How Compounding Pharmacies Fill Critical Gaps in Pediatric Drug Development Processes: Suggested Regulatory Changes to Meet Future Challenges
Abstract
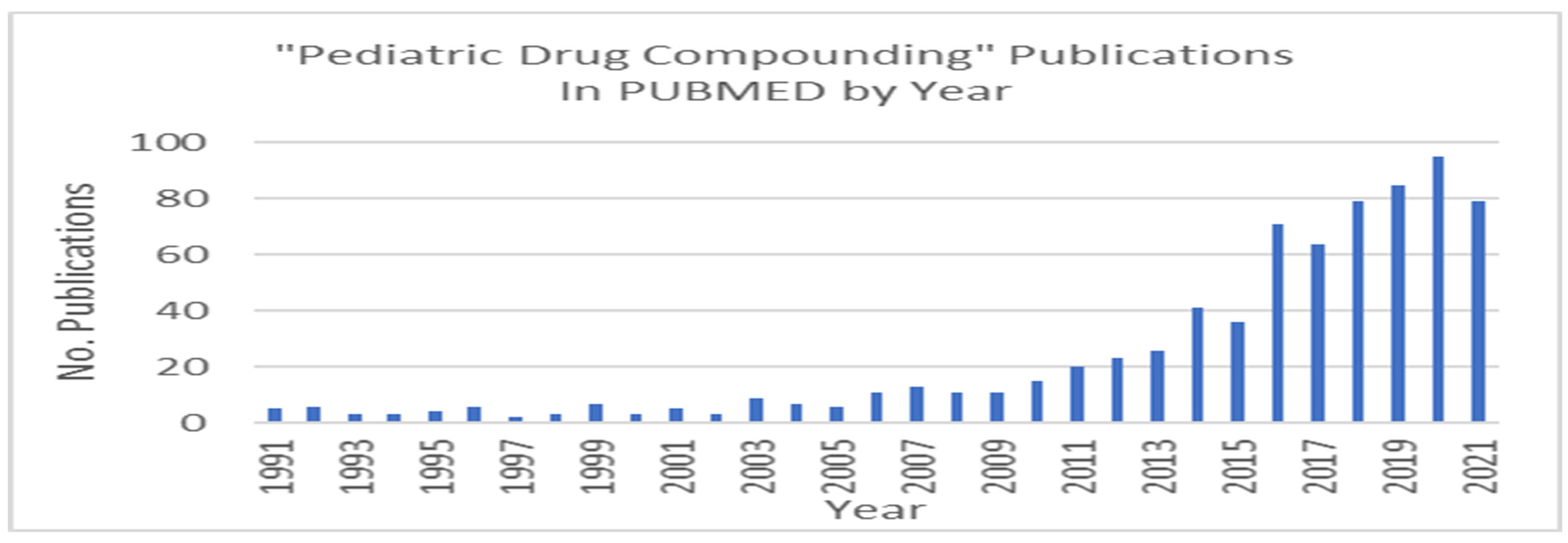
1. Introduction and Statement of the Problem
2. US-FDA Drug Approval Pathway
2.1. Phases for US-FDA Approval
2.1.1. Role of BCPA for Drugs without Active Patents
2.1.2. Use of Supplemental New Drug Applications
3. Drug Compounding Pathway
Drug Quality and Security Act of 2013
- Preparation of drug dosage forms for both human and animal patients;
- Preparation of drugs or devices in anticipation of prescription drug orders based on routine, regularly observed prescribing patterns;
- Reconstitution or manipulation of commercial products that may require the addition of one or more ingredients that is not in the package instructions. [US-FDA does not consider reconstitution or manipulation according to the package instructions compounding; and
- Preparing a medication in accordance with the package insert instructions is compounding under the USP definition, while it is not under US-FDA’s definition [32].
4. Reasons for Needed Regulatory Change
5. Challenges for Meeting These Requirements
6. Suggestions for Regulatory Change
- US-FDA could consider allocating additional resources into properly designed surveillance tools that can begin to measure the depth (number of prescriptions) and breadth (number of different dosage forms dispensed) of compounded preparations to better understand the extent to which pediatric patients rely on them. This surveillance system will generate data that can be used to better understand the compounding industry and guide responsive policy. US-FDA’s Compounding Incidents Program that reviews MedWatch adverse event entries for compounded drugs is a first step towards better compounding surveillance in general [40].
- US-FDA could consider either (a) the creation of a pharmacy compounding grant program for 503A pharmacies and 503B outsourcing facilities in addition to the current grants directed to academic institutions that are involved in compounding practice or (b) foster partnerships between pediatric academic medical centers and registered 503A pharmacies and 503B outsourcing facilities to test and validate evidence-based formulations designed specifically for children.
- A simplified, safe, and reliable pathway for the authorization of compounded preparations for children, and other special populations, could be developed in conjunction with USP. This can include well defined active ingredient (API) and excipient specifications, along with methods of preparation and finished dosage form tests. Additionally, this pathway can include PK studies (bioequivalence studies, population PK and modeling, among others) to bridge pediatric findings to adult populations, as is currently allowed for many sNDAs.
- US-FDA could consider facilitating compounded pediatric preparations by not including frequently compounded APIs on the Bulk Substances Used in Compounding list [27]. The Bulks List should be used to incentivize safe compounding practice.
- US-FDA could encourage the inclusion of alternate dosage form compounding instructions into the labeling of products that are commonly used in pediatrics. The palonosetron CRL, provided previously, is an example. This is especially important when an approved dosage form cannot be used in children, yet the active ingredient is commonly used in pediatrics, and the stability of compounded preparations limited.
- ANDA filers (e.g., generic drug companies) could be permitted to augment labeling with compounding instructions. This would simply require additional in vitro stability studies of compounded formulations, for example a crushed tablet in a well characterized pharmaceutical vehicle. These studies are relatively simple for the generic manufacturers to perform and could add additional value and labeled uses for their specific product. US-FDA could facilitate dissemination of alternate dosage form stability into the product’s labeling when it becomes known, especially when a product is unstable in aqueous or non-aqueous vehicles.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| ADME | Absorption, distribution, metabolism, and excretion |
| ANDA | Abbreviated new drug application |
| API | Active pharmaceutical ingredient |
| BLA | Biologics license application |
| BPCA | Best Pharmaceuticals for Children Act of 2002 |
| CGMP | Current Good Manufacturing Practice |
| CINV-MEC | Chemotherapy-induced nausea and vomiting |
| CRL | Complete response letter |
| DQSA | Drug Quality and Security Act of 2013 |
| FDAMA | Food and Drug Administration Modernization Act of 1998 |
| IND | Investigational new drug |
| NCE | New chemical entity |
| NDA | New drug application |
| NICHD | National Institute for Child Health and Human Development |
| NME | New molecular entity |
| PK | Pharmacokinetic |
| PREA | Pediatric Research Equity Act of 2003 |
| PRV | Rare Pediatric Disease Priority Review Voucher Program of 2012 |
| PSP | Pediatric study plan |
| RCT | Randomized clinical trial |
| sNDA | Supplementary NDA |
| US-FDA | United States Food and Drug Administration |
References
- Carmack, M.; Hwang, T.; Bourgeois, F.T. Pediatric Drug Policies Supporting Safe and Effective Use of Therapeutics in Children: A Systematic Analysis. Health Aff. 2020, 39, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Bucci-Rechtweg, C. Enhancing the Pediatric Drug Development Framework to Deliver Better Pediatric Therapies Tomorrow. Clin. Ther. 2017, 39, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Bourgeois, F.; Franklin, J.; Kesselheim, A. Impact of the Priority Review Voucher Program on Drug Development for Rare Pediatric Diseases. Health Aff. 2019, 38, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.P.; Fein, K.C.; Whitehead, K.A. Oral Delivery of Peptide Therapeutics in Infants: Challenges and Opportunities. Adv. Drug Deliv. Rev. 2021, 173, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Heitman, T.; Day, A.J.; Bassani, A.S. Pediatric Compounding Pharmacy: Taking on the Responsibility of Providing Quality Customized Prescriptions. Children 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Nahata, M.C.; Allen, L.V. Extemporaneous Drug Formulations. Clin. Ther. 2008, 30, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Mattison, D.R.; Parker, R.M.; Jackson, L.M. (Eds.) The Clinical Utility of Compounded Bioidentical Hormone Therapy; National Academies Press: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Carvalho, M.; Almeida, I. The Role of Pharmaceutical Compounding in Promoting Medication Adherence. Pharmaceuticals 2022, 15, 1091. [Google Scholar] [CrossRef] [PubMed]
- Witten, C.; Batra, A.; Durfor, C.N.; Hilbert, S.L.; Kaplan, D.S.; Fink, D.; Lavoie, D.; Maher, E.; McFarland, R. Overview of FDA Regulatory Process. In Principles of Regenerative Medicine; Atala, A., Lanza, R., Mikos, T., Nerem, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 77; pp. 1345–1365. [Google Scholar] [CrossRef]
- Brown, D.G.; Wobst, H.J.; Kapoor, A.; Kenna, L.A.; Southall, N. Clinical Development Times for Innovative Drugs. Nat. Rev. Drug Discov. 2021, 21, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Office of the Inspector General. Compounded Drugs under Medicare Part B: Payment and Oversight. Available online: https://oig.hhs.gov/oei/reports/oei-03-13-00270.pdf (accessed on 2 October 2022).
- United States Food and Drug Administration. Orphan Drug Act—Relevant Excerpts. Available online: https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/orphan-drug-act-relevant-excerpts (accessed on 19 October 2022).
- United States Food and Drug Administration. Development and Approval Process|Drugs. Available online: https://www.fda.gov/drugs/development-approval-process-drugs#:~:text=A%20team%20of%20CDER%20physicians,drug%20is%20approved%20for%20sale (accessed on 17 November 2022).
- United States Food and Drug Administration. Guidance for Industry E11 Clinical Investigation of Medicinal Products in the Pediatric Population. Available online: https://www.fda.gov/media/71355/download (accessed on 2 October 2022).
- United States Food and Drug Administration. FDA Drug Approval Process. Available online: https://www.fda.gov/media/82381/download (accessed on 2 October 2022).
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009–2018. JAMA 2020, 323, 844. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, F.T.; Kesselheim, A.S. Promoting Pediatric Drug Research and Labeling—Outcomes of Legislation. N. Eng. J. Med. 2019, 381, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.S.; Najafzadeh, M.; Rajasingh, E.K.; Love, J.; Kesselheim, A.S. Labeling Changes and Costs for Clinical Trials Performed under the US Food and Drug Administration Pediatric Exclusivity Extension, 2007 to 2012. JAMA Intern. Med. 2018, 178, 1458. [Google Scholar] [CrossRef] [PubMed]
- Zisowsky, J.; Krause, A.; Dingemanse, J. Drug Development for Pediatric Populations: Regulatory Aspects. Pharmaceutics 2010, 2, 364–388. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Child Health and Human Development. BPCA Funded Clinical Trials. Available online: https://www.nichd.nih.gov/sites/default/files/inline-files/BPCA-funded-clinical-trials.pdf (accessed on 19 October 2022).
- United States Food and Drug Administration. Pediatric Study Plans: Content of and Process for Submitting Initial Pediatric Study Plans and Amended Initial Pediatric Study Plans Guidance for Industry. Available online: https://www.fda.gov/media/86340/download (accessed on 2 October 2022).
- Letter from Julie Beitz to Craig Lehmann, re: NDAs 021372 and 022233. August 2010; p. 7. Available online: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM396087.pdf (accessed on 19 October 2022).
- Boccia, R.; Grunberg, S.; Franco-Gonzales, E.; Rubenstein, E.; Voisin, D. Efficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: A phase 3 trial. Support Care Cancer 2013, 21, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Akynzeo® Prescribing Information. Available online: https://www.akynzeo.com/assets/pdf/Akynzeo-USPI.pdf (accessed on 19 October 2022).
- Watson, C.J.; Whitledge, J.D.; Siani, A.M.; Burns, M.M. Pharmaceutical Compounding: A History, Regulatory Overview, and Systematic Review of Compounding Errors. J. Med. Tox. 2021, 17, 197–217. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. Center for Drug Evaluation and Research. Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product under Section 503A of the Federal Food, Drug, and Cosmetic Act: Guidance for Industry. January 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/compounded-drug-products-are-essentially-copies-commercially-available-drug-product-under-section (accessed on 15 September 2022).
- United States Food and Drug Administration. Center for Drug Evaluation and Research. Bulk Drug Substances Used in Compounding under Section 503B of the FD&C Act. Available online: https://www.fda.gov/drugs/human-drug-compounding/bulk-drug-substances-used-compounding-under-section-503b-fdc-act (accessed on 15 September 2022).
- Kim, S.H. The Drug Quality and Security Act of 2013: Compounding Consistently. J. Health Care Law Policy 2017, 19, 5. [Google Scholar]
- United States Food and Drug Administration. Center for Drug Evaluation and Research. Pharmacy Compounding of Human Drug Products under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance. Bethesda, MD, USA, June 2016. Available online: https://www.fda.gov/media/94393/download (accessed on 2 October 2022).
- United States Food and Drug Administration, Centers for Drug Evaluation and Research. Outsourcing Facility Information. Available online: https://www.fda.gov/media/107569/download (accessed on 20 October 2022).
- United States Pharmacopeial Convention. USP Compounding Compendium. 2019. Available online: https://www.usp.org/products/usp-compounding-compendium (accessed on 12 October 2022).
- American Society of Health-System Pharmacists. ASHP Compounding Frequently Asked Questions. Available online: https://www.ashp.org/-/media/assets/advocacy-issues/docs/compounding-guidances-frequently-asked-questions.pdf (accessed on 15 September 2022).
- McPherson, T.; Fontane, P.; Iyengar, R.; Henderson, R. Utilization and Costs of Compounded Medications for Commercially Insured Patients, 2012–2013. J. Manag. Care Spec. Pharm. 2016, 22, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Karesh, A. Pediatric Drug Development: Regulatory Expectations. Available online: https://www.fda.gov/files/drugs/published/Pediatric-Drug-Development--Regulatory-Expectations (accessed on 2 October 2022).
- Government Accounting Office. Drug Development: FDA’s Priority Review Voucher Program. Available online: https://www.gao.gov/assets/gao-20-251.pdf (accessed on 2 October 2022).
- Morgan, S.; Grootendorst, P.; Lexchin, J.; Cunningham, C.; Greyson, D. The Cost of Drug Development: A Systematic Review. Health Policy N. Y. 2011, 100, 4–17. [Google Scholar] [CrossRef]
- Drug Watch. Pharmaceutical Companies with the Most “Pediatric Exclusivity” Drugs. Available online: https://www.drugpatentwatch.com/trends/pediatric-exclusivity.php (accessed on 2 October 2022).
- United States Food and Drug Administration, Center for Drug Evaluation and Research. Division of Cardiovascular and Renal Products, Application Number 211340Orig1s000, KATERZIA (Amlodipine Benzoate) Oral Suspension 1 mg/mL. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211340Orig1s000OtherR.pdf (accessed on 17 November 2022).
- United States Food and Drug Administration, Center for Drug Evaluation and Research. Application Number 022571Orig1s000, Cuvposa 1 mg/5 mL. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022571Orig1s000SumR.pdf (accessed on 17 November 2022).
- Janusziewicz, A.N.; Glueck, S.N.; Park, S.Y.; Nguyen, D.N.; Rimmel, S.C.; Cascio, L.A.; Doh, G.Y.; Martin-Yeboah, G.F. A pharmacist-driven Food and Drug Administration incident surveillance and response program for compounded drugs. Am. J. Health Syst. Pharm. 2021, 78, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Grabb, M.C.; Gobburu, J.V.S. Challenges in Developing Drugs for Pediatric CNS Disorders: A Focus on Psychopharmacology. Prog. Neurobiol. 2017, 152, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Bristol Myers Squibb. Srycel® (Dasatinib Tablets for Oral Use) Package Insert. Available online: https://packageinserts.bms.com/pi/pi_sprycel.pdf (accessed on 2 October 2022).
- Parrish, R.H., II; Ashworth, L.D.; Löbenberg, R.; Benavides, S.; Cies, J.J.; MacArthur, R.B. Compounded Nonsterile Preparations and FDA-Approved Commercially Available Liquid Products for Children: A North American Update. Pharmaceutics 2022, 14, 1032. [Google Scholar] [CrossRef] [PubMed]
| •E11 Clinical Investigation of Medicinal Products in the Pediatric Population |
| •How to Comply with the Pediatric Research Equity Act |
| •Nonclinical Safety Evaluation of Pediatric Drug Products |
| •Over-the-Counter Pediatric Oral Liquid Drug Products Containing Acetaminophen |
| •Pediatric Gastroesophageal Reflux Disease: Developing Drugs for Treatment Guidance for Industry |
| •Pediatric Rare Diseases—A Collaborative Approach for Drug Development Using Gaucher Disease as a Model; Draft Guidance for Industry |
| •Clarification of Orphan Designation of Drugs and Biologics for Pediatric Subpopulations of Common Diseases: Guidance for Industry |
| •E11(R1) Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population |
| •Atopic Dermatitis: Timing of Pediatric Studies During Development of Systemic Drugs |
| •Pediatric HIV Infection: Drug Development for Treatment |
| •Pediatric Information Incorporated Into Human Prescription Drug and Biological Products Labeling Good Review Practice |
| •Rare Pediatric Disease Priority Review Vouchers: Draft Guidance for Industry |
| •Drugs for Treatment of Partial Onset Seizures: Full Extrapolation of Efficacy from Adults to Pediatric Patients 2 Years of Age and Older Guidance for Industry |
| •Cancer Clinical Trial Eligibility Criteria: Minimum Age Considerations for Inclusion of Pediatric Patients |
| •Pediatric Study Plans: Content of and Process for Submitting Initial Pediatric Study Plans and Amended Initial Pediatric Study Plans |
| •S11 Nonclinical Safety Testing In Support of Development of Pediatric Pharmaceuticals: International Council for Harmonization; Draft Guidance for Industry |
| •FDARA Implementation Guidance for Pediatric Studies of Molecularly Targeted Oncology Drugs: Amendments to Sec. 505B of the FD&C Act: Guidance for Industry |
| •Development of Anti-Infective Drug Products for the Pediatric Population: Guidance for Industry |
| •E11A Pediatric Extrapolation |
| •General Clinical Pharmacology Considerations for Pediatric Studies of Drugs, Including Biological Products |
| •Ethical Considerations for Clinical Investigations of Medical Products Involving Children: Draft Guidance for Industry, Sponsors, and IRBs |
| Policy | Description |
|---|---|
| Pediatric Rule (1994) | - Permitted additions to pediatric labeling based upon extrapolation of efficacy in adult populations together with additional pediatric PK, pharmacodynamic, and safety studies. - Requires that disease and drug response in adults is known to be similar in children. - Compliance is voluntary. |
| Pediatric Rule in The Food and Drug Administration Modernization Act (1997) (not currently in effect) | - Required sponsors of a new drug to submit, prior to approval, safety and effectiveness information in relevant pediatric population(s) for the claimed indications. However, submission of the pediatric data could be deferred if certain criteria applied. - US-FDA developed a list of drugs where additional of pediatric information would be beneficial. - Sponsors received a Written Request (WR) for pediatric studies along with a required time to completion. - Sponsors that completed the WR requirement received an additional 6 months of marketing exclusivity. |
| Best Pharmaceuticals for Children Act (2002) | - Provides a financial incentive to companies to voluntarily conduct pediatric studies. - Incentive extends market exclusivity for 6 months after patents expire for pediatrics. - The exclusivity is granted for an active pharmaceutical ingredient and applies to all US-FDA approved drug products containing the ingredient. - US-FDA requests may apply to both approved and unapproved indications. - BPCA is evaluated for renewal every 5 years. |
| Pediatric Research Equity Act (PREA) (2003) | - Requires sponsors to perform studies involving children to assess the safety and effectiveness of a drug or biologic product for the claimed indications. - Requires studies only for the indication(s) under review in adults. - Applies to applications and supplements for a new active ingredient, new indication, new dosage form, new dosing regimen, and new route(s) of administration. - US-FDA may issue a waiver or deferral for some or all studies involving children. Orphan indications are exempt. - Results guide labeling. - Applies to drugs and biologics. |
| Rare Pediatric Disease Priority Review Voucher Program (2012) | - Permits US-FDA to issue priority review vouchers to sponsors who receive approval for a product to treat rare diseases in children. - Voucher can be sold and then redeemed to receive a 6-month priority review of a subsequent marketing application for a different product, including products intended only for adult use (e.g., larger patient populations). |
| Federal Food, Drug, and Cosmetic Act 5059b0(2) | - Other post-approval market exclusivities available after patents expire
|
| Trade and Generic Name | Dosage Form | No. of Patents * | Latest Patent Date | Post Marketing Commitment (Required under PREA) (https://www.accessdata.fda.gov/scripts/cder/pmc/index.cfm) |
|---|---|---|---|---|
| Carospir® (spironolactone) | Suspension | 5 | 28 October 2036 | PMR 3256-1: Conduct a single-dose PK study in pediatric patients 0 to <17 years of age with edematous conditions. PMR 3256-2: Conduct a multiple- dose PK, pharmacodynamics, and safety study in pediatric patients 0 to <17 years of age with edematous conditions. |
| Kapspargo Sprinkle® (metoprolol succinate) | Capsule, ER ^ | 2 | 9 July 2035 | PMR 3638-1: Conduct a randomized, dose-ranging, double-blind, placebo-controlled, parallel group, multi-center clinical study with an open-label 52-week safety extension to evaluate efficacy, safety, tolerability and pharmacokinetics of metoprolol succinate extended release (ER) oral dosage form in hypertensive pediatric subjects from birth to less than 6 years of age |
| Qbrelis® (lisinopril solution) | Solution | 8 | 6 November 2035 | PMR 3099-1: An efficacy, safety and dose-finding study of Qbrelis in hypertensive pediatric patients two years to less than six years of age |
| Katerzia® (amlodipine benzoate) | Suspension | 5 | 11 April 2039 | PMR 3640-1: Conduct non-clinical toxicity studies in juvenile rats to evaluate developmental toxicity to include assessment of the effects of amlodipine benzoate suspension on reproductive and learning development to support dosing in humans down to birth. PMR 3640-2: Conduct a dose-ranging, safety, tolerability, and efficacy study with amlodipine benzoate oral suspension in hypertensive pediatric patients age birth to less than 6 years of age. |
| Norliqva® (amlodipine besylate) | Solution | 1 | 24 February 2041 | PMR 4239-1: Conduct a dose-ranging juvenile toxicology and toxicokinetic study to support the definitive toxicology study, and conduct a toxicity study in juvenile rats to evaluate developmental toxicity, including potential effects on reproductive development and learning. PMR 4239-2: Conduct an open-label, randomized, single oral dose, two-treatment, two-period, two-sequence crossover bioequivalence and bioavailability study of amlodipine (ethanol-containing) oral solution versus amlodipine (ethanol-free) oral solution in healthy adults under fasting conditions. PMR 4239-3: Conduct a dose-ranging, safety, tolerability, and efficacy study of amlodipine besylate oral solution for the treatment of hypertension in pediatric patients, birth to <6 years of age. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacArthur, R.B.; Ashworth, L.D.; Zhan, K.; Parrish, R.H., II. How Compounding Pharmacies Fill Critical Gaps in Pediatric Drug Development Processes: Suggested Regulatory Changes to Meet Future Challenges. Children 2022, 9, 1885. https://doi.org/10.3390/children9121885
MacArthur RB, Ashworth LD, Zhan K, Parrish RH II. How Compounding Pharmacies Fill Critical Gaps in Pediatric Drug Development Processes: Suggested Regulatory Changes to Meet Future Challenges. Children. 2022; 9(12):1885. https://doi.org/10.3390/children9121885
Chicago/Turabian StyleMacArthur, Robert B., Lisa D. Ashworth, Keming Zhan, and Richard H. Parrish, II. 2022. "How Compounding Pharmacies Fill Critical Gaps in Pediatric Drug Development Processes: Suggested Regulatory Changes to Meet Future Challenges" Children 9, no. 12: 1885. https://doi.org/10.3390/children9121885
APA StyleMacArthur, R. B., Ashworth, L. D., Zhan, K., & Parrish, R. H., II. (2022). How Compounding Pharmacies Fill Critical Gaps in Pediatric Drug Development Processes: Suggested Regulatory Changes to Meet Future Challenges. Children, 9(12), 1885. https://doi.org/10.3390/children9121885






