A Survey to Understand Parent/Caregiver and Children’s Views on Devices Used for the Administration of Oral Pediatric Medicines in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Study Population
2.1.2. Composition of the Questionnaire
2.2. Research Setting and Recruitment
2.3. Analysis of the Data
2.4. Ethics Approval
3. Results
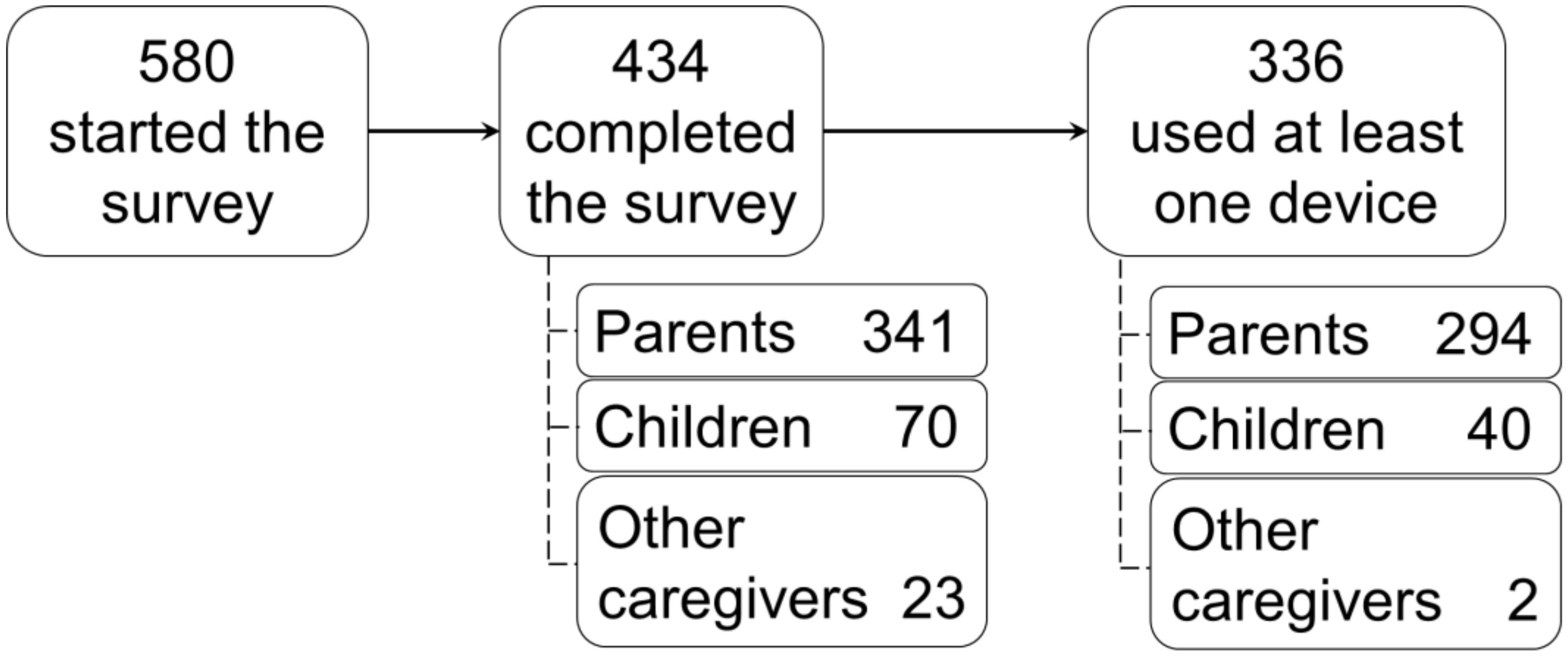
3.1. Participants
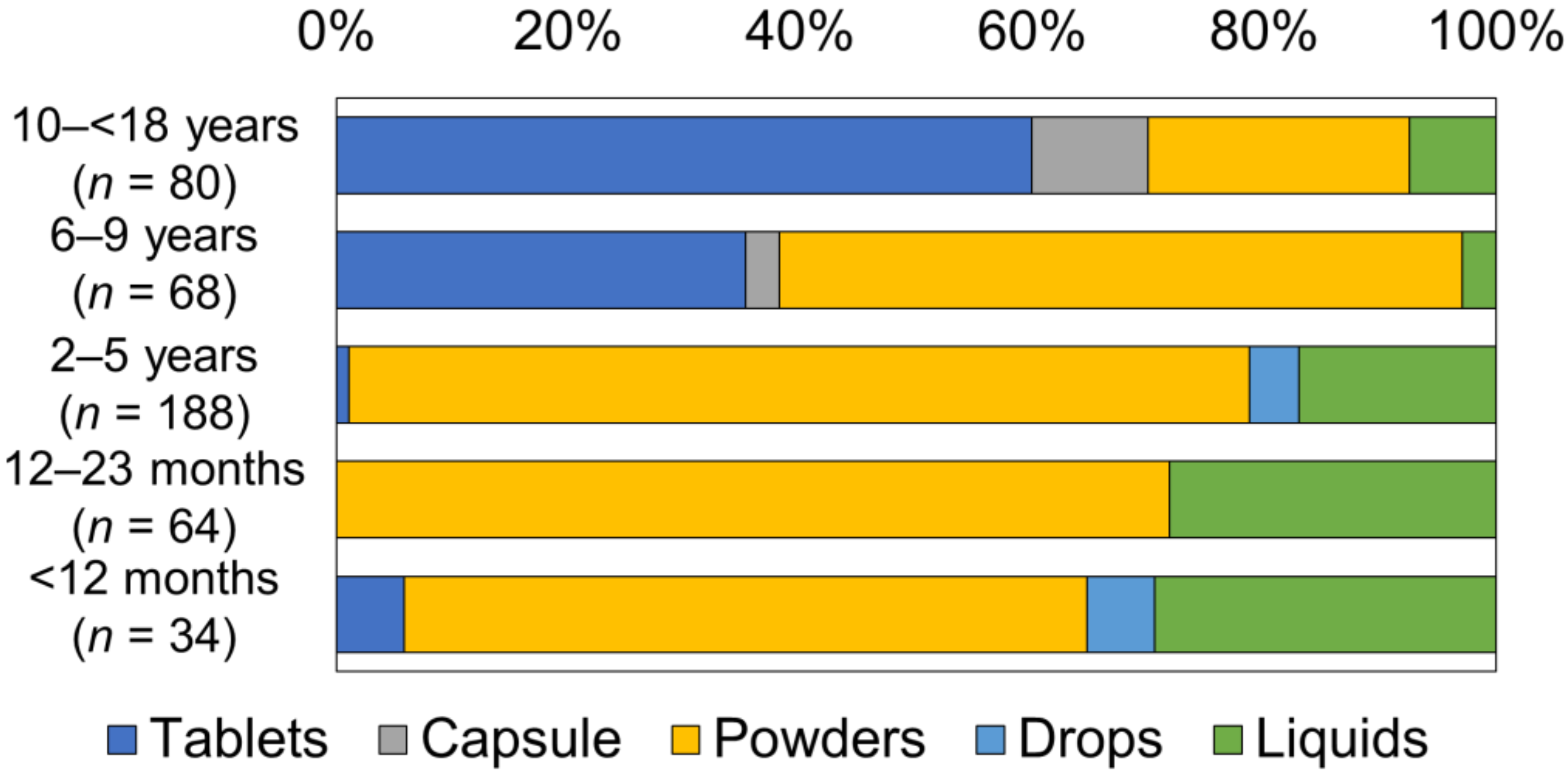
3.2. Dosage Form of Oral Medicine the Child Had Taken
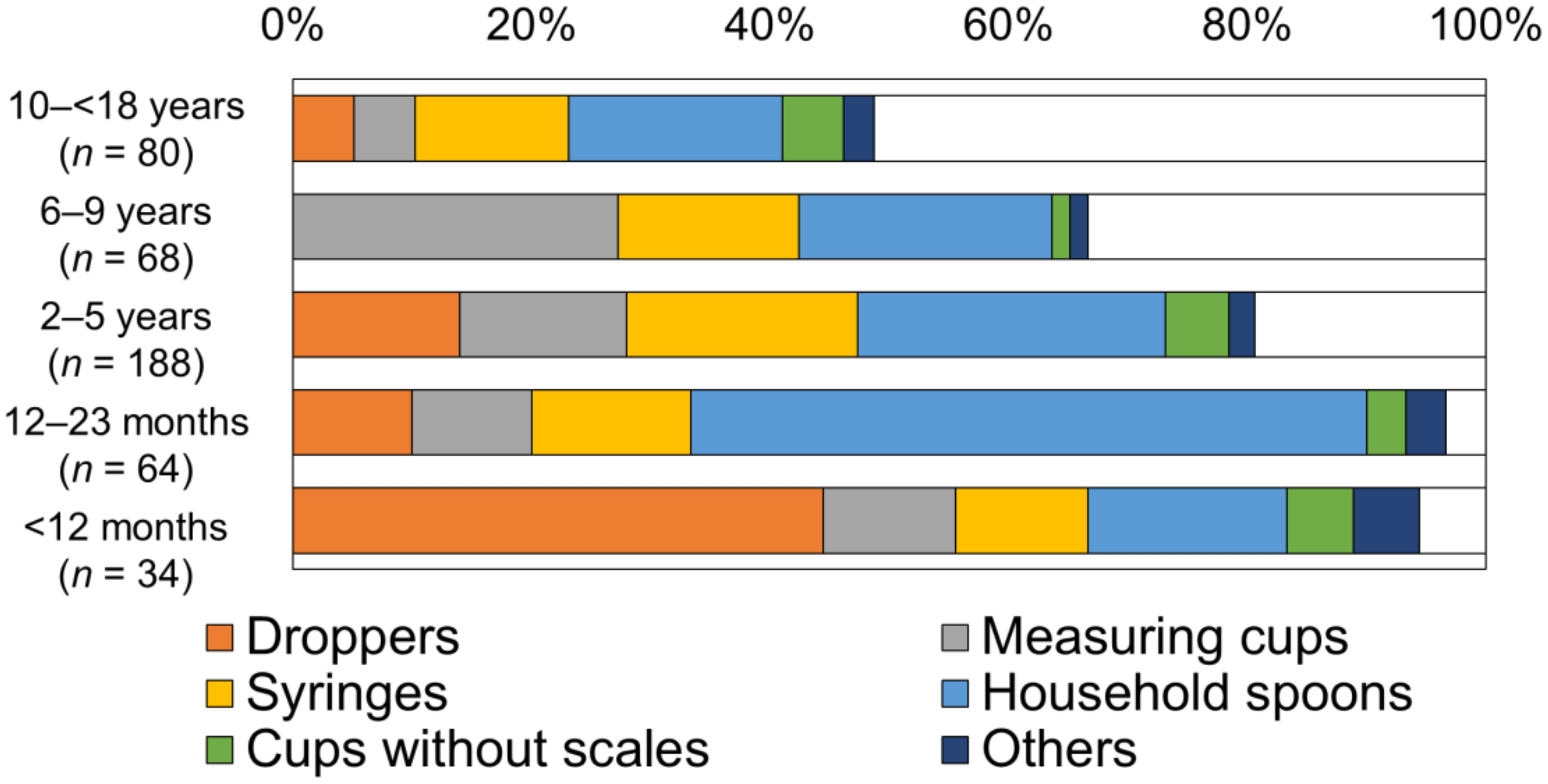
3.3. Type of Device for Medicine Administration
3.4. Duration of Device Use
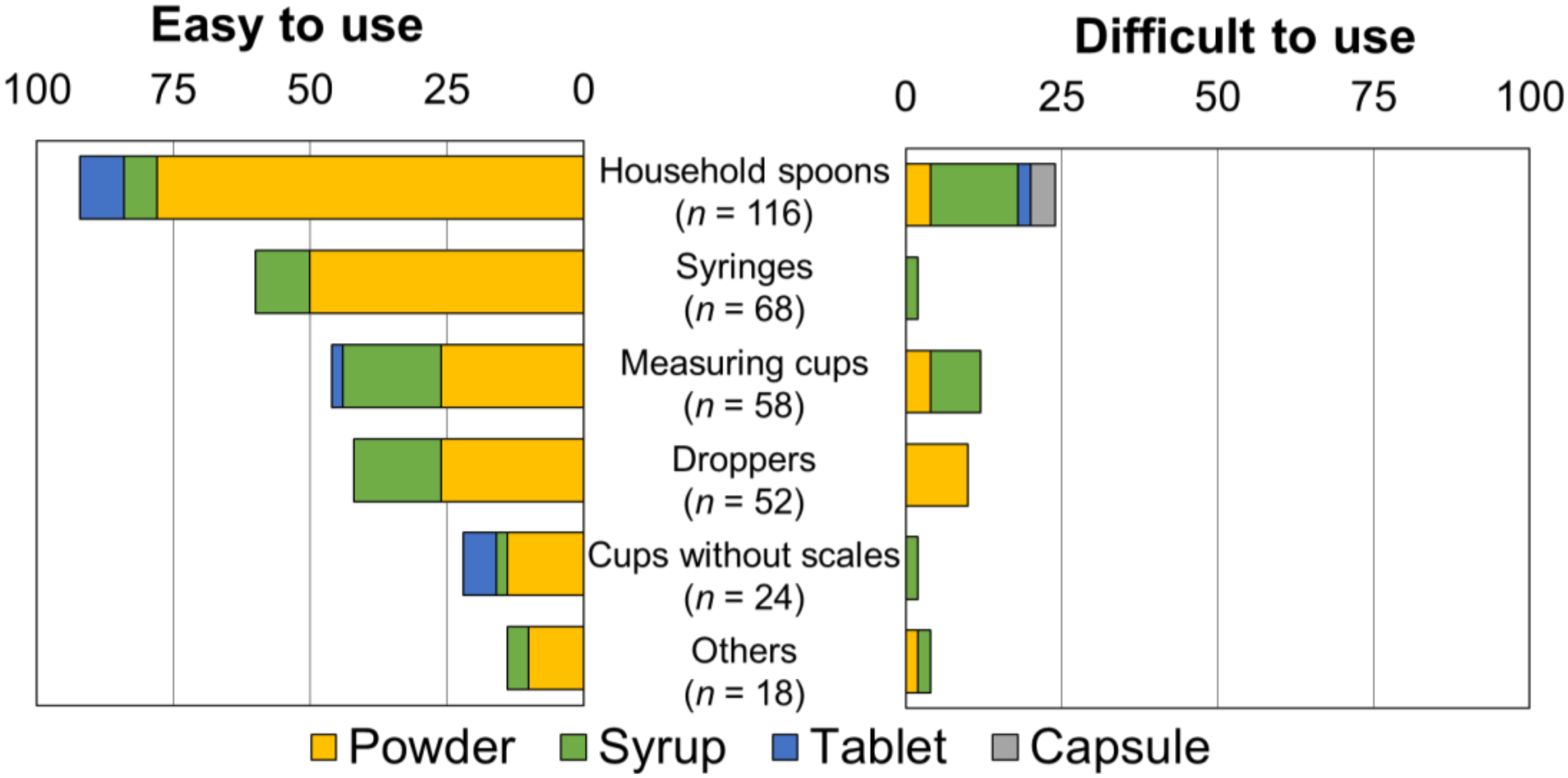
3.5. User-Friendliness of the Selected Device
3.6. Clarity of the Instruction for Devices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beckett, V.L.; Tyson, L.D.; Carroll, D.; Gooding, N.M.; Kelsall, A.W. Accurately administering oral medication to children isn’t child’s play. Arch. Dis. Child. 2012, 97, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Mendelsohn, A.L.; Wolf, M.S.; Parker, R.M.; Fierman, A.; van Schaick, L.; Bazan, I.S.; Kline, M.D.; Dreyer, B.P. Parents’ medication administration errors: Role of dosing instruments and health literacy. Arch. Pediatr. Adolesc. Med. 2010, 164, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Pineda, A.; Gonzalez de Dios, J.; Guilabert Mora, M.; Mira-Perceval Juan, G.; Mira Solves, J.J. A systematic review on pediatric medication errors by parents or caregivers at home. Expert Opin. Drug Saf. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Bickmann, D.; Breitkreutz, J.; Chariot-Goulet, M.; European Paediatric Formulation Initiative (EuPFI). Delivery devices for the administration of paediatric formulations: Overview of current practice, challenges and recent developments. Int. J. Pharm. 2011, 415, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Wachtel, H. Regulatory aspects of devices. Int. J. Pharm. 2012, 435, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Arenas-López, S.; Gurung, K.; Tibby, S.M.; Calleja Hernández, M.Á.; Tuleu, C. Accuracy of enteral syringes with commonly prescribed paediatric liquid medicines. Arch. Dis. Child. 2017, 102, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, J. Novel approaches to the route of administration. In Paediatric Clinical Pharmacology, 1st ed.; Jacqz-Aigrain, E., Choonara, I., Eds.; CRC Press: London, UK, 2021; pp. 235–243. [Google Scholar]
- Grießmann, K.; Brietkreutz, J.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Dosing accuracy of measuring devices provided with antibiotic oral suspensions. Paediatr. Perinat. Drug Ther. 2007, 8, 61–70. [Google Scholar] [CrossRef]
- Sobhani, P.; Christopherson, J.; Ambrose, P.J.; Corelli, R.L. Accuracy of oral liquid measuring devices: Comparison of dosing cup and oral dosing syringe. Ann. Pharmacother. 2008, 42, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Math, M.C.; Breitkreutz, J.; Zerback, T.; Wachtel, H.; European Paediatric Formulation Initiative (EuPFI). Devices for oral and respiratory paediatric medicines: What do healthcare professionals think? Int. J. Pharm. 2015, 492, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Chitlangia, A.; Kaur, N.; Pradhan, V.; Salunke, S. Voice Your Views—A workshop on Use of Traditional and Innovative Devices for Administration of Liquid Oral Paediatric Medicines. In Proceedings of the 13th EuPFI Conference, Virtual, 22–23 September 2021. [Google Scholar]
- Abidi, S.; Talegaonkar, S.; Pokharkar, V.; Sajith, M.; Pradhan, V.; Walsh, J.; Salunke, S. Use and Acceptability of administration devices for oral and respiratory medicine—A pan India survey among parents and children. In Proceedings of the 13th EuPFI Conference, Virtual, 22–23 September 2021. [Google Scholar]
- Alessandrini, E.; Walsh, J.; Hermans, E.; Salunke, S. Usability of administration devices for oral and respiratory paediatric medicines: Opinions of caregivers and children. In Proceedings of the 13th EuPFI Conference, Virtual, 22–23 September 2021. [Google Scholar]
- Marascuilo, L.A. Large-sample multiple comparisons. Psychol. Bull. 1996, 65, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ishikawa, Y. How do Japanese children take their medicines, and what are pharmacists and paediatricians doing about it? Int. J. Pharm. 2014, 469, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, E.; Brako, F.; Scarpa, M.; Lupo, M.; Bonifazi, D.; Pignataro, V.; Cavallo, M.; Cullufe, O.; Enache, C.; Nafria, B.; et al. Children’s Preferences for Oral Dosage Forms and Their Involvement in Formulation Research via EPTRI (European Paediatric Translational Research Infrastructure). Pharmaceutics 2021, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Chitlangia, A.; Pradhan, V.; Salunke, S. Use of traditional and innovative oral devices in domestic and hospital settings in India—A systematic review. In Proceedings of the 13th EuPFI Conference, Virtual, 22–23 September 2021. [Google Scholar]
- Falagas, M.E.; Vouloumanou, E.K.; Plessa, E.; Peppas, G.; Rafailidis, P.I. Inaccuracies in dosing drugs with teaspoons and tablespoons. Int. J. Clin. Pract. 2010, 64, 1185–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Duration | N (%) | Daily Frequencies | N (%) | ||
|---|---|---|---|---|---|
| >7 days | 35 | (10.4) | Once-daily | 86 | (25.6) |
| 1 to 2 weeks | 32 | (9.5) | Twice daily | 183 | (54.5) |
| 3 to 4 weeks | 18 | (5.4) | Three times a day | 50 | (14.9) |
| 1 to 11 months | 46 | (13.7) | Four times a day | 7 | (2.1) |
| >1 years | 197 | (58.6) | Others | 10 | (3.0) |
| Unknown | 8 | (2.4) | |||
| Total | 336 | Total | 336 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, J.; Nakamura, H.; Walsh, J.; Yamatani, A.; Salunke, S. A Survey to Understand Parent/Caregiver and Children’s Views on Devices Used for the Administration of Oral Pediatric Medicines in Japan. Children 2022, 9, 196. https://doi.org/10.3390/children9020196
Saito J, Nakamura H, Walsh J, Yamatani A, Salunke S. A Survey to Understand Parent/Caregiver and Children’s Views on Devices Used for the Administration of Oral Pediatric Medicines in Japan. Children. 2022; 9(2):196. https://doi.org/10.3390/children9020196
Chicago/Turabian StyleSaito, Jumpei, Hidefumi Nakamura, Jennifer Walsh, Akimasa Yamatani, and Smita Salunke. 2022. "A Survey to Understand Parent/Caregiver and Children’s Views on Devices Used for the Administration of Oral Pediatric Medicines in Japan" Children 9, no. 2: 196. https://doi.org/10.3390/children9020196
APA StyleSaito, J., Nakamura, H., Walsh, J., Yamatani, A., & Salunke, S. (2022). A Survey to Understand Parent/Caregiver and Children’s Views on Devices Used for the Administration of Oral Pediatric Medicines in Japan. Children, 9(2), 196. https://doi.org/10.3390/children9020196






