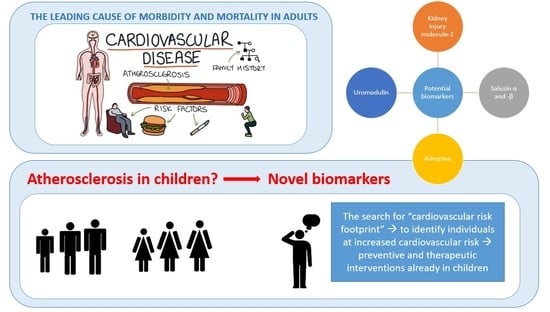
Current Knowledge of Selected Cardiovascular Biomarkers in Pediatrics: Kidney Injury Molecule-1, Salusin-α and -β, Uromodulin, and Adropin
Abstract
1. Introduction
2. Kidney Injury Molecule 1
2.1. What Is It?
2.2. How Is It Involved in Cardiovascular Risk?
2.3. What Is Its Role in Cardiovascular Risk in Pediatric Patients?
3. Salusin-α and -β
3.1. What Are They?
3.2. How Are They Involved in Cardiovascular Risk?
3.3. What Are Their Roles in Cardiovascular Risk in Pediatric Patients?
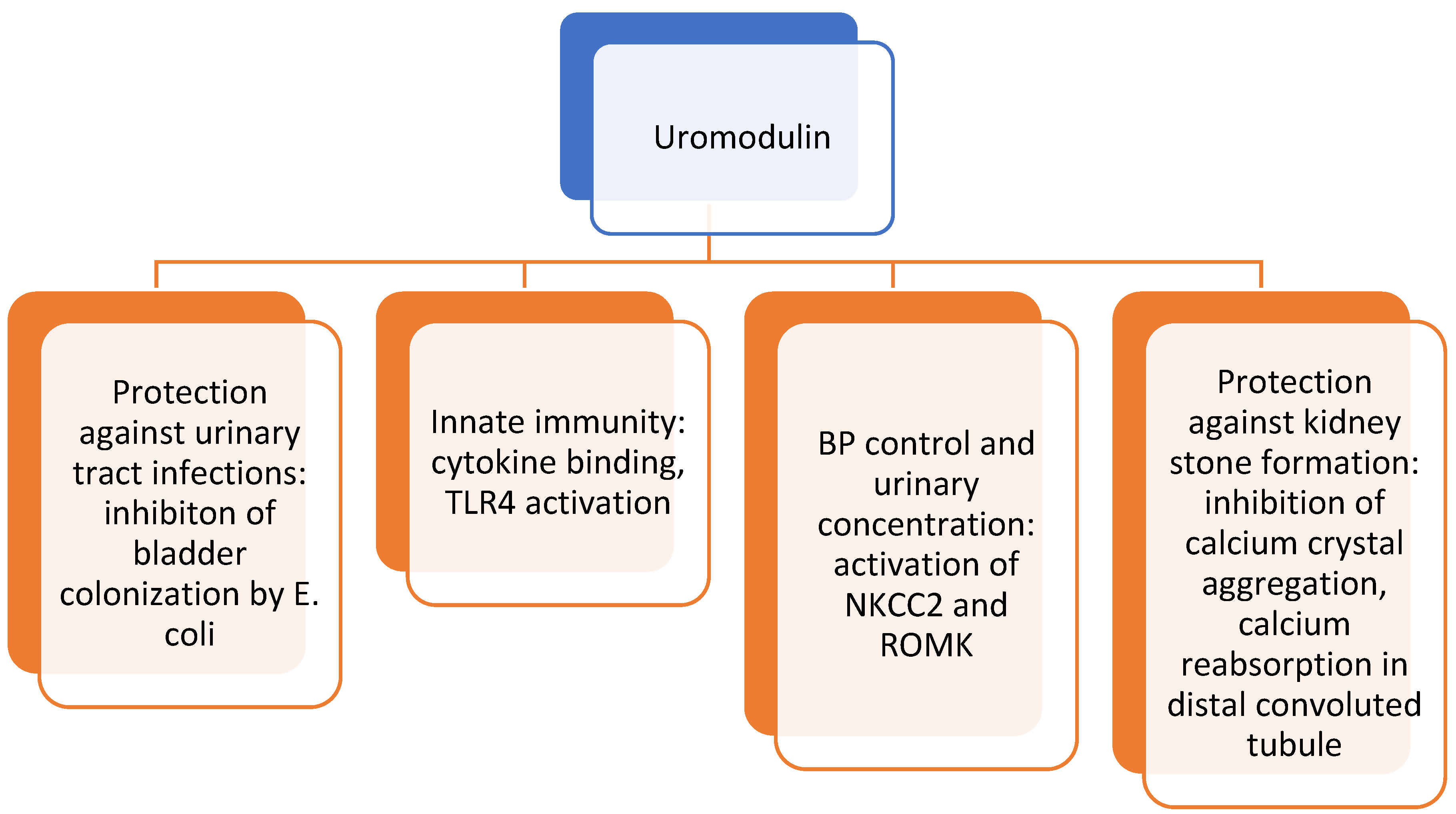
4. Uromodulin
4.1. What Is It?
4.2. How Is It Involved in Cardiovascular Risk?
4.3. What Is Its Role in Cardiovascular Risk in Pediatric Patients?
5. Adropin
5.1. What Is It?
5.2. How Is It Involved in Cardiovascular Risk?
5.3. What Is Its Role in Cardiovascular Risk in Pediatric Patients?
6. Other Investigated Potential Cardiovascular Biomarkers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability Statement
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Piko, N.; Ekart, R.; Bevc, S.; Hojs, R. Atherosclerosis, epigenetic modifications and arterial stiffness. Acta Med.-Biotech. 2017, 10, 10–17. [Google Scholar]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Santi, M.; Lava, S.A.; Simonetti, G.D.; Stettbacher, A.; Bianchetti, M.G.; Muggli, F. Clustering of cardiovascular disease risk factors among male youths in Southern Switzerland: Preliminary study. Swiss Med. Wkly. 2016, 146, w14338. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Czober, T.; Lepich, T.; Sierka, O.; Bajor, G. Selected biomarkers of atherosclerosis—Clinical aspects. Acta Angiol. 2020, 26, 28–39. [Google Scholar] [CrossRef]
- De Ferranti, S.D.; Steinberger, J.; Ameduri, R.; Baker, A.; Gooding, H.; Kelly, A.S.; Mietus-Snyder, M.; Mitsnefes, M.M.; Peterson, A.L.; St-Pierre, J.; et al. Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association. Circulation. Circulation. 2019, 139, e603–e634. [Google Scholar] [CrossRef]
- Danhof, M.; Alvan, G.; Dahl, S.G.; Kuhlmann, J.; Paintaud, G. Mechanism-based pharmacokinetic-pharmacodynamic modeling—A new classification of biomarkers. Pharm. Res. 2005, 22, 1432–1437. [Google Scholar] [CrossRef]
- Song, J.; Yu, J.; Prayogo, G.W.; Cao, W.; Wu, Y.; Jia, Z.; Zhang, A. Understanding kidney injury molecule 1: A novel immune factor in kidney pathophysiology. Am. J. Transl. Res. 2019, 11, 1219–1229. [Google Scholar]
- Yin, C.; Wang, N. Kidney injury molecule-1 in kidney disease. Ren Fail. 2016, 38, 1567–1573. [Google Scholar] [CrossRef]
- Waikar, S.S.; Sabbisetti, V.; Arnlov, J.; Carlsson, A.C.; Coresh, J.; Feldman, H.I.; Foster, M.C.; Fufaa, G.D.; Helmersson-Karlqvist, J.; Hsu, C.Y.; et al. Chronic Kidney Disease Biomarkers Consortium Investigators. Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule-1 in five cohort studies. Nephrol. Dial. Transplant. 2016, 31, 1460–1470. [Google Scholar] [CrossRef]
- Carter, J.L.; Parker, C.T.; Stevens, P.E.; Eaglestone, G.; Knight, S.; Farmer, C.K.; Lamb, E.J. Biological variation of plasma and urinary markers of acute kidney injury in patients with CKD. Clin. Chem. 2016, 62, 876–883. [Google Scholar] [CrossRef]
- Castillo-Rodriguez, E.; Fernandez-Prado, R.; Martin-Cleary, C.; Pizarro-Sanchez, M.S.; Sanchez-Nino, M.D.; Sanz, A.B.; Fernandez-Fernandez, B.; Ortiz, A. Kidney injury marker 1 and neutrophil gelatinase-associated lipocalin in chronic kidney disease. Nephron 2017, 136, 263–267. [Google Scholar] [CrossRef]
- Alderson, H.V.; Ritchie, J.P.; Pagano, S.; Middleton, R.J.; Pruijm, M.; Vuilleumier, N.; Kalra, P.A. The associations of blood kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin with progression from CKD to ESRD. Clin. J. Am. Soc. Nephrol. 2016, 11, 2141–2149. [Google Scholar] [CrossRef]
- Kwon, S.H.; Park, M.Y.; Jeon, J.S.; Noh, H.; Choi, S.J.; Kim, J.K.; Hwang, S.D.; Jin, S.Y.; Han, D.C. KIM-1 expression predicts renal outcomes in IgA nephropathy. Clin. Exp. Nephrol. 2013, 17, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, M.M.; Bakker, S.J.; Vaidya, V.S.; Bailly, V.; Schuurs, T.A.; Damman, J.; Stegeman, C.A.; Bonventre, J.V.; van Goor, H. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am. J. Physiol. Renal Physiol. 2006, 291, F456–F464. [Google Scholar] [CrossRef] [PubMed]
- Medić, B.; Rovčanin, B.; Basta Jovanović, G.; Radojević-Škodrić, S.; Prostran, M. Kidney injury molecule-1 and cardiovascular diseases: From basic science to clinical practice. Biomed. Res. Int. 2015, 2015, 854070. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R. New biomarkers of acute kidney injury and the cardio-renal syndrome. Korean J. Lab. Med. 2011, 31, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ghatanatti, R.; Teli, A.; Tirkey, S.S.; Bhattacharya, S.; Sengupta, G.; Mondal, A. Role of renal biomarkers as predictors of acute kidney injury in cardiac surgery. Asian Cardiovasc. Thorac. Ann. 2014, 22, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, C.G.; Birner, C.; Jung, B.; Buchner, S.; Lubnow, M.; von Bary, C.; Endemann, D.; Banas, B.; Mack, M.; Böger, C.A.; et al. Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur. J. Heart Fail. 2011, 13, 1104–1110. [Google Scholar] [CrossRef]
- Hosohata, K. Biomarkers for chronic kidney disease associated with high salt intake. Int. J. Mol. Sci. 2017, 18, 2080. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.C.; Larsson, A.; Helmersson-Karlqvist, J.; Lind, L.; Ingelsson, E.; Larsson, T.E.; Bottai, M.; Sundström, J.; Ärnlöv, J. Urinary kidney injury molecule-1 and the risk of cardiovascular mortality in elderly men. Clin. J. Am. Soc. Nephrol. 2014, 9, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Nowak, C.; Carlsson, A.C.; Östgren, C.J.; Nyström, F.H.; Alam, M.; Feldreich, T.; Sundström, J.; Carrero, J.J.; Leppert, J.; Hedberg, P.; et al. Multiplex proteomics for prediction of major cardiovascular events in type 2 diabetes. Diabetologia. 2018, 6, 1748–1757. [Google Scholar] [CrossRef]
- Fazel, M.; Sarveazad, A.; Mohamed Ali, K.; Yousefifard, M.; Hosseini, M. Accuracy of urine kidney injury molecule-1 in predicting acute kidney injury in children; a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 2020, 8, e44. [Google Scholar]
- Carvalho Pedrosa, D.; Macedo de Oliveira Neves, F.; Cavalcante Meneses, G.; Pinheiro Gomes Wirtzbiki, G.; da Costa Moraes, C.A.; Costa Martins, A.M.; Braga Libório, A. Urinary KIM-1 in children undergoing nephrotoxic antineoplastic treatment: A prospective cohort study. Pediatr. Nephrol. 2015, 30, 2207–2213. [Google Scholar] [CrossRef]
- Greenberg, J.H.; Abraham, A.G.; Xu, Y.; Schelling, J.R.; Feldman, H.I.; Sabbisetti, V.S.; Gonzalez, M.C.; Coca, S.; Schrauben, S.J.; Waikar, S.S.; et al. CKD Biomarkers Consortium. Plasma biomarkers of tubular injury and inflammation are associated with CKD progression in children. J. Am. Soc. Nephrol. 2020, 31, 1067–1077. [Google Scholar] [CrossRef]
- Ucakturk, A.; Avci, B.; Genc, G.; Ozkaya, O.; Aydin, M. Kidney injury molecule-1 and neutrophil gelatinase associated lipocalin in normoalbuminuric diabetic children. J. Pediatr. Endocrinol. Metab. 2016, 29, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Ziypak, T.; Orsal, E.; Laloglu, E.; Bedir, F.; Aksoy, Y. Is urinary kidney injury molecule-1 a noninvasive marker for renal scarring in children with vesicoureteral reflux? Urology 2013, 81, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Goknar, N.; Oktem, F.; Ozgen, I.T.; Torun, E.; Kuçukkoc, M.; Demir, A.D.; Cesur, Y. Determination of early urinary renal injury markers in obese children. Pediatr. Nephrol. 2015, 30, 139–144. [Google Scholar] [CrossRef]
- Polidori, N.; Giannini, C.; Salvatore, R.; Pelliccia, P.; Parisi, A.; Chiarelli, F.; Mohn, A. Role of urinary NGAL and KIM-1 as biomarkers of early kidney injury in obese prepubertal children. J. Pediatr. Endocrinol. Metab. 2020, 33, 1183–1189. [Google Scholar] [CrossRef]
- Gul, A.; Yilmaz, R.; Ozmen, Z.C.; Gumuser, R.; Demir, O.; Unsal, V. Assessment of renal function in obese and overweight children with NGAL and KIM-1 biomarkers. Nutr. Hosp. 2020, 34, 436–442. [Google Scholar] [PubMed]
- Sato, K.; Watanabe, R.; Itoh, F.; Shichiri, M.; Watanabe, T. Salusins: Potential use as a biomarker for atherosclerotic cardiovascular diseases. Int. J. Hypertens. 2013, 2013, 965140. [Google Scholar] [CrossRef]
- Niepolski, L.; Grzegorzewska, A.E. Salusins and adropin: New peptides potentially involved in lipid metabolism and atherosclerosis. Adv. Med. Sci. 2016, 61, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nishio, K.; Kanome, T.; Matsuyama, T.A.; Koba, S.; Sakai, T.; Sato, K.; Hongo, S.; Nose, K.; Ota, H.; et al. Impact of salusin-alpha and -beta on human macrophage foam cell formation and coronary atherosclerosis. Circulation. 2008, 117, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sato, K.; Itoh, F.; Iso, Y.; Nagashima, M.; Hirano, T.; Shichiri, M. The roles of salusins in atherosclerosis and related cardiovascular diseases. J. Am. Soc. Hypertens. 2011, 5, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Izumiyama, H.; Tanaka, H.; Egi, K.; Sunamori, M.; Hirata, Y.; Shichiri, M. Synthetic salusins as cardiac depressors in rat. Hypertension. 2005, 45, 419–425. [Google Scholar] [CrossRef]
- Citil, C.; Konar, V.; Aydin, S.; Yilmaz, M.; Albayrak, S.; Ozercan, I.H.; Ozkan, Y. Brain, liver, and serum salusin-alpha and -beta alterations in Sprague-Dawley rats with or without metabolic syndrome. Med. Sci. Monit. 2014, 20, 1326–1333. [Google Scholar]
- Watanabe, T.; Suguro, T.; Sato, K.; Koyama, T.; Nagashima, M.; Kodate, S.; Hirano, T.; Adachi, M.; Shichiri, M.; Miyazaki, A. Serum salusin-alpha levels are decreased and correlated negatively with carotid atherosclerosis in essential hypertensive patients. Hypertens. Res. 2008, 31, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Ti, Y.; Wang, F.; Wang, Z.H.; Wang, X.L.; Zhang, W.; Zhang, Y.; Bu, P.L. Associations of serum salusin-alpha levels with atherosclerosis and left ventricular diastolic dysfunction in essential hypertension. J. Hum. Hypertens. 2012, 26, 603–609. [Google Scholar] [CrossRef]
- Alpsoy, S.; Dogan, B.; Ozkaramanli Gur, D.; Akyüz, A.; Fidan, Ç.; Guzel, S.; Ozkoyuncu, B. Assessment of salusin alpha and salusin beta levels in patients with newly diagnosed dipper and non-dipper hypertension. Clin. Exp. Hypertens. 2021, 43, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Zhang, J.; Zhang, M.; Zhang, H.; Gong, G.; Luo, M.; Wang, T.; Mao, X. Salusin-β is superior to salusin-α as a marker for evaluating coronary atherosclerosis. J. Int. Med. Res. 2020, 48, 300060520903868. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, Y.G.; Zhang, L.H.; Tong, Y.W.; Kang, L. Serum salusin-β levels are associated with the presence and severity of coronary artery disease. J. Investig. Med. 2015, 63, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Minasian, V.; Hovsepian, S. Effects of two types of moderate- and high-intensity interval training on serum salusin-α and salusin-β levels and lipid profile in women with overweight/obesity. Diabetes Metab. Syndr. Obes. 2020, 13, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Argun, D.; Argun, F.; Borku Uysal, B. Evaluation of salusin-α and salusin-β levels in patients with type 2 diabetes mellitus and determination of the impact of severity of hyperglycemia on salusin levels. Ir. J. Med. Sci. 2021, 190, 1403–1411. [Google Scholar] [CrossRef]
- Kołakowska, U.; Kuroczycka-Saniutycz, E.; Wasilewska, A.; Olański, W. Is the serum level of salusin-β associated with hypertension and atherosclerosis in the pediatric population? Pediatr. Nephrol. 2015, 30, 523–531. [Google Scholar] [CrossRef][Green Version]
- Kołakowska, U.; Kuroczycka-Saniutycz, E.; Olański, W.; Wasilewska, A. Correlation of salusin beta with hs-CRP and ADMA in hypertensive children and adolescents. Curr. Pharm. Des. 2018, 24, 3551–3557. [Google Scholar] [CrossRef]
- Dervişoğlu, P.; Elmas, B.; Kösecik, M.; İşgüven, Ş.P.; Büyükavcı, M.; Köroğlu, M. Salusin-α levels are negatively correlated with diastolic blood pressure in children with obesity. Cardiol. Young. 2019, 29, 1225–1229. [Google Scholar] [CrossRef]
- Paahoo, A.; Tadibi, V.; Behpoor, N. Effectiveness of continuous aerobic versus high-intensity interval training on atherosclerotic and inflammatory markers in boys with overweight/obesity. Pediatr. Exerc. Sci. 2021, 33, 132–138. [Google Scholar] [CrossRef]
- Scherberich, J.E.; Gruber, R.; Nockher, W.A.; Christensen, E.I.; Schmitt, H.; Herbst, V.; Block, M.; Kaden, J.; Schlumberger, W. Serum uromodulin—A marker of kidney function and renal parenchymal integrity. Nephrol. Dial. Transplant. 2018, 33, 284–295. [Google Scholar] [CrossRef]
- Devuyst, O.; Olinger, E.; Rampoldi, L. Uromodulin: From physiology to rare and complex kidney disorders. Nat. Rev. Nephrol. 2017, 13, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Vyletal, P.; Bleyer, A.J.; Kmoch, S. Uromodulin biology and pathophysiology—An update. Kidney Blood Press. Res. 2010, 33, 456–475. [Google Scholar] [CrossRef]
- Kipp, A.; Olinger, E. What does uromodulin do? Clin. J. Am. Soc. Nephrol. 2020, 16, 150–153. [Google Scholar] [CrossRef]
- Graham, L.A.; Padmanabhan, S.; Fraser, N.J.; Kumar, S.; Bates, J.M.; Raffi, H.S.; Welsh, P.; Beattie, W.; Hao, S.; Leh, S.; et al. Validation of uromodulin as a candidate gene for human essential hypertension. Hypertension 2014, 63, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Pattaro, C.; Köttgen, A.; Teumer, A.; Garnaas, M.; Böger, C.A.; Fuchsberger, C.; Olden, M.; Chen, M.H.; Tin, A.; Taliun, D.; et al. Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet. 2012, 8, e1002584. [Google Scholar] [CrossRef]
- Algharably, E.A.H.; Bolbrinker, J.; Lezius, S.; Reibis, R.; Wegscheider, K.; Völler, H.; Kreutz, R. Uromodulin associates with cardiorenal function in patients with hypertension and cardiovascular disease. J. Hypertens. 2017, 35, 2053–2058. [Google Scholar] [CrossRef]
- Garimella, P.S.; Biggs, M.L.; Katz, R.; Ix, J.H.; Bennett, M.R.; Devarajan, P.; Kestenbaum, B.R.; Siscovick, D.S.; Jensen, M.K.; Shlipak, M.G.; et al. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int. 2015, 88, 1126–1134. [Google Scholar] [CrossRef]
- Steubl, D.; Buzkova, P.; Ix, J.H.; Devarajan, P.; Bennett, M.R.; Chaves, P.H.M.; Shlipak, M.G.; Bansal, N.; Sarnak, M.J.; Garimella, P.S. Association of serum and urinary uromodulin and their correlates in older adults—The Cardiovascular Health Study. Nephrology 2020, 25, 522–526. [Google Scholar] [CrossRef]
- Delgado, G.E.; Kleber, M.E.; Scharnagl, H.; Krämer, B.K.; März, W.; Scherberich, J.E. Serum uromodulin and mortality risk in patients undergoing coronary angiography. J. Am. Soc. Nephrol. 2017, 28, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Buzkova, P.; Garimella, P.S.; Ix, J.H.; Devarajan, P.; Bennett, M.R.; Chaves, P.H.M.; Shlipak, M.G.; Bansal, N.; Sarnak, M.J. Association of serum uromodulin with mortality and cardiovascular disease in the elderly—The Cardiovascular Health Study. Nephrol. Dial. Transplant. 2020, 35, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Leiherer, A.; Muendlein, A.; Saely, C.H.; Ebner, J.; Brandtner, E.M.; Fraunberger, P.; Drexel, H. Serum uromodulin is a predictive biomarker for cardiovascular events and overall mortality in coronary patients. Int. J. Cardiol. 2017, 231, 6–12. [Google Scholar] [CrossRef]
- Steubl, D.; Schneider, M.P.; Meiselbach, H.; Nadal, J.; Schmid, M.C.; Saritas, T.; Krane, V.; Sommerer, C.; Baid-Agrawal, S.; Voelkl, J.; et al. GCKD Study Investigators. Association of serum uromodulin with death, cardiovascular events, And kidney failure in CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 616–624. [Google Scholar] [CrossRef]
- Sandokji, I.; Greenberg, J.H. Novel biomarkers of acute kidney injury in children: An update on recent findings. Curr. Opin. Pediatr. 2020, 32, 354–359. [Google Scholar] [CrossRef]
- Uwaezuoke, S.N. Uromodulin and YKL-40 as biomarkers in pediatric acute kidney injury: A review of current evidence. J. Integr. Nephrol. Androl. 2017, 4, 115–120. [Google Scholar] [CrossRef]
- Wiromrat, P.; Bjornstad, P.; Roncal, C.; Pyle, L.; Johnson, R.J.; Cherney, D.Z.; Lipina, T.; Bishop, F.; Maahs, D.M.; Wadwa, R.P. Serum uromodulin is associated with urinary albumin excretion in adolescents with type 1 diabetes. J. Diabetes Complicat. 2019, 33, 648–650. [Google Scholar] [CrossRef]
- Li, L.; Xie, W.; Zheng, X.L.; Yin, W.D.; Tang, C.K. A novel peptide adropin in cardiovascular diseases. Clin. Chim. Acta. 2016, 453, 107–113. [Google Scholar] [CrossRef]
- Gao, S.; Stevens, J.R.; Butler, A.A. Adropin—A circulating factor in metabolic control or a drop in the ocean? Expert Rev. Endocrinol. Metab. 2016, 11, 239–241. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhao, P.; Wu, M.C.; Liu, J.; Yin, W. Serum adropin levels are decreased in patients with acute myocardial infarction. Regul. Pept. 2014, 190–191, 46–49. [Google Scholar] [CrossRef]
- Zhao, L.P.; Xu, W.T.; Wang, L.; You, T.; Chan, S.P.; Zhao, X.; Yang, X.J. Serum adropin level in patients with stable coronary artery disease. Heart Lung Circ. 2015, 24, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Demircelik, B.; Cakmak, M.; Nazli, Y.; Gurel, O.M.; Akkaya, N.; Cetin, M.; Cetin, Z.; Selcoki, Y.; Kurtul, A.; Eryonucu, B. Adropin: A new marker for predicting late saphenous vein graft disease after coronary artery bypass grafting. Clin. Invest. Med. 2014, 37, E338–E344. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Çakmak, T.; Eren, M.N. Elevated adropin: A candidate diagnostic marker for myocardial infarction in conjunction with troponin-I. Peptides. 2014, 58, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.T.; Bilen, M.; Kazancı, F.; Yildirim, M.E.; İncebay, İ.B.; Erdamar, H. Serum adropin as a predictive biomarker of erectile dysfunction in coronary artery disease patients. Cent. Eur. J. Urol. 2019, 72, 302–306. [Google Scholar]
- Fan, Z.; Zhang, Y.; Zou, F.; Xu, T.; Pan, P.; Hu, C.; Su, X. Serum adropin level is associated with endothelial dysfunction in patients with obstructive sleep apnea and hypopnea syndrome. Sleep Breath. 2021, 25, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as a fat-burning hormone with multiple functions—Review of a decade of research. Molecules. 2020, 25, 549. [Google Scholar] [CrossRef]
- Altamimi, T.R.; Gao, S.; Karwi, Q.G.; Fukushima, A.; Rawat, S.; Wagg, C.S.; Zhang, L.; Lopaschuk, G.D. Adropin regulates cardiac energy metabolism and improves cardiac function and efficiency. Metabolism. 2019, 98, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Mushala, B.A.S.; Scott, I. Adropin: A hepatokine modulator of vascular function and cardiac fuel metabolism. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H238–H244. [Google Scholar] [CrossRef]
- Gao, S.; McMillan, R.P.; Zhu, Q.; Lopaschuk, G.D.; Hulver, M.W.; Butler, A.A. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 2015, 4, 310–324. [Google Scholar] [CrossRef]
- Wu, L.; Fang, J.; Chen, L.; Zhao, Z.; Luo, Y.; Lin, C.; Fan, L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 751–758. [Google Scholar] [CrossRef]
- Kałużna, M.; Pawlaczyk, K.; Schwermer, K.; Hoppe, K.; Człapka-Matyasik, M.; Ibrahim, A.Y.; Sawicka-Gutaj, N.; Minczykowski, A.; Ziemnicka, K.; Oko, A.; et al. Adropin and irisin: New biomarkers of cardiac status in patients with end-stage renal disease? A preliminary study. Adv. Clin. Exp. Med. 2019, 28, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Sayın, O.; Tokgöz, Y.; Arslan, N. Investigation of adropin and leptin levels in pediatric obesity-related nonalcoholic fatty liver disease. J. Pediatr. Endocrinol. Metab. 2014, 27, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Altincik, A.; Sayin, O. Evaluation of the relationship between serum adropin levels and blood pressure in obese children. J. Pediatr. Endocrinol. Metab. 2015, 28, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, L.; Yang, Y.J.; Ge, R.K.; Zhou, M.; Hu, H.; Liu, H.; Cui, J.; Li, L.L.; Dong, Y.F.; et al. Aerobic exercise improves endothelial function and serum adropin levels in obese adolescents independent of body weight loss. Sci. Rep. 2017, 7, 17717. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Pei, Q.; Zhang, J.; Weng, H.; Jing, F.; Yi, Q. Association between adropin and coronary artery lesions in children with Kawasaki disease. Eur. J. Pediatr. 2021, 180, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Molero-Ramirez, H.; Tan, H.L.; Bandla, H.P. Circulating adropin concentrations in pediatric obstructive sleep apnea: Potential relevance to endothelial function. J. Pediatr. 2013, 163, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Lyass, A.; Courchesne, P.; Chen, G.; Liu, C.; Yin, X.; Hwang, S.J.; Massaro, J.M.; Larson, M.G.; Levy, D. Protein biomarkers of cardiovascular disease and mortality in the community. J. Am. Heart Assoc. 2018, 7, e008108. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, A.I.; Olza, J.; Gil-Campos, M.; Leis, R.; Bueno, G.; Aguilera, C.M.; Gil, A.; Moreno, L.A. Cardiovascular risk biomarkers and metabolically unhealthy status in prepubertal children: Comparison of definitions. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.H.; Maddaloni, E.; Buzzetti, R. Risk factors and predictive biomarkers of early cardiovascular disease in obese youth. Diabetes Metab. Res. Rev. 2019, 35, e3134. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.; Latronico, M.V.; Cavarretta, E. MicroRNAs in cardiovascular diseases: Current knowledge and the road ahead. J. Am. Coll. Cardiol. 2014, 63, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Oses, M.; Margareto Sanchez, J.; Portillo, M.P.; Aguilera, C.M.; Labayen, I. Circulating miRNAs as biomarkers of obesity and obesity-associated comorbidities in children and adolescents: A systematic review. Nutrients 2019, 11, 2890. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Močnik, M.; Marčun Varda, N. Current Knowledge of Selected Cardiovascular Biomarkers in Pediatrics: Kidney Injury Molecule-1, Salusin-α and -β, Uromodulin, and Adropin. Children 2022, 9, 102. https://doi.org/10.3390/children9010102
Močnik M, Marčun Varda N. Current Knowledge of Selected Cardiovascular Biomarkers in Pediatrics: Kidney Injury Molecule-1, Salusin-α and -β, Uromodulin, and Adropin. Children. 2022; 9(1):102. https://doi.org/10.3390/children9010102
Chicago/Turabian StyleMočnik, Mirjam, and Nataša Marčun Varda. 2022. "Current Knowledge of Selected Cardiovascular Biomarkers in Pediatrics: Kidney Injury Molecule-1, Salusin-α and -β, Uromodulin, and Adropin" Children 9, no. 1: 102. https://doi.org/10.3390/children9010102
APA StyleMočnik, M., & Marčun Varda, N. (2022). Current Knowledge of Selected Cardiovascular Biomarkers in Pediatrics: Kidney Injury Molecule-1, Salusin-α and -β, Uromodulin, and Adropin. Children, 9(1), 102. https://doi.org/10.3390/children9010102