 178 | 444 ± 176 | 407 ± 182 | 0.37 |
| TST (min) | 249 ± 88 | 249 ± 103 | 250 ± 81 | 0.75 | 115 ± 65 | 127 ± 60 | 109 ± 68 | 0.36 |
| Sleep efficiency (%) | 53.3 ± 17.9 | 50.4 ± 10.0 | 54.9 ± 21.2 | 0.29 | 28.5 ± 14.1 | 29.3 ± 13.5 | 28.1 ± 14.7 | 0.93 |
| Quiet sleep (% of TST) | 36.6 ± 18.6 | 37.6 ± 12.4 | 36.0 ± 21.6 | 0.45 | 71.6 ± 22.1 | 85.1 ± 8.5 | 65.1 ± 23.8 | 0.02 |
| Active sleep (% of TST) | 49.8 ± 21.7 | 44.8 ± 24.0 | 52.6 ± 20.3 | 0.19 | 27.9 ±22.1 | 14.6 ± 8.3 | 34.2 ± 23.9 | 0.02 |
| Arousal index (/h of TST) | 14.6 ± 8.8 | 8.5 ± 8.6 | 18.0 ± 7.1 | 0.0004 | 11.0 ± 7.9 | 5.1 ± 4.9 | 13.8 ± 7.6 | 0.004 |
| Arousal in quiet sleep (/h of TST) | 13.4 ± 17.3 | 21.8 ± 22.5 | 8.5 ± 11.4 | 0.007 | 7.7 ± 9.5 | 6.2 ± 6.3 | 8.5 ± 10.8 | 0.51 |
| Arousal in active sleep (/h of TST) | 21.8 ± 12.0 | 10.1 ± 9.9 | 28.5 ± 6.9 | 0.0002 | 18.3 ± 15.0 | 11.5 ± 10.6 | 21.6 ± 16.0 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guyon, A.; Ravet, F.; Champavert, A.; Thieux, M.; Patural, H.; Plancoulaine, S.; Franco, P. Maturation of Arousals during Day and Night in Preterm Infants. Children 2022, 9, 223. https://doi.org/10.3390/children9020223
Guyon A, Ravet F, Champavert A, Thieux M, Patural H, Plancoulaine S, Franco P. Maturation of Arousals during Day and Night in Preterm Infants. Children. 2022; 9(2):223. https://doi.org/10.3390/children9020223
Chicago/Turabian StyleGuyon, Aurore, Francoise Ravet, Alex Champavert, Marine Thieux, Hugues Patural, Sabine Plancoulaine, and Patricia Franco. 2022. "Maturation of Arousals during Day and Night in Preterm Infants" Children 9, no. 2: 223. https://doi.org/10.3390/children9020223
APA StyleGuyon, A., Ravet, F., Champavert, A., Thieux, M., Patural, H., Plancoulaine, S., & Franco, P. (2022). Maturation of Arousals during Day and Night in Preterm Infants. Children, 9(2), 223. https://doi.org/10.3390/children9020223





