The Impact of Vitamin K2 (Menaquionones) in Children’s Health and Diseases: A Review of the Literature
Abstract
1. Introduction
2. The Beneficial Role of Vitamin K2 in Physiological Processes
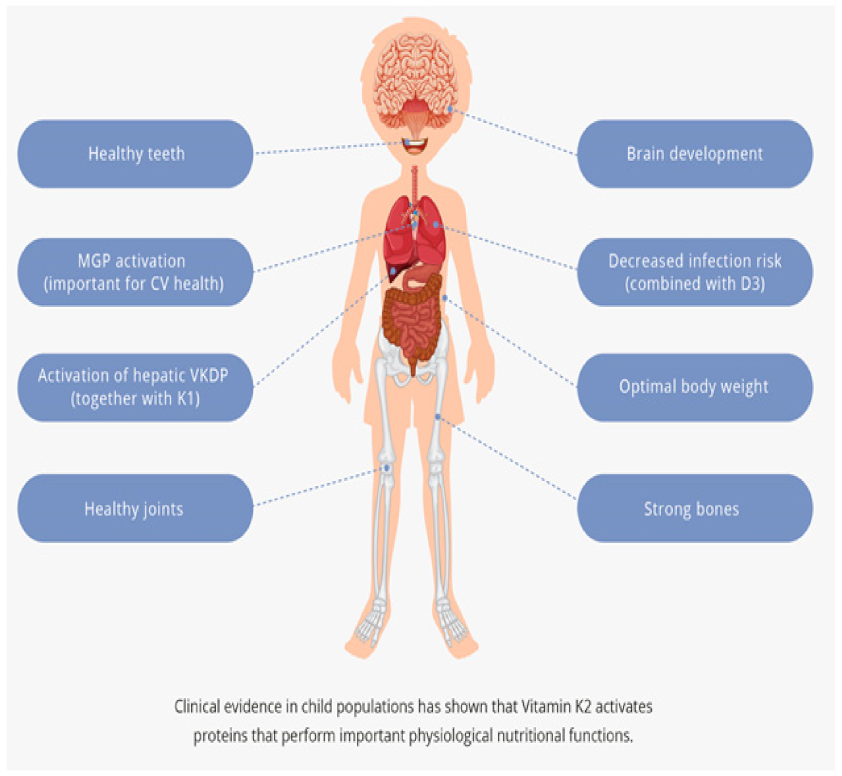
2.1. Strong Bones
2.2. Healthy Teeth
2.3. Cardiovascular Health
2.4. Brain Development
2.5. Activation of VKDP in the Liver
2.6. Joint Health
2.7. Anti-Infection with D3
3. The Importance of Vitamin K2 Supplementation in Various Pathological Conditions
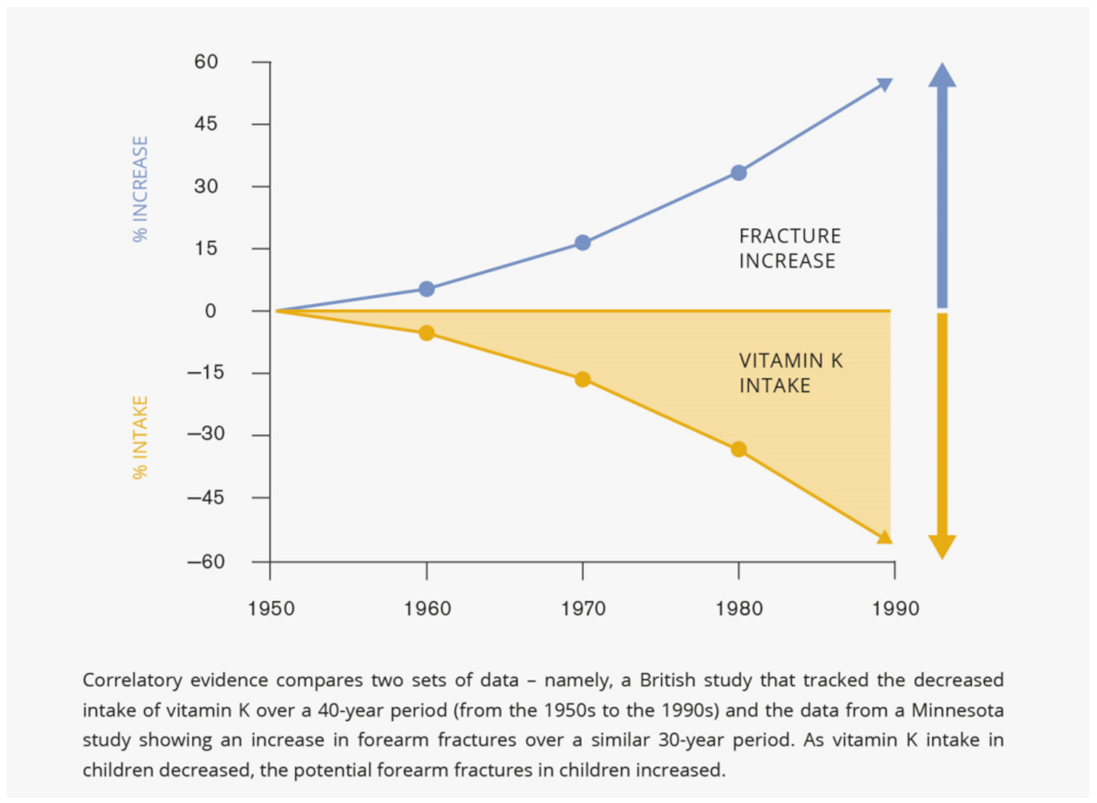
3.1. Bone Fractures
3.2. Optimal Body Weight
3.3. Cooley’s Anemia
3.4. Cystic Fibrosis
3.5. Inflammatory Bowel Diseases (IBD)
3.6. Liver Diseases
3.7. Severe Disability
4. The Negative Impact of Prolonged/Chronic Medication on Vitamin K Status
4.1. Long-Term Antibiotic Use
4.2. Long-Term Glucocorticoid Use
5. Children Have the Highest Needs for Vitamin K
6. Infants and K Deficiency
7. VKDB Is More Common in Asia than Western Countries
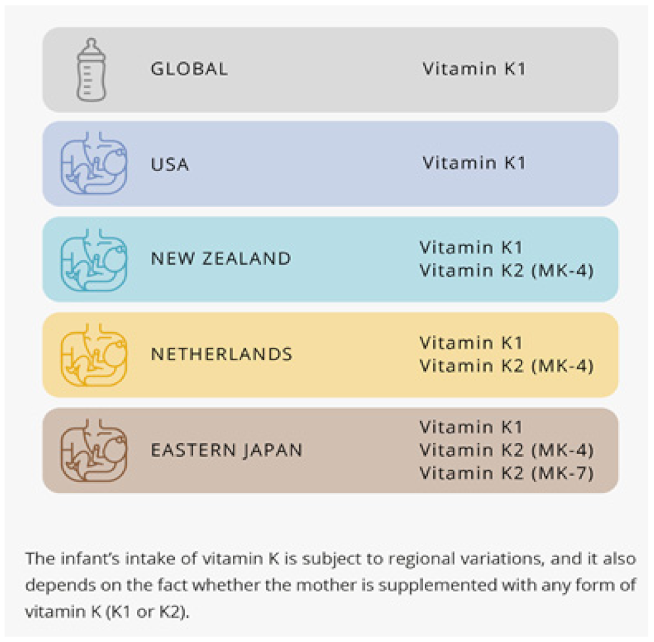
8. The Recommendations for Infant’s Intake
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Akbulut, A.C.; Pavlic, A.; Petsophonsakul, P.; Halder, M.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients 2020, 12, 1852. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vita-min K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef]
- El Asmar, M.S.; Naoum, J.J.; Arbid, E.J. Vitamin K Dependent Proteins and the Role of Vitamin K2 in the Modulation of Vascular Calcification: A Review. Oman Med. J. 2014, 29, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P. Novel Role of Vitamin K in Preventing Oxidative Injury to Developing Oligodendrocytes and Neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.; Sato, A.; Ohashi, A.; Iribe, M.; Goto, T.; Komai, M. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Ambrożewicz, E.; Muszyńska, M.; Tokajuk, G.; Grynkiewicz, G.; Žarković, N.; Skrzydlewska, E. Beneficial Effects of Vitamins K and D3 on Redox Balance of Human Osteoblasts Cultured with Hydroxyapatite-Based Biomaterials. Cells 2019, 8, 325. [Google Scholar] [CrossRef]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grün, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin K2 Regulation of Bone Homeostasis Is Mediated by the Steroid and Xenobiotic Receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; Van Meensel, S.; Schaap, O.; De Strooper, B.; Meganathan, R.; et al. Vitamin K 2 Is a Mitochondrial Electron Carrier That Rescues Pink1 Deficiency. Science 2012, 336, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Weber, P. Management of osteoporosis: Is there a role for vitamin K? Int. J. Vitam. Nutr. Res. 1997, 67, 350–356. [Google Scholar] [PubMed]
- Baily, D.A. The Saskatchewan paediatric bone mineral accrual study: Bone mineral acquisition during the growing years. Int. J. Sports Med. 1997, 18, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Diet, nutrition and bone health. J. Nutr. 2007, 137, 2507–2512. [Google Scholar] [CrossRef]
- Cummings, S.R.; Browner, W.; Black, D.M.; Nevitt, M.C.; Genant, H.K.; Cauley, J.; Ensrud, K.; Scott, J.; Vogt, T.M. Bone density at various sites for predic-tion of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 1993, 341, 72–75. [Google Scholar] [CrossRef]
- Southward, K. A hypothetical role for vitamin K2 in the endocrine and exocrine aspects of dental caries. Med. Hypotheses 2015, 84, 276–280. [Google Scholar] [CrossRef]
- Gordeladze, J.O.; Landin, M.A.; Johnsen, G.F.; Osmundsen, H. Vitamin K2 and its Impact on Tooth Epigenetics. In Vitamin K2—Vital for Health and Wellbeing; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Meyer, W.W.; Lind, J. Calcification of arteries: Frequent findings in childhood. Monatssch. Kinderheilkd. 1971, 119, 298–300. [Google Scholar]
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Origin of atherosclerosis in child-hood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307–1315. [Google Scholar]
- Kavey, R.E.; Allada, V.; Daniels, S.R.; Hayman, L.L.; McCrindle, B.W.; Newburger, J.W.; Parekh, R.S.; Steinberger, J. Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation 2006, 114, 2710–2738. [Google Scholar] [PubMed]
- Schurgers, L.J.; Cranenburg, E.C.; Vermeer, C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar]
- Schurgers, L.J.; Dissel, P.E.; Spronk, H.M.; Soute, B.A.; Dhore, C.R.; Cleutjens, J.P.; Vermeer, C. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z. Kardiol. 2001, 90, 57–63. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.J.; Van Der Meer, I.M.; Hofman, A.; Witteman, J.C.M. Dietary Intake of Menaquinone Is Associated with a Reduced Risk of Coronary Heart Disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin K, an emerging nutrient in brain function. BioFactors 2012, 38, 151–157. [Google Scholar] [CrossRef]
- Gorgels, T.G.; Waarsing, J.H.; Herfs, M.; Versteeg, D.; Schoensiegel, F.; Sato, T.; Schlingemann, R.O.; Ivandic, B.; Vermeer, C.; Schurgers, L.J.; et al. Vitamin K supplementation increases vitamin K tissue levels but fails to counteract ectopic cal-cification in a mouse model for pseudoxanthoma elasticum. J. Mol. Med. 2011, 89, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Carrié, I.; Portoukalian, J.; Vicaretti, R.; Rochford, J.; Potvin, S.; Ferland, G. Menaquinone-4 Concentration Is Correlated with Sphingolipid Concentrations in Rat Brain. J. Nutr. 2004, 134, 167–172. [Google Scholar] [CrossRef]
- Hill, L.M.; Kleinberg, F. Effects of drugs and chemicals on the fetus and newborn (2). Mayo Clin. Proc. 1984, 59, 755–765. [Google Scholar] [CrossRef]
- Araki, S.; Shirahata, A. Vitamin K Deficiency Bleeding in Infancy. Nutrients 2020, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, A.; Itou, S.; Takahashi, Y.; Nishiguchi, T.; Mastuda, Y. Modified guidelline of vitamin K administration for vita-min K deficiency in infancy. J. Jpn. Pediatric Soc. 2011, 115, 705–712. [Google Scholar]
- Abadi, S.; Einarson, A.; Koren, G. Use of warfarin during pregnancy. Can. Fam. Physician Med. Fam. Can. 2002, 48, 695–697. [Google Scholar]
- Loeser, R.; Carlson, C.S.; Tulli, H.; Jerome, W.G.; Miller, L.; Wallin, R. Articular-cartilage matrix gamma-carboxyglutamic acid-containing protein. Characterization and immunolocalization. Biochem. J. 1992, 282, 1–6. [Google Scholar] [CrossRef]
- Shea, M.K.; Kritchevsky, S.B.; Hsu, F.-C.; Nevitt, M.; Booth, S.L.; Kwoh, C.K.; McAlindon, T.E.; Vermeer, C.; Drummen, N.; Harris, T.B.; et al. The association between vitamin K status and knee osteoarthritis features in older adults: The Health, Aging and Body Composition Study. Osteoarthr. Cartil. 2015, 23, 370–378. [Google Scholar] [CrossRef]
- Esposito, S.; Lelii, M. Vitamin D and respiratory tract infections in childhood. BMC Infect. Dis. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Goddek, S. Vitamin D3 and K2 and their potential contribution to reducing the COVID-19 mortality rate. Int. J. Infect. Dis. 2020, 99, 286–290. [Google Scholar] [CrossRef]
- Szmodis, M.; Bosnyák, E.; Protzner, A.; Szőts, G.; Trájer, E.; Tóth, M. Relationship between physical activity, dietary intake and bone parameters in 10-12 years old Hungarian boys and girls. Central. Eur. J. Public Health 2019, 27, 10–16. [Google Scholar] [CrossRef]
- Karpiński, M.; Popko, J.; Maresz, K.; Badmaev, V.; Stohs, S.J. Roles of Vitamins D and K, Nutrition, and Lifestyle in Low-Energy Bone Fractures in Children and Young Adults. J. Am. Coll. Nutr. 2017, 36, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Popko, J.; Karpinski, M.; Chojnowska, S.; Maresz, K.; Milewski, R.; Badmaev, V.; Schurgers, L.J. Decreased Levels of Circu-lating Carboxylated Osteocalcin in Children with Low Energy Fractures: A Pilot Study. Nutrients 2018, 10, 734. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT03871322 (accessed on 21 October 2021).
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the Metabolic Syndrome in Children and Adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Kim, M.; Na, W.; Sohn, C. Menaquinone benefits weight control and improves inflammatory biomarkers in high-fat diet-induced obese rats (815.1). FASEB J. 2014, 28, 815.1. [Google Scholar] [CrossRef]
- Dam, V.; Dalmeijer, G.W.; Vermeer, C.; Drummen, N.E.; Knapen, M.H.; van der Schouw, Y.T.; Beulens, J.W. Association Be-tween Vitamin K and the Metabolic Syndrome: A 10-Year Follow-Up Study in Adults. J. Clin. Endocrinol. Metab. 2015, 100, 2472–2479. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Available online: https://clinicaltrials.gov/ct2/show/NCT01972113?term=vitamin+k2+and+children&draw=2&rank=2 (accessed on 21th of October 2021).
- Ozdemir, M.A.; Yilmaz, K.; Abdulrezzak, U.; Muhtaroglu, S.; Patiroglu, T.; Karakukcu, M.; Unal, E. The Efficacy of Vitamin K2 and Calcitriol Combination on Thalassemic Osteopathy. J. Pediatr. Hematol. 2013, 35, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.P. Vitamin K in cystic fibrosis. J. R. Soc. Med. 2004, 97, 48–51. [Google Scholar] [PubMed]
- Conway, S.P.; Wolfe, S.P.; Brownlee, K.G.; White, H.; Oldroyd, B.; Truscott, J.G.; Harvey, J.M.; Shearer, M.J. Vitamin K status among children with cystic fibrosis and its relationship to bone mineral density and bone turnover. Pediatrics 2005, 115, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska, P.; Lisowska, A.; Woś, H.; Trawińska-Bartnicka, M.; Bober, L.; Rohovyk, N.; Rachel, M.; Walkowiak, J. Vita-min K status in young children with cystic fibrosis. Acta Sci. Pol. Technol. Aliment. 2011, 10, 399–406. [Google Scholar]
- Krasinski, S.D.; Russell, R.M.; Furie, B.C.; Kruger, S.F.; Jacques, P.F.; Furie, B. The prevalence of vitamin K deficiency in chronic gastrointestinal disorders. Am. J. Clin. Nutr. 1985, 41, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Grzybowska-Chlebowczyk, U.; Landowski, P.; Szaflarska-Poplawska, A.; Klincewicz, B.; Adamczak, D.M.; Banasiewicz, T.; Plawski, A.; Walkowiak, J. Prevalence and correlates of vitamin K deficiency in children with inflammatory bowel disease. Sci. Rep. 2014, 4, 4768. [Google Scholar] [CrossRef]
- Kozioł-Kozakowska, A.; Stochel-Gaudyn, A.; Mazuryk, O.; Brindell, M. Osteocalcin status as an indicator of vitamin K2 defi-ciency in inflammatory bowel disease children—A pilot study. Hygeia Public Health 2018, 53, 206–211. (In Polish) [Google Scholar]
- Shea, M.K.; Booth, S.L.; Massaro, J.M.; Jacques, P.F.; D’Agostino Sr, R.B.; Dawson-Hughes, B.; Ordovas, J.M.; O’Donnell, C.J.; Kathiresan, S.; Keaney, J.F.; et al. Vitamin K and Vitamin D Status: Associations with Inflammatory Markers in the Framingham Offspring Study. Am. J. Epidemiol. 2008, 167, 313–320. [Google Scholar] [CrossRef]
- Iijima, H.; Shinzaki, S.; Takehara, T. The importance of vitamins D and K for the bone health and immune function in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 635–640. [Google Scholar] [CrossRef]
- Eman, R.A.; Wesam, M.R. Ashour, Novel Role of Vitamin K in Amelioration of Experimental Induced Ulcerative Colitis in Rats. Am. J. Biomed. Sci. 2020, 12, 27–36. [Google Scholar]
- Shearer, M.J.; Bechtold, H.; Andrassy, K.; Koderisch, J.; McCarthy, P.T.; Trenk, D.; Jähnchen, E.; Ritz, E. Mechanism of cepha-losporin-induced hypoprothrombinemia: Relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J. Clin. Pharmacol. 1988, 28, 88–95. [Google Scholar] [CrossRef]
- Bhat, R.V.; Deshmukh, C.T. A study of Vitamin K status in children on prolonged antibiotic therapy. Indian Pediatr. 2003, 40, 36–40. [Google Scholar]
- Wong, R.S.M.; Cheng, G.; Chan, N.P.H.; Wong, W.-S.; Ng, M.H.L. Use of cefoperazone still needs a caution for bleeding from induced vitamin K deficiency. Am. J. Hematol. 2006, 81, 76. [Google Scholar] [CrossRef] [PubMed]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteopo-rosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef]
- Wędrychowicz, A.; Zając, A.; Tomasik, P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J. Gastroenterol. 2016, 22, 1045–1066. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Schoon, E.J.; Müller, M.C.; Vermeer, C.; Schurgers, L.J.; Brummer, R.J.; Stockbrügger, R.W. Low serum and bone vitamin K status in patients with longstanding Crohn’s disease: Another pathogenetic factor of osteoporosis in Crohn’s disease? Gut 2001, 48, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Vitamin K status in patients with Crohn’s dis-ease and relationship to bone turnover. Am. J. Gastroenterol. 2004, 99, 2178–2185. [Google Scholar] [CrossRef]
- Inaba, N.; Sato, T.; Yamashita, T. Low-Dose Daily Intake of Vitamin K(2) (Menaquinone-7) Improves Osteocalcin γ-Carboxylation: A Double-Blind, Randomized Controlled Trials. J. Nutr. Sci. Vitaminol. 2015, 61, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Emaus, N.; Gjesdal, C.G.; Almås, B.; Christensen, M.; Grimsgaard, A.S.; Berntsen, G.K.R.; Salomonsen, L.; Fønnebø, V. Vita-min K2 supplementation does not influence bone loss in early menopausal women: A randomised double-blind place-bocontrolled trial. Osteoporos. Int. 2010, 21, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.R.; McGee, P.L.; Furuya, K.N.; Roberts, E.A. Prevalence of Vitamin K Deficiency in Children with Mild to Moderate Chronic Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 71–76. [Google Scholar] [CrossRef]
- Strople, J.; Lovell, G.; Heubi, J. Prevalence of Subclinical Vitamin K Deficiency in Cholestatic Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 78–84. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Yamazaki, S.; Watanabe, T.; Abe, T. Vitamin K Deficiency in Severely Disabled Children. J. Child Neurol. 2003, 18, 93–97. [Google Scholar] [CrossRef]
- Quinn, L.; Sheh, A.; Ellis, J.L.; Smith, D.E.; Booth, S.L.; Fu, X.; Muthupalani, S.; Ge, Z.; Puglisi, D.A.; Wang, T.C.; et al. Helicobacterpylori antibiotic eradication coupled with a chemically defined diet in INS-GAS mice triggers dysbiosis and vitamin K deficiency resulting in gastric hemorrhage. Gut Microb. 2020, 19, 1–22. [Google Scholar]
- Henry, N.K.; Hoecker, J.L.; Rhodes, K.H. Antimicrobial therapy for infants and children: Guidelines for the inpatient and outpatient practice of pediatriac infectious diseases. Mayo Clin. Proc. 2000, 75, 86–97. [Google Scholar] [CrossRef]
- Aljebab, F.; Choonara, I.; Conroy, S. Systematic Review of the Toxicity of Long-Course Oral Corticosteroids in Children. PLoS ONE 2017, 12, e0170259. [Google Scholar] [CrossRef]
- Ward, L.M. Osteoporosis due to Glucocorticoid Use in Children with Chronic Illness. Horm. Res. Paediatr. 2005, 64, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.B. Glucocorticoid-Induced Osteoporosis in Children: Impact of the Underlying Disease. Pediatrics 2007, 119, S166–S174. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, I.; Oshima, H. Vitamin K2 as a potential therapeutic agent for glucocorticoid-induced osteoporosis. Clin. Calcium 2007, 17. [Google Scholar]
- Jayasena, A.; Atapattu, N.; Lekamwasam, S. Treatment of glucocorticoid-induced low bone mineral density in children: A sys-tematic review. Int. J. Rheum. Dis. 2015, 18, 287–293. [Google Scholar] [CrossRef]
- Inoue, T.; Sugiyama, T.; Matsubara, T.; Kawai, S.; Furukawa, S. Inverse correlation between the changes of lumbar bone min-eral density and serum undercarboxylated osteocalcin after vitamin K2 (menatetrenone) treatment in children treated with glu-cocorticoid and alfacalcidol. Endocr. J. 2001, 48, 11–18. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Shi, X.; Weng, S.-J.; Xie, J.; Tang, J.-H.; Yan, D.-Y.; Wang, B.-Z.; Xie, Z.-J.; Wu, Z.-Y.; Yang, L. Vitamin K2 Can Rescue the Dexamethasone-Induced Downregulation of Osteoblast Autophagy and Mitophagy Thereby Restoring Osteoblast Function In Vitro and In Vivo. Front. Pharmacol. 2020, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Mandatori, D.; Pelusi, L.; Schiavone, V.; Pipino, C.; Di Pietro, N.; Pandolfi, A. The Dual Role of Vitamin K2 in “Bone-Vascular Crosstalk”: Opposite Effects on Bone Loss and Vascular Calcification. Nutrients 2021, 13, 1222. [Google Scholar] [CrossRef]
- Prynne, C.J.; Thane, C.W.; Prentice, A.; Wadsworth, M.E.J. Intake and sources of phylloquinone (vitamin K1) in 4-year-old British children: Comparison between 1950 and the 1990s. Public Health Nutr. 2005, 8, 171–180. [Google Scholar] [CrossRef]
- Khosla, S.; Melton, L.J., 3rd; Dekutoski, M.B.; Achenbach, S.J.; Oberg, A.L.; Riggs, BL. Incidence of childhood distal forearm fractures over 30 years: A population-based study. JAMA 2003, 290, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- van Summeren, M.; Braam, L.; Noirt, F.; Kuis, W.; Vermeer, C. Pronounced Elevation of Undercarboxylated Osteocalcin in Healthy Children. Pediatr. Res. 2007, 61, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Magdeleyns, E.J.; Braam, L.A.J.L.M.; Teunissen, K.J.; Knapen, M.H.; Binnekamp, I.A.G.; van Summeren, M.J.H.; Vermeer, C. Vitamin K status in healthy volunteers. Food Funct. 2013, 5, 229–234. [Google Scholar] [CrossRef]
- Ferland, G.; Vitamin, K. Present Knowledge in Nutrition, 9th ed.; Bowman, B.A., Russell, R.M., Eds.; ILSI Press: Washington, DC, USA, 2006; Volume 1, pp. 220–230. [Google Scholar]
- Olson, R.E. Vitamin K. Modern Nutrition in Health and Disease, 9th ed.; Shils, M., Olson, J.A., Shike, M., Ross, A.C., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1999; pp. 363–380. [Google Scholar]
- Shirahata, A.; Nakamura, T.; Ariyoshi, N. Vitamin K1 and K2 contents in blood, stool, and liver tissues of nenotes and young infants. In Perinatal Trombosis and Hemostasis; Suzuki, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 213–223. [Google Scholar]
- Oldenburg, J.; von Brederlow, B.; Fregin, A.; Rost, S.; Wolz, W.; Eberl, W.; Eber, S.; Lenz, E.; Schwaab, R.; Brackmann, H.H.; et al. Congenital deficiency of vitamin K dependent coagulation factors in two families presents as a genetic defect of the vitamin K-epoxide-reductase-complex. Thromb. Haemost. 2000, 84, 937–941. [Google Scholar] [PubMed]
- Bevans, C.G.; Fregin, A.; Geisen, C.; Müller-Reible, C.; Watzka, M.; Oldenburg, J. Current pharmacogenetic developments in oral anticoagulation therapy: The influence of variant VKORC1 and CYP2C9 alleles. Thromb. Haemost. 2007, 98, 570–578. [Google Scholar] [CrossRef][Green Version]
- Greer, F.R.; Marshall, S.; Cherry, J.; Suttie, J.W. Vitamin K status of lactating mothers, human milk, and breast-feeding infants. Pediatrics 1991, 88, 751–756. [Google Scholar] [PubMed]
- Beutler, E.; Lichtman, M.A.; Coller, B.S. Disorders of the vitamin K dependent coagulation factors. In Williams Hematology, 5th ed.; McGraw-Hill: New York, NY, USA, 1995; pp. 1481–1485. [Google Scholar]
- Lippi, G.; Franchini, M. Vitamin K in neonates: Facts and myths. Blood Transfus. 2011, 9, 4–9. [Google Scholar] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv. Nutr. 2018, 9 (Suppl. 1), 278S–294S.
- Canfield, L.M.; Hopkinson, J.M.; Lima, A.F.; Martin, G.S.; Sugimoto, K.; Burr, J.; Clark, L.; McGee, D.L. Quantitation of vita-min K in human milk. Lipids 1990, 25, 406–411. [Google Scholar] [CrossRef]
- Kojima, T.; Asoh, M.; Yamawaki, N.; Kanno, T.; Hasegawa, H.; Yonekubo, A. Vitamin K concentrations in the maternal milk of Japanese women. Acta Paediatr. 2004, 93, 457–463. [Google Scholar] [PubMed]
- Kamao, M.; Tsugawa, N.; Suhara, Y.; Wada, A.; Mori, T.; Murata, K.; Nishino, R.; Ukita, T.; Uenishi, K.; Tanaka, K.; et al. Quantification of fat-soluble vitamins in human breast milk by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 859, 192–200. [Google Scholar] [CrossRef]
- Greer, F.R. Are breast-fed infants vitamin K deficient? Adv. Exp. Med. Biol. 2001, 501, 391–395. [Google Scholar] [PubMed]
- Food and Nutrition Board, Institute of Medicine. Vitamin K. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001; pp. 162–196. [Google Scholar]
- Thijssen, H.H.W.; Drittij, M.-J.; Vermeer, C.; Schoffelen, E. Menaquinone-4 in breast milk is derived from dietary phylloqui-none. Br. J. Nutr. 2002, 87, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kazzi, N.J.; Ilagan, N.B.; Liang, K.C.; Kazzi, G.M.; Grietsell, L.A.; Brans, Y.W. Placental transfer of vitamin K1 in preterm pregnancy. Obstet. Gynecol. 1990, 75. [Google Scholar]
- Liu, J.; Wang, Q.; Gao, F.; He, J.W.; Zhao, J.H. Maternal antenatal administration of vitamin K1 results in increasing the activities of vitamin K-dependent coagulation factors in umbilical blood and in decreasing the incidence rate of periventricular-intraventricular hemorrhage in premature infants. J. Perinat Med. 2006, 34, 173–176. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Chen, Y.-H.; Qin, G.-L.; Zhao, J.-H.; Zhu, L.-C. Level of vitamin K-dependent coagulation factors in premature infants and the influence of maternal antenatal administration of vitamin K1 on their activity. Chin. J. Pediatr. 2005, 43, 908–910. [Google Scholar]
- El-Ganzoury, M.M.; El-Farrash, R.A.; Saad, A.A.; Ali, M.S.; El-Bhbiti, A.R.; Selem, A.M. Antenatal administration of vitamin K1: Relationship to vitamin K-dependent coagulation factors and incidence rate of periventricular-intraventricular hemorrhage in preterm infants; Egyptian randomized controlled trial. J. Matern. Neonatal Med. 2013, 27, 816–820. [Google Scholar] [CrossRef]
- Shearer, M.J. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev. 2009, 23, 49–59. [Google Scholar] [CrossRef]
- Hey, E. Neonatal Formulary, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA; Limited European Distribution Centre New Era Estate, Oldlands WayBognor Regis: West Sussex, UK, 2011; pp. 531–532. [Google Scholar]
- Chuansumrit, A.; Plueksacheeva, T.; Hanpinitsak, S.; Sangwarn, S.; Chatvutinun, S.; Suthutvoravut, U.; Herabutya, Y.; Shearer, M.J. Prevalence of subclinical vitamin K deficiency in Thai newborns: Relationship to maternal phylloquinone intakes and delivery risk. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 95, F104–F108. [Google Scholar] [CrossRef]
- Shearer, M.J.; Fu, X.; Booth, S.L. Vitamin K Nutrition, Metabolism, and Requirements: Current Concepts and Future Research. Adv. Nutr. Int. Rev. J. 2012, 3, 182–195. [Google Scholar] [CrossRef]
- von Kries, R.; Hanawa, Y. Neonatal vitamin K prophylaxis. Report of Scientific and Standardization Subcommittee on Perinatal Haemostasis. Thromb. Haemost. 1993, 69, 293–295. [Google Scholar] [CrossRef]
- Nagao, T.; Nakayama, K. Vitamin K deficiency in infancy in Japan. Pediatrics 1984, 74, 315–316. [Google Scholar] [CrossRef]
- Hanawa, Y.; Maki, M.; Murata, B.; Matsuyama, E.; Yamamoto, Y.; Nagao, T.; Yamada, K.; Ikeda, I.; Terao, T.; Mikami, S.; et al. The second nation-wide survey in Japan of vitamin K deficiency in infancy. Eur. J. Nucl. Med. Mol. Imaging 1988, 147, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Fewtrell, M.; Mis, N.F.; Hojsak, I.; Hulst, J.; Indrio, F.; et al. ESPGHAN Committee on Nutrition. Prevention of Vitamin K Deficiency Bleeding in Newborn Infants: A Position Paper by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes for Japanese. Authorized Guidelines Published by the Ministry of Health, Labour and Welfare of Japan. 2015. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Full_DRIs2015.pdf (accessed on 23 October 2021).
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Vermeer, C.; Beulens, J.W. Abstract P071: The Effect of Menaquinone -7 Supplementation on Circulating Species of Matrix-Gla Protein. Circulation 2012, 125. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Braam, L.A.; Drummen, N.E.; Bekers, O.; Hoeks, A.P.G.; Vermeer, C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Knapen, M.H.J.; Drummen, N.E.; Smit, E.; Vermeer, C.; Theuwissen, E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos. Int. 2013, 24, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion: Vitamin K2 added for nutritional purposes in foods for particular nutritional uses, food supplements and foods intended for the general population and vitamin K2 as a source of vitamin K added for nutritional purposes to foodstuffs, in the context of Regulation (EC) No. 258/971. EFSA J. 2008, 822, 1–31. [Google Scholar]
- World Health Organization; Food and Agriculture Organization of the United Nations. Vitamin K. In Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; WHO: Geneva, Switzerland, 2004; pp. 108–129. [Google Scholar]
- Marles, R.J.; Roe, A.L.; Oketch-Rabah, H.A. US Pharmacopeial Convention safety evaluation of menaquinone-7, a form of vitamin K. Nutr. Rev. 2017, 75, 553–557. [Google Scholar] [CrossRef] [PubMed]
| Vitamin K1 (Phylloquinone) | Vitamin K2 (Menaquinones) | |
|---|---|---|
| Major source | Leafy greens, fruits, vegetable oils | Fermented food (natto), animal products (meat, dairy) |
| Half time | ∼3 h | MK-4: ∼1.5 h MK-7: ∼70 h |
| Efficacy | Low. High dosages (mg) are needed to improve vitamin K status | MK-4: Low. High dosages (mg) are needed to improve vitamin K status MK-7: High. Low dosages (mcg) are needed to improve vitamin K status |
| Mechanism of action | Activation of VKDPs (mainly hepatic) Antioxidant Anti-inflammatory effects | Activation of VKDPs (hepatic and extra hepatic) Antioxidant Anti-inflammatory effects Transcriptional regulator Mitochondrial electron carrier, helping to maintain normal ATP levels |
| Utilization | Primarily used by liver, helps maintaining healthy blood clotting | Can be used by liver, helps maintaining healthy blood clotting Available for extra hepatic tissues. Essential for bone strength, blood vessel health, brain development and more |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozioł-Kozakowska, A.; Maresz, K. The Impact of Vitamin K2 (Menaquionones) in Children’s Health and Diseases: A Review of the Literature. Children 2022, 9, 78. https://doi.org/10.3390/children9010078
Kozioł-Kozakowska A, Maresz K. The Impact of Vitamin K2 (Menaquionones) in Children’s Health and Diseases: A Review of the Literature. Children. 2022; 9(1):78. https://doi.org/10.3390/children9010078
Chicago/Turabian StyleKozioł-Kozakowska, Agnieszka, and Katarzyna Maresz. 2022. "The Impact of Vitamin K2 (Menaquionones) in Children’s Health and Diseases: A Review of the Literature" Children 9, no. 1: 78. https://doi.org/10.3390/children9010078
APA StyleKozioł-Kozakowska, A., & Maresz, K. (2022). The Impact of Vitamin K2 (Menaquionones) in Children’s Health and Diseases: A Review of the Literature. Children, 9(1), 78. https://doi.org/10.3390/children9010078








